Structural Variations in Biobased Polyfurfuryl Alcohol Induced by Polymerization in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PFA Resins
2.3. Characterization of the PFA Resins
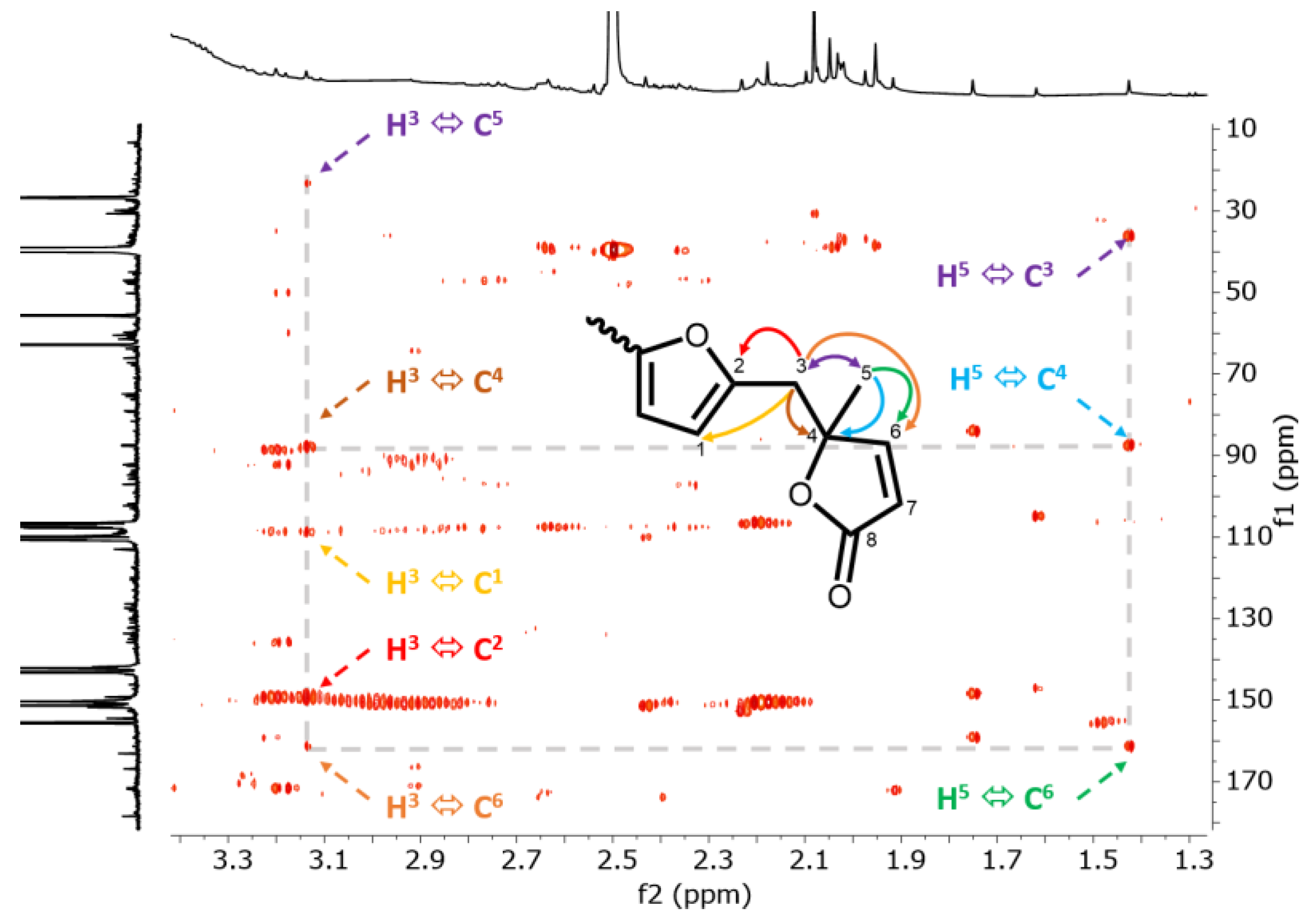
2.4. Nuclear Magnetic Resonance (NMR)
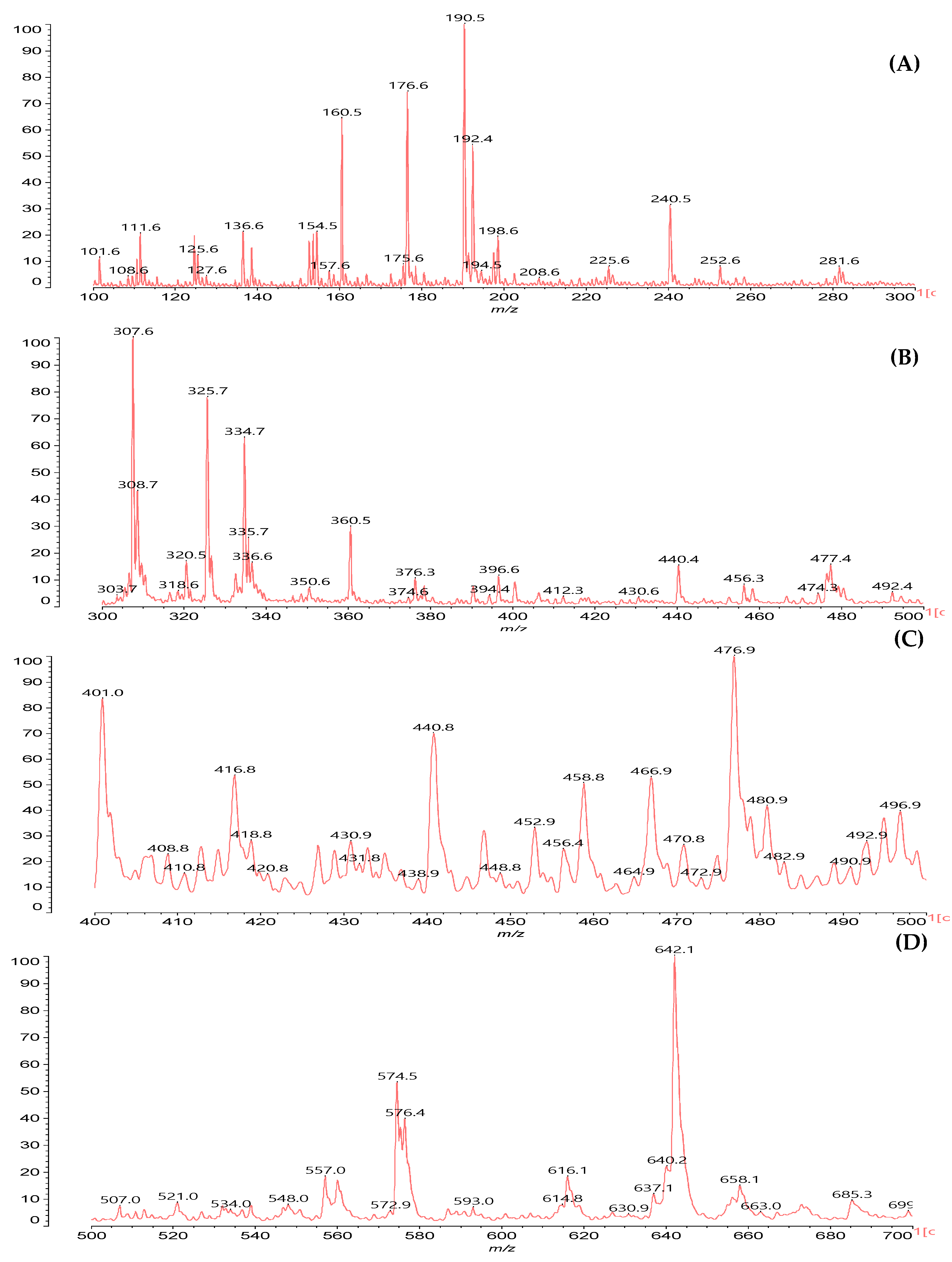
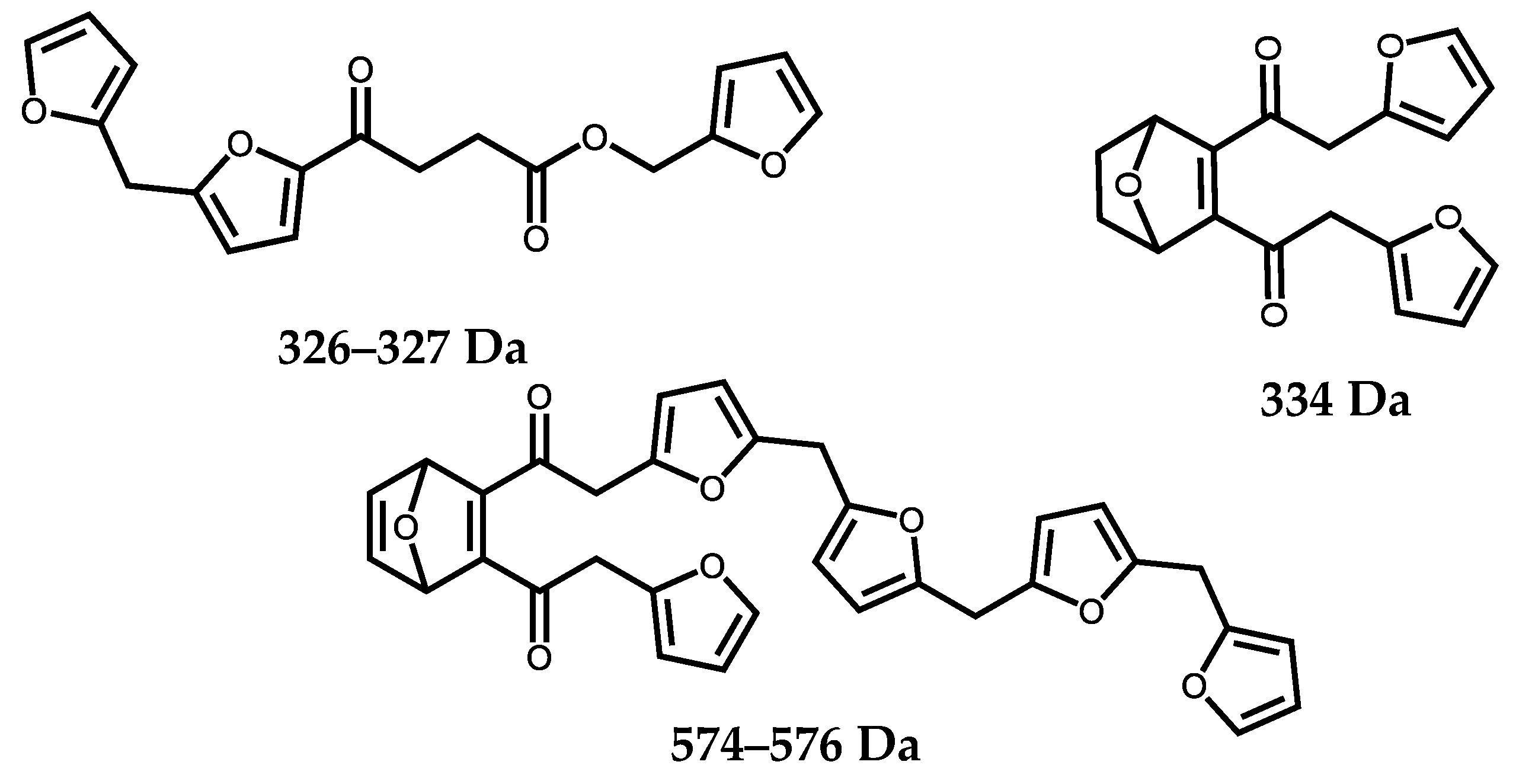
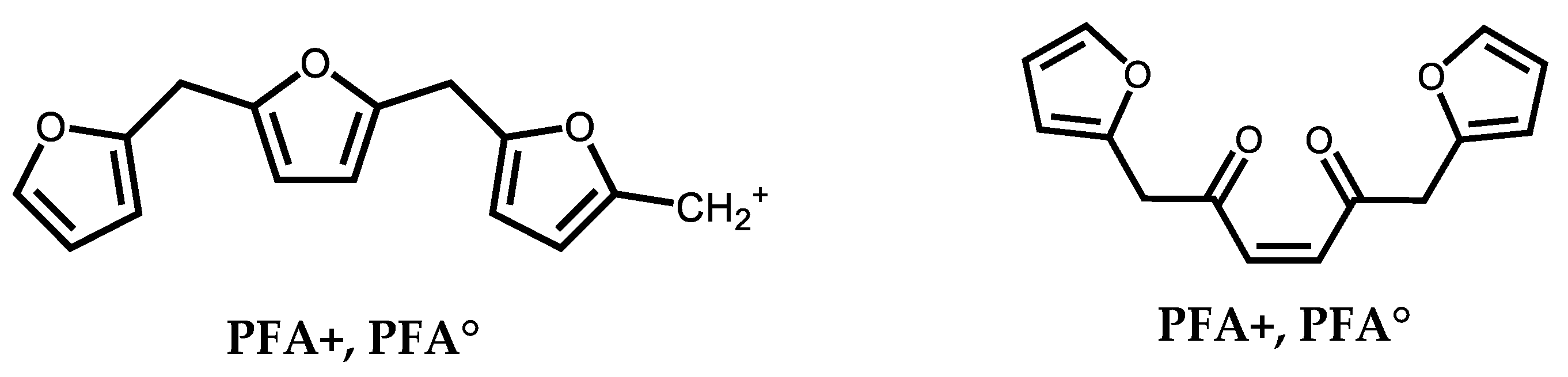
2.5. MALDI ToF Mass Spectrometry
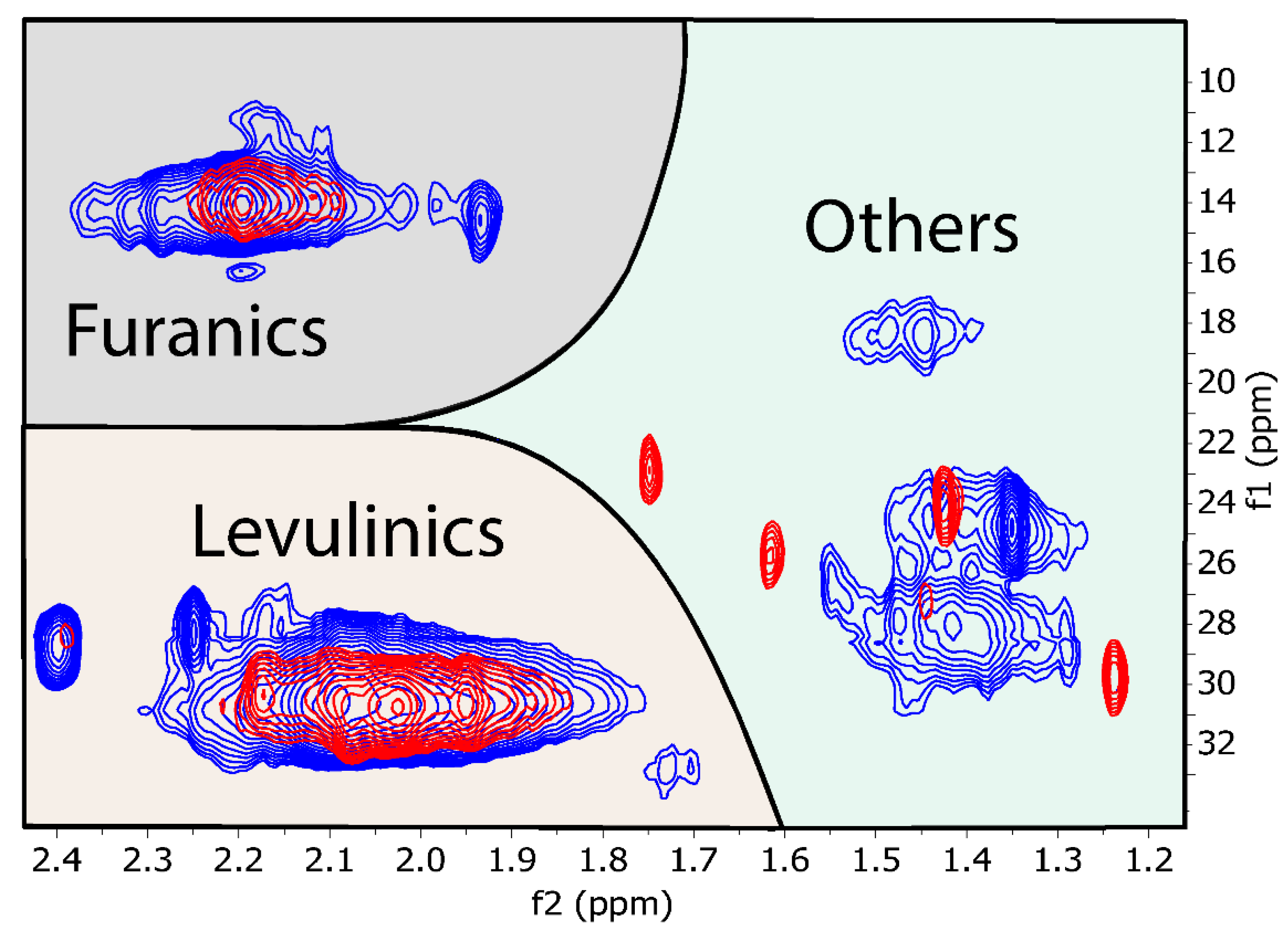
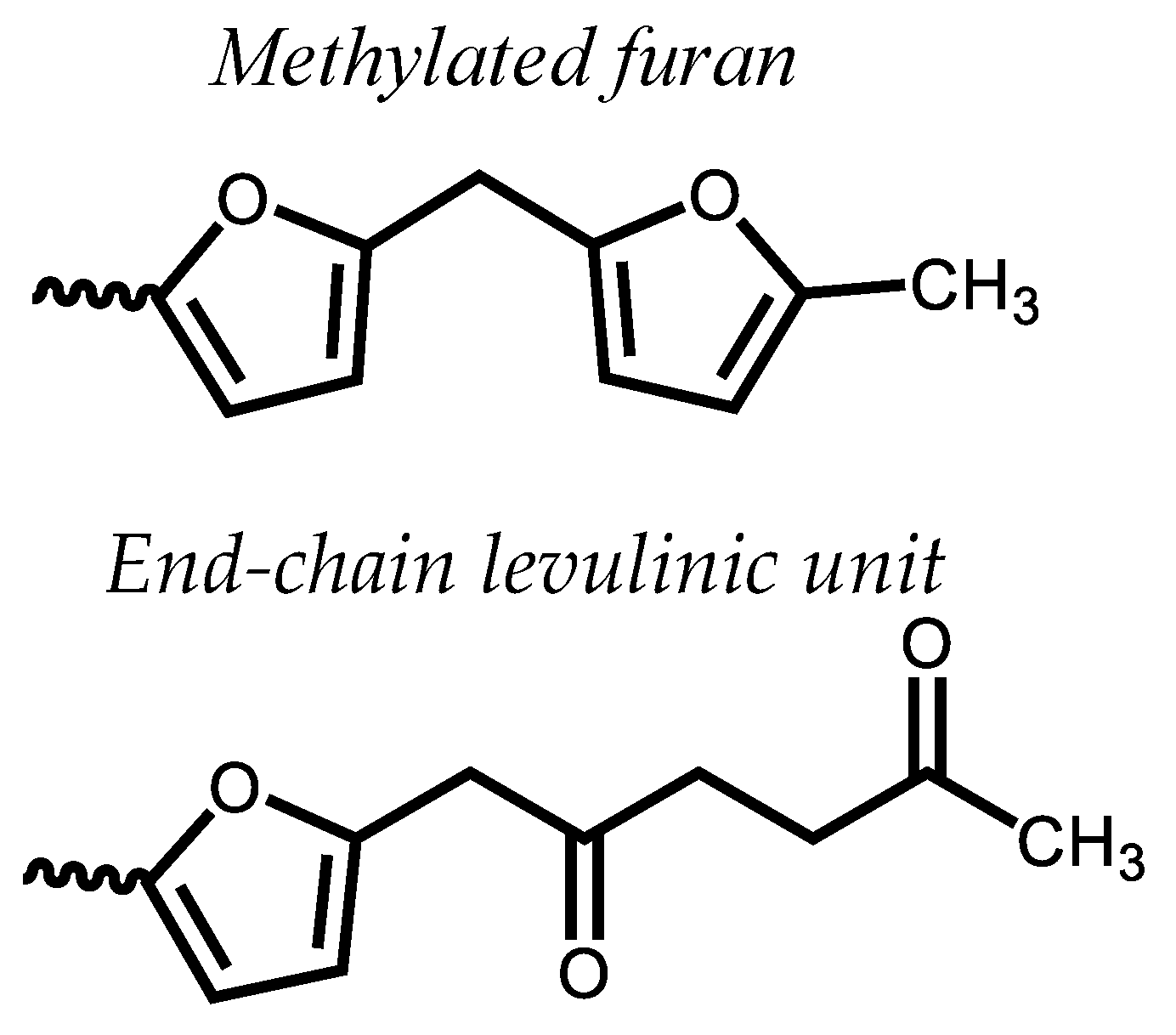
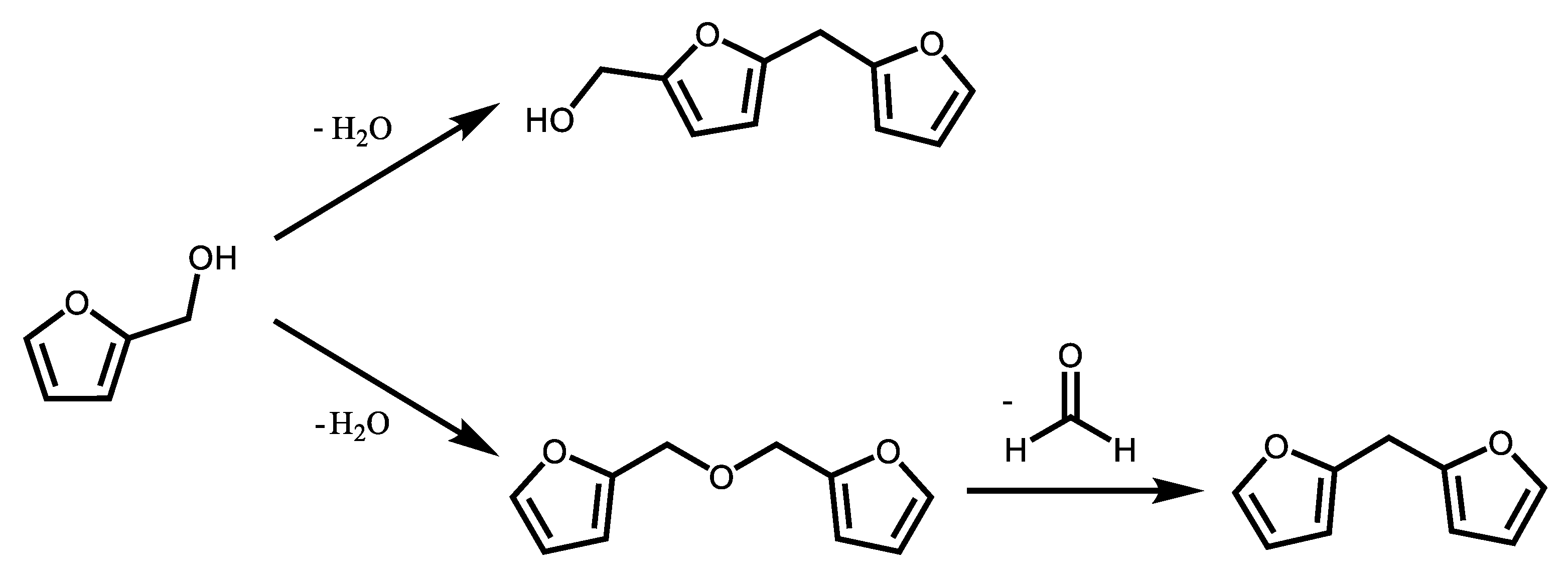
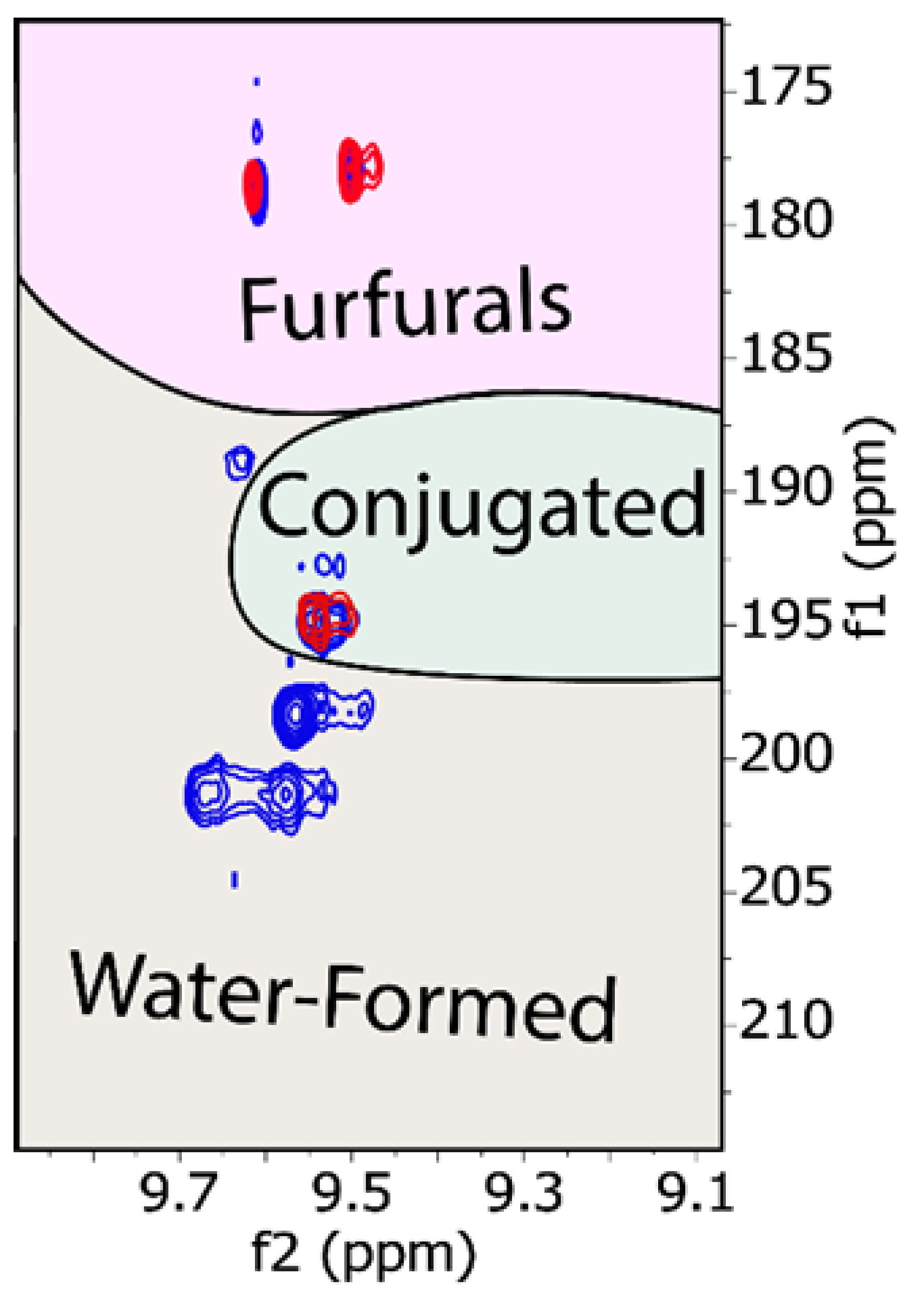
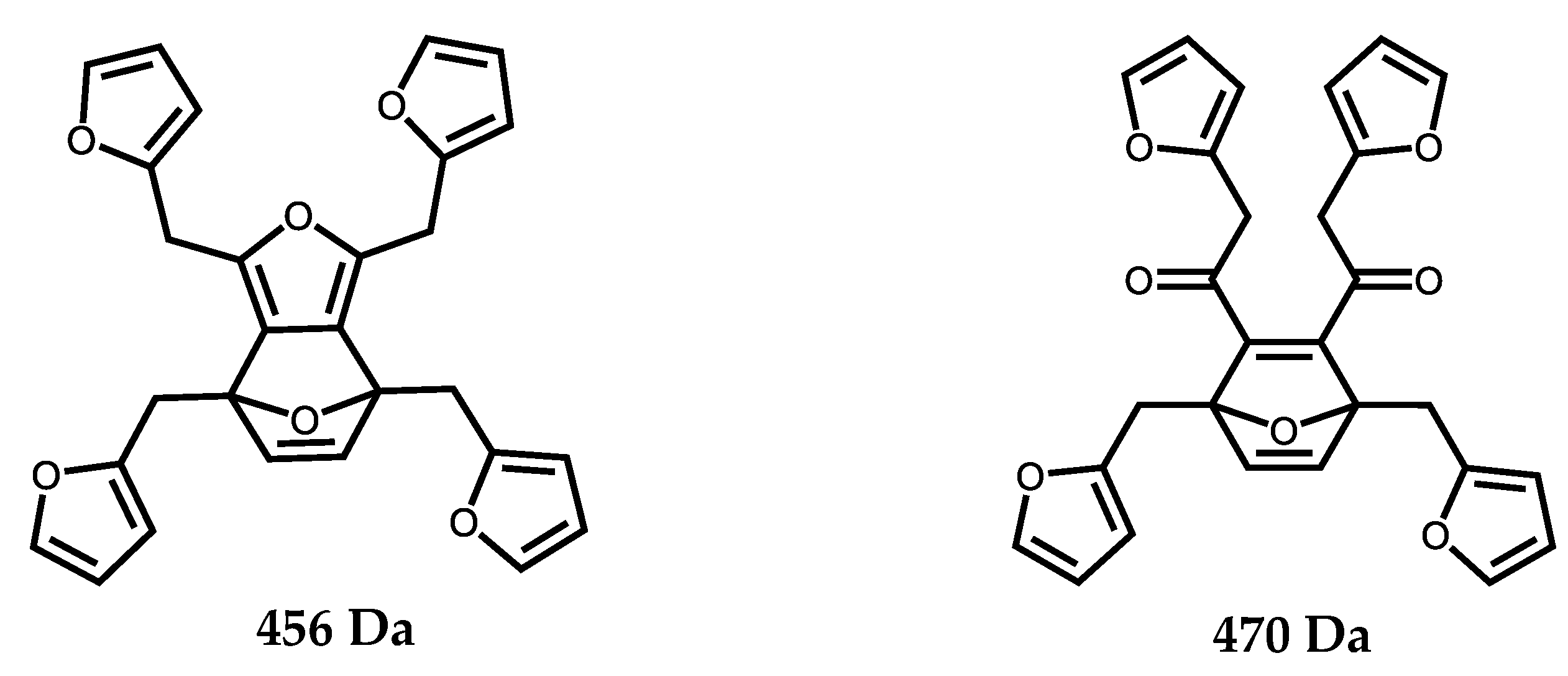
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Biron, M. The Plastics Industry: Economic Overview. In Thermosets and Composites, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014; pp. 25–104. [Google Scholar]
- Mahajan, J.S.; O’Dea, R.M.; Norris, J.B.; Korley, L.S.T.J.; Epps, T.H. Aromatics from Lignocellulosic Biomass: A Platform for High-Performance Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 15072–15096. [Google Scholar] [CrossRef]
- Pizzi, A.; Ibeh, C.C. Phenol-Formaldehyde Resins. In Handbook of Thermosets and Plastics, 4th ed.; Elsevier Inc.: Oxford, UK, 2022; pp. 13–40. [Google Scholar]
- Abdullah, U.H.B.; Pizzi, A. Tannin-Furfuryl Alcohol Wood Panel Adhesives without Formaldehyde. Eur. J. Wood Wood Prod. 2013, 71, 131–132. [Google Scholar] [CrossRef]
