Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Expression and Purification
2.3. Material Characterization
2.4. Cell Lines and Animals
2.5. Preparation of CD@PICsomes
2.6. Preparation of Fluorescence Modified CD-Loaded PICsomes
2.7. Interaction of Pre-CL PICsomes and CD
2.8. Evaluation of Stability of CD@PICsome Based on Enzyme Activity Assay
2.9. Evaluation of In Vitro Cytotoxicity
2.10. Evaluation of Plasma Clearance and Biodistribution and Hepatotoxicity of CD@PICsome
2.11. Evaluation of In Vivo Therapeutic Effect
2.12. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Crosslinked PICsomes Loaded with CD (CD@PICsomes) by SWCL
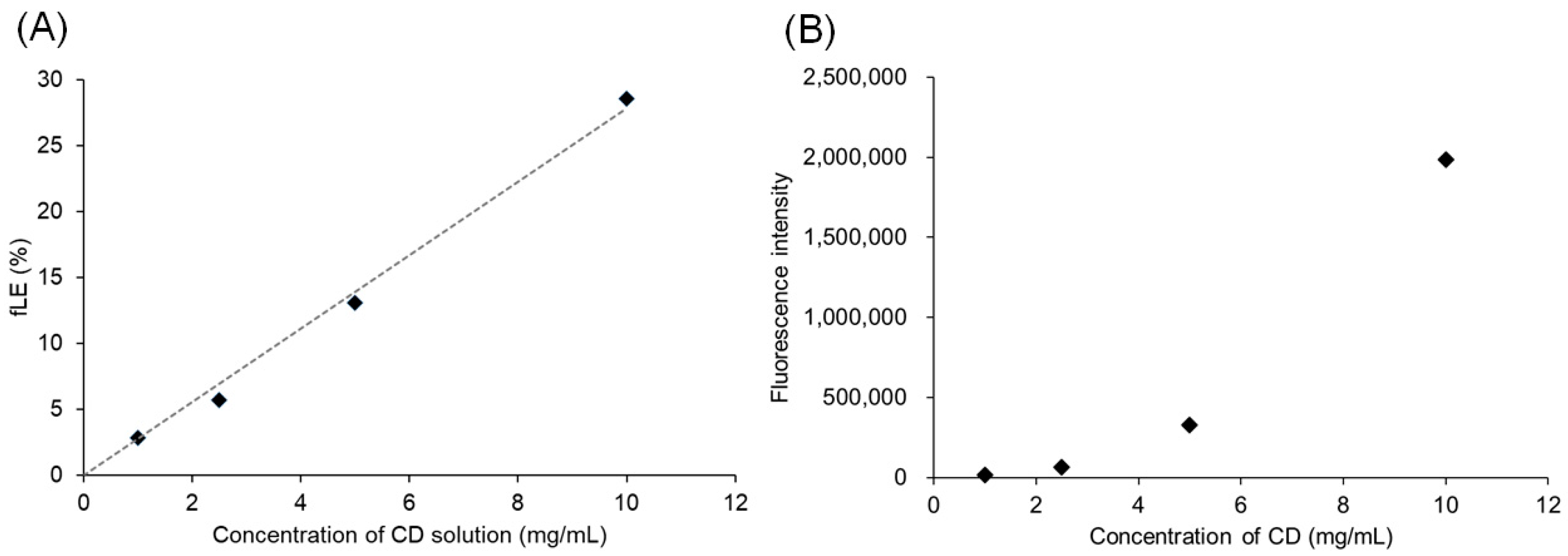
3.2. Interaction of Pre-CL PICsomes and CD
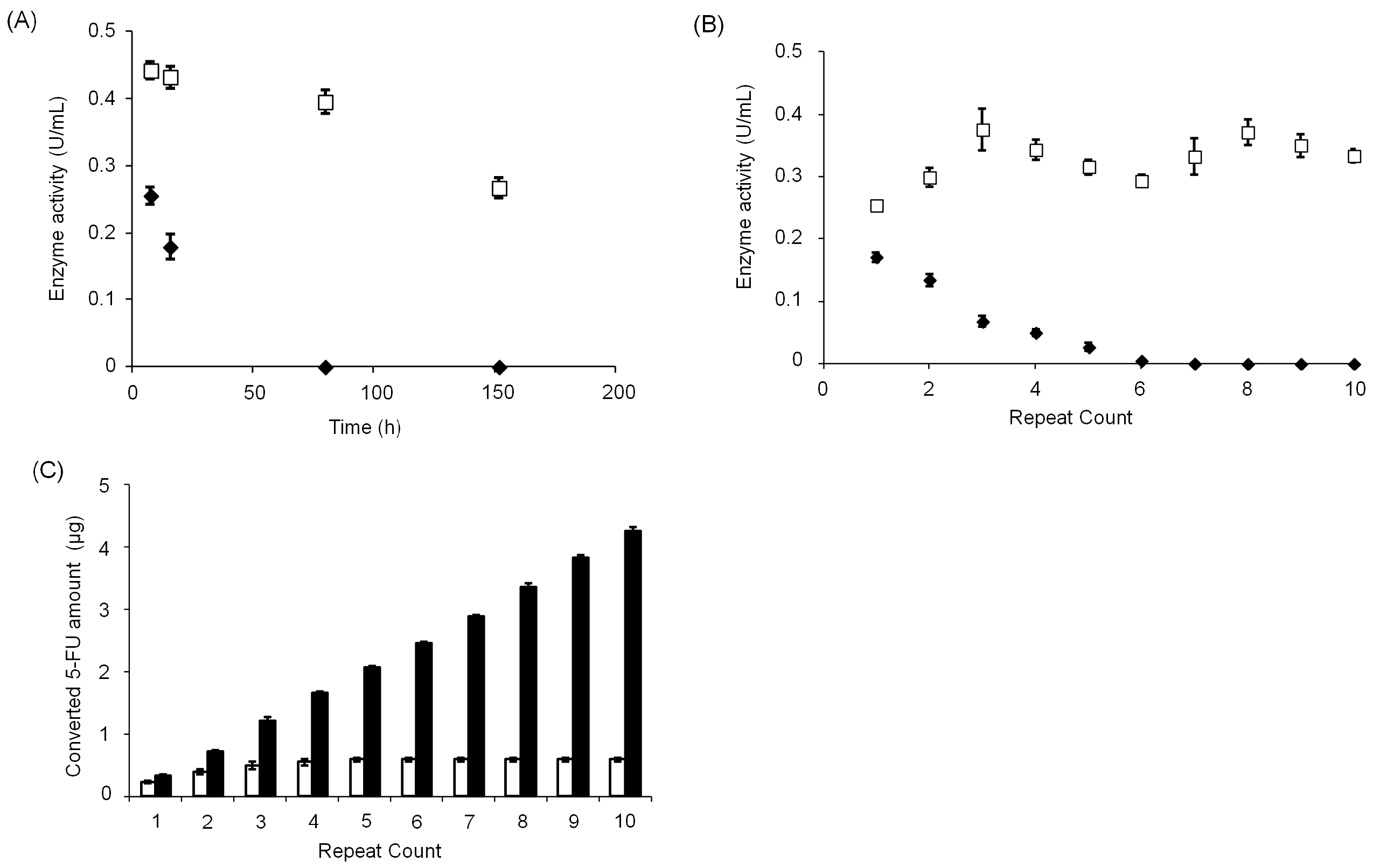
3.3. Stability of CD@PICsome Based on Enzyme Activity
3.4. Evaluation of Cytotoxicity of CD@PICsome
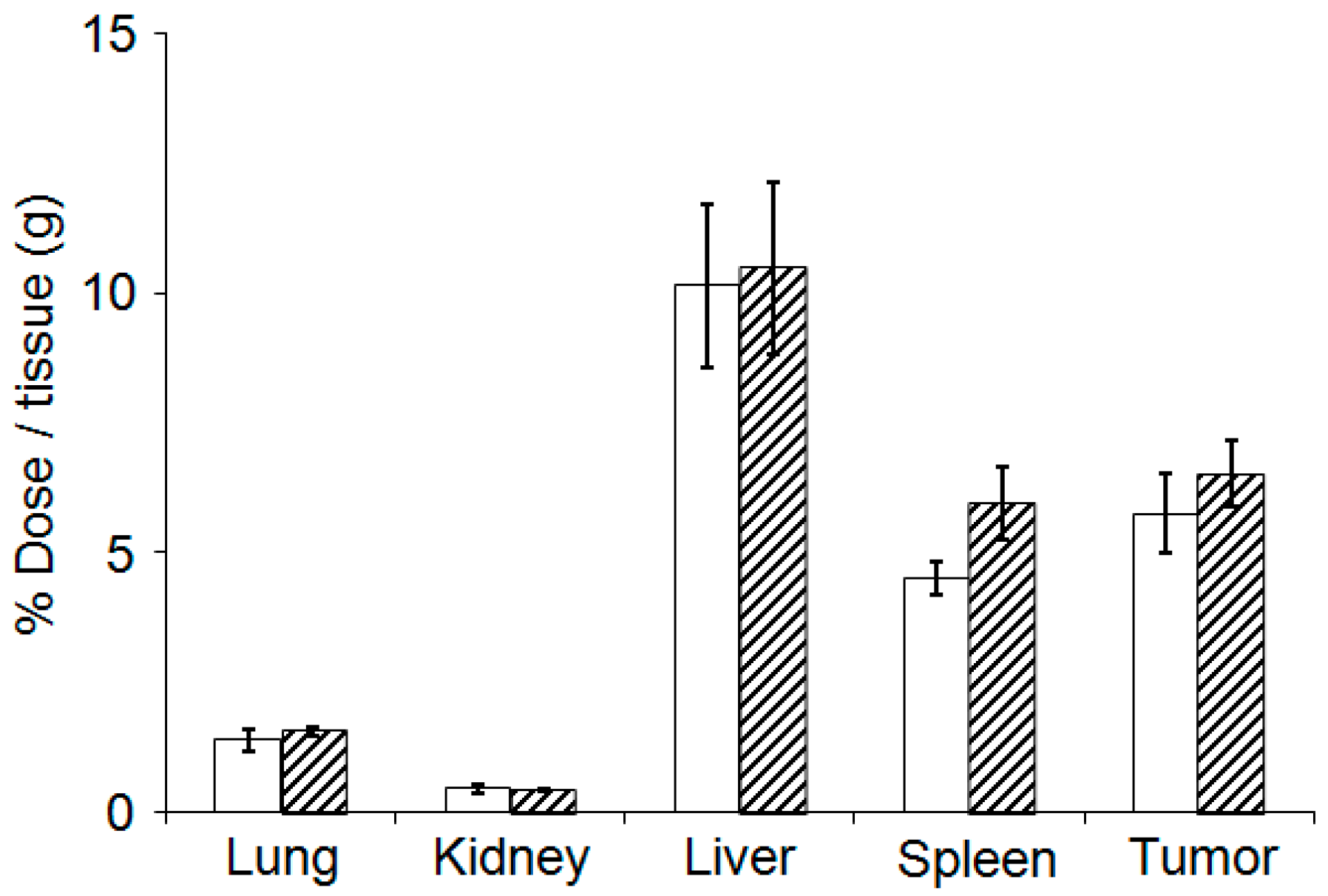
3.5. Evaluation of Plasma Clearance, Biodistribution, and Hepatotoxicity of CD@PICsome
3.6. Evaluation of In Vivo Therapeutic Effect of CD@PICsome
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Lomora, M.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Chuanoi, S.; Anraku, Y.; Hori, M.; Kishimura, A.; Kataoka, K. Fabrication of Polyion Complex Vesicles with Enhanced Salt and Temperature Resistance and Their Potential Applications as Enzymatic Nanoreactors. Biomacromolecules 2014, 15, 2389–2397. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Kamiya, M.; Tanaka, S.; Nomoto, T.; Toh, K.; Matsumoto, Y.; Fukushima, S.; Sueyoshi, D.; Kano, M.R.; et al. Systemically Injectable Enzyme-Loaded Polyion Complex Vesicles as In Vivo Nanoreactors Functioning