Aging of Industrial Polypropylene Surfaces in Detergent Solution and Its Consequences for Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Polypropylene Samples and Aging Process
2.1.1. Injection-Moulded Polypropylene Samples
2.1.2. Aging Process
2.2. Physical Methods
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Atomic Force Microscopy (AFM)
2.2.3. ATR-FTIR
2.2.4. DSC
2.3. Biological Methods
2.3.1. Bacterial Strains
2.3.2. SEM Investigation of Attached Cells and Biofilm Formation
2.3.3. Colony Forming Unit Assay (CFU)
2.3.4. Microtiter Plate Assay (MTP)
3. Results
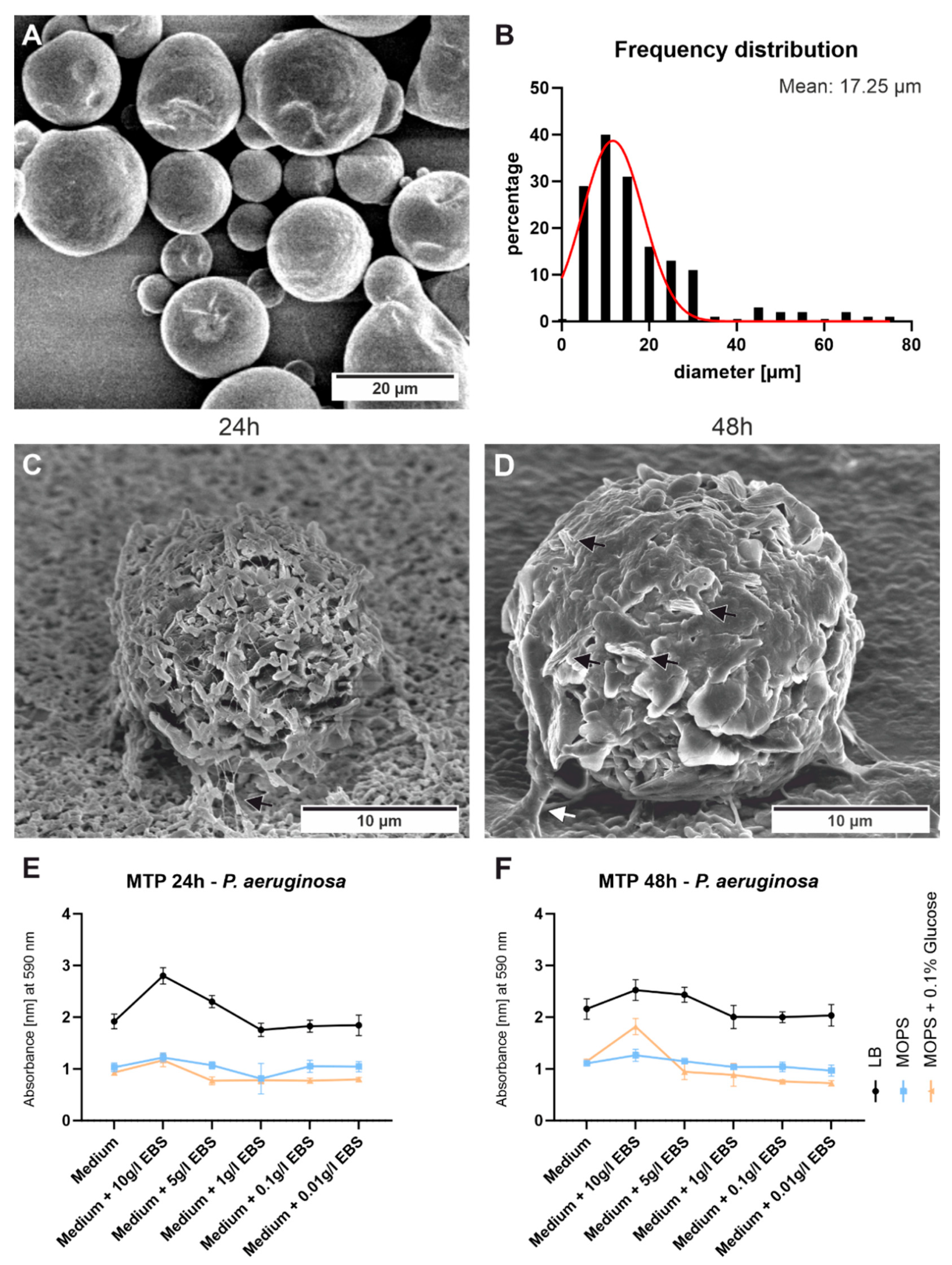
3.1. Microscopy
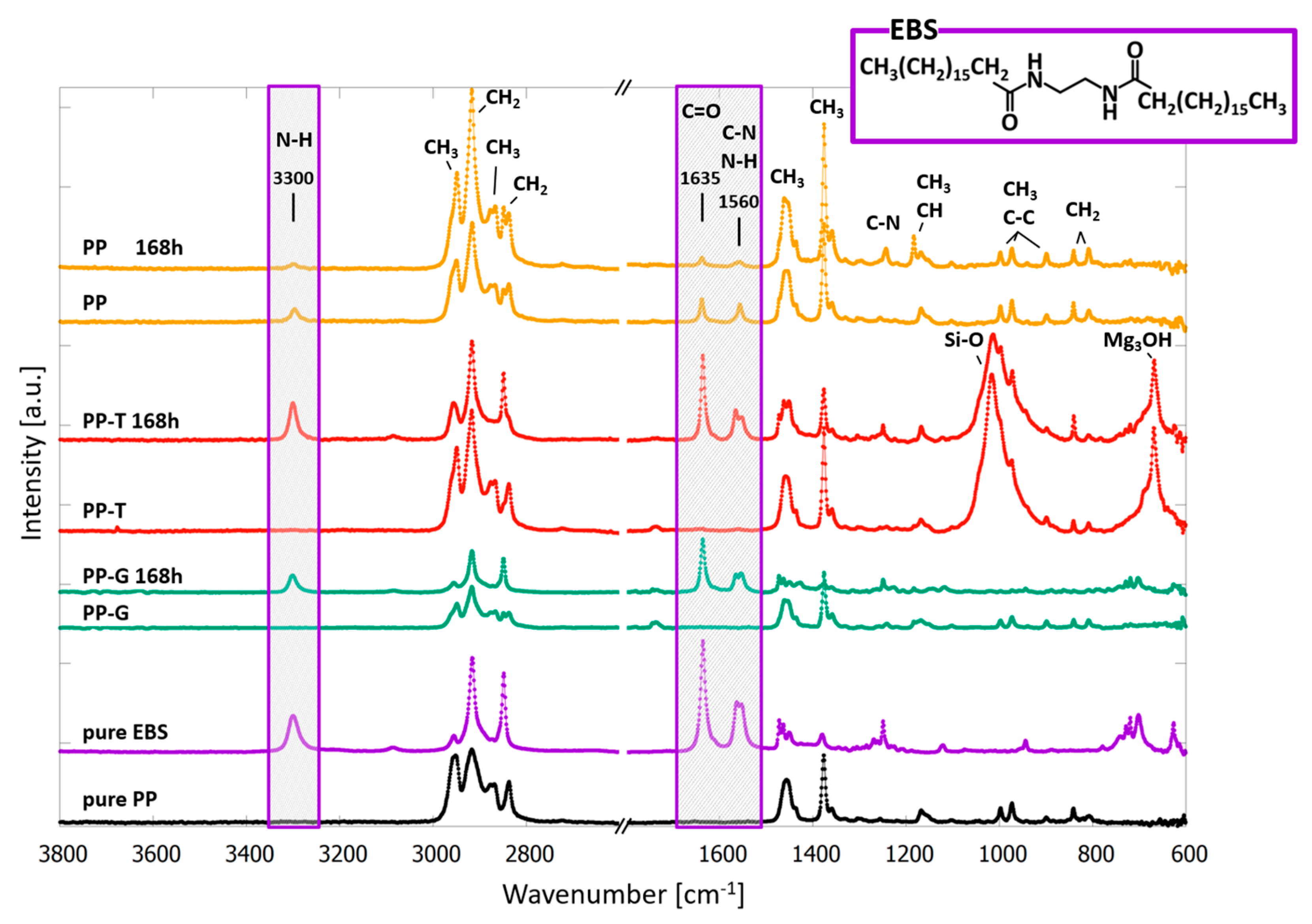
3.2. ATR-FTIR Spectroscopy
3.3. DSC
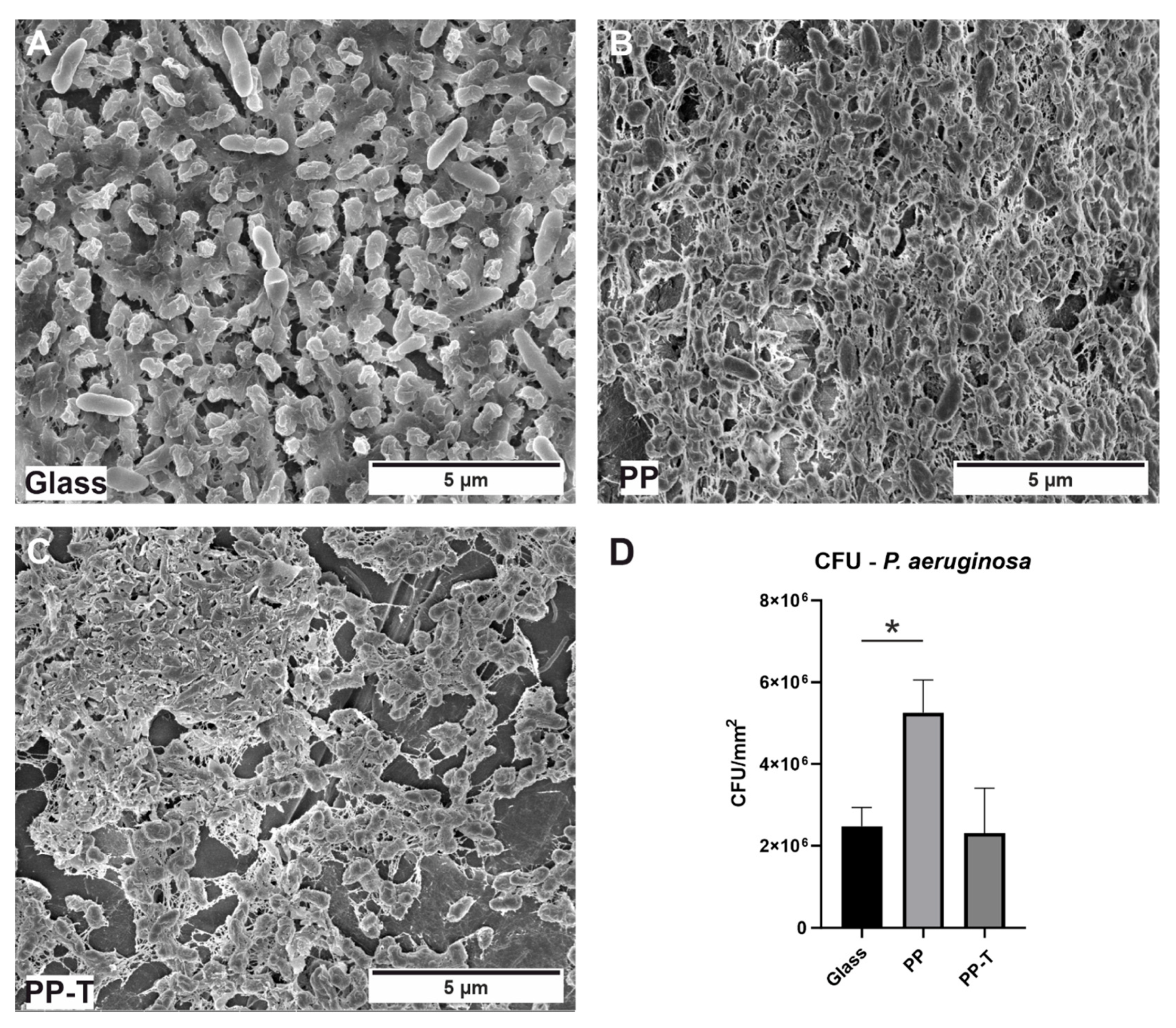
3.4. Bacterial Cultivation/CFUs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fink, J.K. A Concise Introduction to Additives for Thermoplastic Polymers; Wiley: Hoboken, NJ, USA, 2010; ISBN 9780470609552. [Google Scholar]