- Gonçalves, D.; Bordado, J.M.; Marques, A.C.; Dos Santos, R.G. Non-Formaldehyde, Bio-Based Adhesives for Use in Wood-Based Panel Manufacturing Industry—A Review. Polymers 2021, 13, 4086. [Google Scholar] [CrossRef] [PubMed]
- Foyer, G.; Chanfi, B.H.; Boutevin, B.; Caillol, S.; David, G. New Method for the Synthesis of Formaldehyde-Free Phenolic Resins from Lignin-Based Aldehyde Precursors. Eur. Polym. J. 2016, 74, 296–309. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Thébault, M.; Pizzi, A.; Celzard, A. Structure and Properties of Poly(Furfuryl Alcohol)-Tannin PolyHIPEs. Eur. Polym. J. 2016, 78, 195–212. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Bahabri, F.; Ganash, A. Furanic Copolymers with Synthetic and Natural Phenolic Materials for Wood Adhesives—A MALDI TOF Study. Maderas Cienc. Tecnol. 2015, 17, 99–104. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-Friendly Composite Resins Based on Renewable Biomass Resources: Polyfurfuryl Alcohol/Lignin Thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]
- Zhu, X.; Bruijnaers, B.; Lourençon, T.V.; Balakshin, M. Structural Analysis of Lignin-Based Furan Resin. Materials 2022, 15, 350. [Google Scholar] [CrossRef]
- Pin, J.M.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N.; Mija, A. Copolymerization as a Strategy to Combine Epoxidized Linseed Oil and Furfuryl Alcohol: The Design of a Fully Bio-Based Thermoset. ChemSusChem 2015, 8, 4149–4161. [Google Scholar] [CrossRef]