in Tumors. Angew. Chem. Int. Ed. 2016, 55, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, D.; Anraku, Y.; Komatsu, T.; Urano, Y.; Kataoka, K. Enzyme-Loaded Polyion Complex Vesicles as in vivo Nanoreactors Working Sustainably under the Blood Circulation: Characterization and Functional Evaluation. Biomacromolecules 2017, 18, 1189–1196. [Google Scholar] [CrossRef]
- Nishimura, T.; Sasaki, Y.; Akiyoshi, K. Biotransporting Self-Assembled Nanofactories Using Polymer Vesicles with Molecular Permeability for Enzyme Prodrug Cancer Therapy. Adv. Mater. 2017, 29, 1702406. [Google Scholar] [CrossRef]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic Polymersome Nanoreactors with Tumor-Specific Activable Cascade Reactions for Cooperative Cancer Therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Mukerabigwi, J.F.; Yin, W.; Zha, Z.; Ke, W.; Wang, Y.; Chen, W.; Japir, A.A.-W.M.M.; Wang, Y.; Ge, Z. Polymersome nanoreactors with tumor pH-triggered selective membrane permeability for prodrug delivery, activation, and combined oxidationchemotherapy. J. Control. Release 2019, 303, 209–222. [Google Scholar] [CrossRef]
- Varlas, S.; Foster, J.C.; Georgiou, P.G.; Keogh, R.; Husband, J.T.; Williams, D.S.; O’Reilly, R.K. Tuning the membrane permeability of polymersome nanoreactors developed by aqueous emulsion polymerization-induced self-assembly. Nanoscale 2019, 11, 12643–12654. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Kobayashi, A.; Oba, M.; Kataoka, K. Size-controlled long-circulating PICsome as a ruler to measure critical cut-off disposition size into normal and tumor tissues. Chem. Commun. 2011, 47, 6054–6056. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Oba, M.; Yamasaki, Y.; Kataoka, K. Spontaneous formation of nanosized unilamellar polyion complex vesicles with tunable size and properties. J. Am. Chem. Soc. 2010, 132, 1631–1636. [Google Scholar] [CrossRef]