- Klampfl, C.W.; Himmelsbach, M. Advances in the Determination of Hindered Amine Light Stabilizers—A Review. Anal. Chim. Acta 2016, 933, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, J. Mechanistic Action of Phenolic Antioxidants in Polymers—A Review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent Advances in Antioxidant Polymers: From Sustainable and Natural Monomers to Synthesis and Applications. Polymers 2021, 13, 2465. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The Potential of Flavonoids as Natural Antioxidants and UV Light Stabilizers for Polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer Additives. Phys. Sci. Rev. 2019, 2, 1–20. [Google Scholar] [CrossRef]
- Prasert, A.; Sontikaew, S.; Sriprapai, D.; Chuangchote, S. Polypropylene/ZnO Nanocomposites: Mechanical Properties, Photocatalytic Dye Degradation, and Antibacterial Property. Materials 2020, 13, 914. [Google Scholar] [CrossRef]
- Nouman, M.; Saunier, J.; Jubeli, E.; Yagoubi, N. Additive Blooming in Polymer Materials: Consequences in the Pharmaceutical and Medical Field. Polym. Degrad. Stab. 2017, 143, 239–252. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Li, X.; Liu, Y.; Zhao, L. Evaluation of Biofilm Development on Various Pipelines in the Domestic Hot Water System. Water Sci. Technol. Water Supply 2018, 18, 638–647. [Google Scholar] [CrossRef]
- Gattlen, J.; Amberg, C.; Zinn, M.; Mauclaire, L. Biofilms Isolated from Washing Machines from Three Continents and Their Tolerance to a Standard Detergent. Biofouling 2010, 26, 873–882. [Google Scholar] [CrossRef]
- Raghupathi, P.K.; Zupančič, J.; Brejnrod, A.D.; Jacquiod, S.; Houf, K.; Burmølle, M.; Gunde-Cimerman, N.; Sørensen, S.J. Microbial Diversity and Putative Opportunistic Pathogens in Dishwasher Biofilm Communities. Appl. Environ. Microbiol. 2018, 84, e02755-17. [Google Scholar] [CrossRef]
- Asghari, E.; Kiel, A.; Kaltschmidt, B.P.; Wortmann, M.; Schmidt, N.; Hüsgen, B.; Hütten, A.; Knabbe, C.; Kaltschmidt, C.; Kaltschmidt, B. Identification of Microorganisms from Several Surfaces by MALDI-TOF MS: P. Aeruginosa Is Leading in Biofilm Formation. Microorganisms 2021, 9, 992. [Google Scholar] [CrossRef]
- Hisham, A. Maddah Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Antiblocking, Release, and Slip Additives; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9781895198836. [Google Scholar]
- Shubhra, Q.T.H.; Alam, A.K.M.M.; Quaiyyum, M.A. Mechanical Properties of Polypropylene Composites: A Review. J. Thermoplast. Compos. Mater. 2013, 26, 362–391. [Google Scholar] [CrossRef]
- Ceresana Market Research. Available online: www.ceresana.com/de/marktstudien/kunststoffe/polypropylen/polypropylen-markt-anteil-kapazitaet-angebot-nachfrage-prognose-innovation-anwendung-wachstum-produktion-industrie.html (accessed on 11 October 2022).