- Pin, J.M.; Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N.; Van Der Waal, J.C.; De Jong, E. Valorization of Biorefinery Side-Stream Products: Combination of Humins with Polyfurfuryl Alcohol for Composite Elaboration. ACS Sustain. Chem. Eng. 2014, 2, 2182–2190. [Google Scholar] [CrossRef]
- Menager, C.; Guigo, N.; Wu, X.; Vincent, L.; Sbirrazzuoli, N. “Green” Composites Prepared from Polyfurfuryl Alcohol and Cork Residues: Thermal and Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105473. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Du, G. Furfuryl Alcohol-Aldehyde Plywood Adhesive Resins. J. Adhes. 2020, 96, 814–838. [Google Scholar] [CrossRef]
- Deka, H.; Misra, M.; Mohanty, A. Renewable Resource Based “All Green Composites” from Kenaf Biofiber and Poly(Furfuryl Alcohol) Bioresin. Ind. Crops Prod. 2013, 41, 94–101. [Google Scholar] [CrossRef]
- Lems, E.M.; Winklehner, S.; Hansmann, C.; Gindl-Altmutter, W.; Veigel, S. Reinforcing Effect of Poly(Furfuryl Alcohol) in Cellulose-Based Porous Materials. Cellulose 2019, 26, 4431–4444. [Google Scholar] [CrossRef]
- Bosq, N.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. Thermomechanical Behavior of a Novel Biobased Poly(Furfurylalcohol)/Silica Nanocomposite Elaborated by Smart Functionalization of Silica Nanoparticles. Polym. Degrad. Stab. 2015, 118, 137–146. [Google Scholar] [CrossRef]