- Tang, H.; Sakamura, Y.; Mori, T.; Katayama, Y.; Kishimura, A. Development of Enzyme Loaded Polyion Complex Vesicle (PICsome): Thermal Stability of Enzyme in PICsome Compartment and Effect of Coencapsulation of Dextran on Enzyme Activity. Macromol. Biosci. 2017, 17, 1600542. [Google Scholar] [CrossRef]
- Naoyama, K.; Mori, T.; Katayama, Y.; Kishimura, A. Fabrication of Dendrimer-Based Polyion Complex Submicrometer-Scaled Structures with Enhanced Stability under Physiological Conditions. Macromol. Rapid Commun. 2016, 37, 1087–1093. [Google Scholar] [CrossRef]
- Mutaf, O.F.; Anraku, Y.; Kishimura, A.; Kataoka, K. Unilamellar polyion complex vesicles (PICsomes) with tunable permeabilities for macromolecular solutes with different shapes and sizes. Polymer 2017, 133, 1–7. [Google Scholar] [CrossRef]
- Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust polyion complex vesicles (PICsomes) under physiological condition reinforced by multiple hydrogen bond formation derived by guanidinium groups. Biomacromolecules 2018, 19, 4113–4121. [Google Scholar] [CrossRef]
- Austin, E.A.; Huber, B.E. A first step in the development of gene therapy for colorectal carcinoma: Cloning, sequencing, and expression of Escherichia coli cytosine deaminase. Mol. Pharmacol. 1993, 43, 380–387. [Google Scholar] [PubMed]
- Cheung, W.Y.; Fralick, R.A.; Cheng, S. The confused cancer patient: A case of 5-fluorouracil-induced encephalopathy. Curr. Oncol. 2008, 15, 234–236. [Google Scholar] [CrossRef]