- Wang, P.; Deng, G.; Zhu, H.; Zhang, H.; Yin, J.; Xiong, X.; Wu, X. Effect of MWCNT Content on Conductivity and Mechanical and Wear Properties of Copper Foam/Resin Composite. Compos. Part B Eng. 2019, 168, 572–580. [Google Scholar] [CrossRef]
- Montagna, L.S.; da Silva, A.P.B.; de Melo Morgado, G.F.; Ribeiro, B.; Passador, F.R.; Rezende, M.C. PFA Nanocomposites: The Influence of Three Carbon Nanofillers on the Mechanical and Electromagnetic Properties. J. Polym. Res. 2021, 28, 253. [Google Scholar] [CrossRef]
- Shibutani, K.; Nakai, J.; Aphichartsuphapkhajorn, K.; Kayaki, Y.; Kuwata, S.; Arao, Y.; Kubouchi, M. The Activation of Furfuryl Alcohol Polymerization by Oxygen and Its Enhanced Mechanical Properties. J. Appl. Polym. Sci. 2021, 138, 50311. [Google Scholar] [CrossRef]
- Pranger, L.A.; Nunnery, G.A.; Tannenbaum, R. Mechanism of the Nanoparticle-Catalyzed Polymerization of Furfuryl Alcohol and the Thermal and Mechanical Properties of the Resulting Nanocomposites. Compos. Part B Eng. 2012, 43, 1139–1146. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Mechanisms of Chromophore Formation and Cross-Linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Chemorheological Analysis and Model-Free Kinetics of Acid Catalysed Furfuryl Alcohol Polymerization. Phys. Chem. Chem. Phys. 2007, 9, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Zavaglia, R.; Guigo, N.; Sbirrazzuoli, N.; Mija, A.; Vincent, L. Complex Kinetic Pathway of Furfuryl Alcohol Polymerization Catalyzed by Green Montmorillonite Clays. J. Phys. Chem. B 2012, 116, 8259–8268. [Google Scholar] [CrossRef] [PubMed]
- Falco, G.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. FA Polymerization Disruption by Protic Polar Solvents. Polymers 2018, 10, 529. [Google Scholar] [CrossRef]
- Falco, G.; Guigo, N.; Vincent, L.; Sbirrazzuoli, N. Opening Furan for Tailoring Properties of Bio-Based Poly(Furfuryl Alcohol) Thermoset. ChemSusChem 2018, 11, 1805–1812. [Google Scholar] [CrossRef]
- González Maldonado, G.M.; Assary, R.S.; Dumesic, J.; Curtiss, L.A. Experimental and Theoretical Studies of the Acid-Catalyzed Conversion of Furfuryl Alcohol to Levulinic Acid in Aqueous Solution. Energy Environ. Sci. 2012, 5, 6981–6989. [Google Scholar] [CrossRef]
- Chappaz, A.; Lai, J.; De Oliveira Vigier, K.; Morvan, D.; Wischert, R.; Corbet, M.; Doumert, B.; Trivelli, X.; Liebens, A.; Jérôme, F. Selective Conversion of Concentrated Feeds of Furfuryl Alcohol to Alkyl Levulinates Catalyzed by Metal Triflates. ACS Sustain. Chem. Eng. 2018, 6, 4405–4411. [Google Scholar] [CrossRef]
- Conley, R.T.; Metil, I. An Investigation of the Structure of Furfuryl Alcohol Polycondensates with Infrared Spectroscopy. J. Appl. Polym. Sci. 1963, 7, 37–52. [Google Scholar] [CrossRef]
- Barsberg, S.; Thygesen, L.G. Poly(Furfuryl Alcohol) Formation in Neat Furfuryl Alcohol and in Cymene Studied by ATR-IR Spectroscopy and Density Functional Theory (B3LYP) Prediction of Vibrational Bands. Vib. Spectrosc. 2009, 49, 52–63. [Google Scholar] [CrossRef]