- Phillips, T.A.; Howell, A.; Grieve, R.J.; Welling, P.G. Pharmacokinetics of oral and Intravenous fluorouracil in humans. J. Pharm. Sci. 1980, 69, 1428–1431. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.-J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity, and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Koide, A.; Osada, K.; Yamasaki, Y.; Kataoka, K. Encapsulation of myoglobin in PEGylated polyion complex vesicles made from a pair of oppositely charged block ionomers: A physiologically available oxygen carrier. Angew. Chem. Int. Ed. 2007, 46, 6085–6088. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Yamasaki, Y.; Kataoka, K. Living Unimodal Growth of Polyion Complex Vesicles via Two-Dimensional Supramolecular Polymerization. J. Am. Chem. Soc. 2013, 135, 1423–1429. [Google Scholar] [CrossRef]
- Smith, M.J.; Haidar, I.A.; Striegel, A.M. Measuring the size of polymers with negative radii using MALS/QELS: An exploration of the thermodynamic radius. Analyst 2007, 132, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Malet-Martino, M.; Gilard, V.; Desmoulin, F.; Martino, R. Fluorine nuclear magnetic resonance spectroscopy of human biofluids in the field of metabolic studies of anticancer and antifungal fluoropyrimidine drugs. Clin. Chim. Acta 2006, 366, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Yen, H.-C.; Anraku, Y.; Fukushima, S.; Lai, P.-S.; Kato, M.; Kishimura, A.; Kataoka, K. Facile preparation of delivery platform of water-soluble low-molecular-weight drugs based on polyion complex vesicle (PICsome) encapsulating mesoporous silica nanoparticle. ACS Biomater. Sci. Eng. 2017, 3, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Speller, D.C.E. Antifungal Chemotherapy; John Wiley & Sons, Ltd.: Chichester, UK, 1980; pp. 271–344. [Google Scholar]
- Daneshmend, T.K.; Warnock, D.W. Clinical pharmacokinetics of systemic antifungal drugs. Clin. Pharmacokinet. 1983, 8, 17–42. [Google Scholar] [CrossRef] [PubMed]






| Sample | Contents | ||
|---|---|---|---|
| CD@PICsome | 5-FC | 5-FU | |
| 1 | + | − | − |
| 2 | − | + (2 μg/mL) | − |
| 3 | − | + (4 μg/mL) | − |
| 4 | + | + (2 μg/mL) | − |
| 5 | + | + (4 μg/mL) | − |
| 6 | − | − | + (2 μg/mL) |
| 7 | − | − | + (4 μg/mL) |
| 8 | − | − | − |
| Group | Day | ||||
|---|---|---|---|---|---|
| 1 | 4 | 8 | 11 | 15 | |
| 1 | × | ♦ | ♦ | ♦ | ♦ |
| 2 | × | ◊ | ◊ | ◊ | ◊ |
| 3 | − | ■ | ■ | ■ | ■ |
| 4 | − | □ | □ | □ | □ |
| 5 | × | − | − | − | − |
| 6 | − | ♦ | ♦ | ♦ | ♦ |
| 7 | − | ◊ | ◊ | ◊ | ◊ |
| 8 | ∆ | ∆ | ∆ | ∆ | ∆ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, A.; Anraku, Y.; Fukushima, S.; Kishimura, A. Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers 2023, 15, 1368. https://doi.org/10.3390/polym15061368
Goto A, Anraku Y, Fukushima S, Kishimura A. Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers. 2023; 15(6):1368. https://doi.org/10.3390/polym15061368
Chicago/Turabian StyleGoto, Akinori, Yasutaka Anraku, Shigeto Fukushima, and Akihiro Kishimura. 2023. "Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome" Polymers 15, no. 6: 1368. https://doi.org/10.3390/polym15061368
APA StyleGoto, A., Anraku, Y., Fukushima, S., & Kishimura, A. (2023). Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers, 15(6), 1368. https://doi.org/10.3390/polym15061368