- Kim, T.; Assary, R.S.; Marshall, C.L.; Gosztola, D.J.; Curtiss, L.A.; Stair, P.C. Acid-Catalyzed Furfuryl Alcohol Polymerization: Characterizations of Molecular Structure and Thermodynamic Properties. ChemCatChem 2011, 3, 1451–1458. [Google Scholar] [CrossRef]
- Tondi, G.; Cefarin, N.; Sepperer, T.; D’Amico, F.; Berger, R.J.F.; Musso, M.; Birarda, G.; Reyer, A.; Schnabel, T.; Vaccari, L. Understanding the Polymerization of Polyfurfuryl Alcohol: Ring Opening and Diels-Alder Reactions. Polymers 2019, 11, 2126. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Musso, M.E.; Berger, R.J.F.; Cefarin, N.; Birarda, G.; Tondi, G.; Bertoldo Menezes, D.; Reyer, A.; Scarabattoli, L.; Sepperer, T.; et al. Chemical Constitution of Polyfurfuryl Alcohol Investigated by FTIR and Resonant Raman Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120090. [Google Scholar] [CrossRef]
- Delliere, P.; Guigo, N. Monitoring the Degree of Carbonyl-Based Open Structure in a Furanic Macromolecular System. Macromolecules 2022, 55, 1196–1204. [Google Scholar] [CrossRef]
- Quinquet, L.; Delliere, P.; Guigo, N. Conditions to Control Furan Ring Opening during Furfuryl Alcohol Polymerization. Molecules 2022, 27, 3212. [Google Scholar] [CrossRef]
- Delliere, P.; Guigo, N. Exploring New Horizons for Bio-Based Poly(Furfuryl Alcohol) by Exploiting Functionalities Offered by Side Reactions. ACS Macro Lett. 2022, 11, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Delliere, P.; Guigo, N. Revealed Pathways of Furan Ring Opening and Surface Crosslinking in Biobased Polyfurfuryl Alcohol. Eur. Polym. J. 2023, 111869. [Google Scholar] [CrossRef]
- Faix, O.; Andersons, B.; Zakis, G. Determination of Carbonyl Groups of Six Round Robin Lignins by Modified Oximation and FTIR Spectroscopyy. Holzforschung 1998, 52, 268–274. [Google Scholar] [CrossRef]
- Black, S.; Ferrell, J.R. Determination of Carbonyl Groups in Pyrolysis Bio-Oils Using Potentiometric Titration: Review and Comparison of Methods. Energy Fuels 2016, 30, 1071–1077. [Google Scholar] [CrossRef]
- Gonzalez, R.; Martinez, R.; Ortiz, P. Polymerization of Furfuryl Alcohols with Trifluoroacetic Acid: The Influence of Experimental Conditions. Macromol. Chem. Phys. 1992, 193, 1–9. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. The Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Old Puzzles Unravelled. Macromol. Symp. 1997, 122, 263–268. [Google Scholar] [CrossRef]
- González, R.; Figueroa, J.M.; González, H. Furfuryl Alcohol Polymerisation by Iodine in Methylene Chloride. Eur. Polym. J. 2002, 38, 287–297. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Buchwalter, S.L. Polymerization of Furfuryl Acetate in Acetonitrile. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 2897–2911. [Google Scholar] [CrossRef]
- Wewerka, E.M. Study of the γ-Alumina Polymerization of Furfuryl Alcohol. J. Polym. Sci. Part A-1 Polym. Chem. 1971, 9, 2703–2715. [Google Scholar] [CrossRef]
- Banfi, D.; Patiny, L. www.nmrdb.org: Resurrecting and Processing NMR Spectra On-line. Chimia 2008, 62, 280–281. [Google Scholar] [CrossRef]















| External Standard | Left-Over Furans | |||
|---|---|---|---|---|
| Aldehyde Content (mmol/g) | Aldehyde Ratio | Aldehyde Content (mmol/g) | Aldehyde Ratio | |
| 0.00 | 0.04 | 0.21 | 0.04 | 0.20 |
| 0.23 | 0.10 | 0.07 | 0.11 | 0.08 |
| 0.55 | 0.09 | 0.05 | 0.13 | 0.07 |
| 0.76 | 0.13 | 0.07 | 0.22 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delliere, P.; Pizzi, A.; Guigo, N. Structural Variations in Biobased Polyfurfuryl Alcohol Induced by Polymerization in Water. Polymers 2023, 15, 1745. https://doi.org/10.3390/polym15071745
Delliere P, Pizzi A, Guigo N. Structural Variations in Biobased Polyfurfuryl Alcohol Induced by Polymerization in Water. Polymers. 2023; 15(7):1745. https://doi.org/10.3390/polym15071745
Chicago/Turabian StyleDelliere, Pierre, Antonio Pizzi, and Nathanael Guigo. 2023. "Structural Variations in Biobased Polyfurfuryl Alcohol Induced by Polymerization in Water" Polymers 15, no. 7: 1745. https://doi.org/10.3390/polym15071745
APA StyleDelliere, P., Pizzi, A., & Guigo, N. (2023). Structural Variations in Biobased Polyfurfuryl Alcohol Induced by Polymerization in Water. Polymers, 15(7), 1745. https://doi.org/10.3390/polym15071745