- Kantz, M.R.; Newman, H.D.; Stigale, F.H. The Skin-core Morphology and Structure–Property Relationships in Injection-molded Polypropylene. J. Appl. Polym. Sci. 1972, 16, 1249–1260. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.B. Unusual Hierarchical Distribution of β-Crystals and Improved Mechanical Properties of Injection-Molded Bars of Isotactic Polypropylene. RSC Adv. 2014, 4, 25135–25147. [Google Scholar] [CrossRef]
- Yang, H.R.; Lei, J.; Li, L.; Fu, Q.; Li, Z.M. Formation of Interlinked Shish-Kebabs in Injection-Molded Polyethylene under the Coexistence of Lightly Cross-Linked Chain Network and Oscillation Shear Flow. Macromolecules 2012, 45, 6600–6610. [Google Scholar] [CrossRef]
- Blaine, R.L. Determination of Polyethylene Crystallinity by DSC; TA Instruments: New Castle, DE, USA, 2002; pp. 1–3. [Google Scholar]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]
- Neidhardt, F.C.; Bloch, P.L.; Smith, D.F. Culture Medium for Enterobacteria. J. Bacteriol. 1974, 119, 736–747. [Google Scholar] [CrossRef]
- Singh, D.K.; Pradhan, M.; Materny, A. (Eds.) Modern Techniques of Spectroscopy; Springer: Singapore, 2021; Volume 13, ISBN 978-981-33-6083-9. [Google Scholar]
- Gee, D.; Melia, T. Thermal Properties of Melt and Solution Crystallized Isotactic Polypropylene. Makromol. Chem. 1970, 132, 195–201. [Google Scholar] [CrossRef]
- Murmu, U.K.; Adhikari, J.; Naskar, A.; Dey, D.; Roy, A.; Ghosh, A.; Ghosh, M. Mechanical Properties of Crystalline and Semicrystalline Polymer Systems. In Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 917–927. [Google Scholar] [CrossRef]
- Statista. Available online: www.statista.com/statistics/1245169/polypropylene-market-volume-worldwide/ (accessed on 11 October 2022).
- Jubinville, D.; Abdelwahab, M.; Mohanty, A.K.; Misra, M. Comparison in Composite Performance after Thermooxidative Aging of Injection Molded Polyamide 6 with Glass Fiber, Talc, and a Sustainable Biocarbon Filler. J. Appl. Polym. Sci. 2020, 137, 48618. [Google Scholar] [CrossRef]
- Sližová, M.; Stašek, M.; Raab, M. Polypropylene after Thirty Years of Storage: Mechanical Proof of Heterogeneous Aging. Polym. J. 2020, 52, 775–781. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging Effects on Low- and High-Density Polyethylene, Polypropylene and Polystyrene under UV Irradiation: An Insight into Decomposition Mechanism by Py-GC/MS for Microplastic Analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Romano, R.S.G.; Oliani, W.L.; Parra, D.F.; Lugao, A.B. Effects of Environmental Aging in Polypropylene Obtained by Injection Molding. AIP Conf. Proc. 2017, 1914, 140001. [Google Scholar] [CrossRef]
- Díez-Gutiérrez, S.; Rodríguez-Pérez, M.A.; de Saja, J.A.; Velasco, J.I. Dynamic Mechanical Analysis of Injection-Moulded Discs of Polypropylene and Untreated and Silane-Treated Talc-Filled Polypropylene Composites. Polymer 1999, 40, 5345–5353. [Google Scholar] [CrossRef]
- Naiki, M.; Fukui, Y.; Matsumura, T.; Nomura, T.; Matsuda, M. Effect of Talc on the Crystallization of Isotactic Polypropylene. J. Appl. Polym. Sci. 2001, 79, 1693–1703. [Google Scholar] [CrossRef]
- Jahani, Y. Dynamic Rheology, Mechanical Performance, Shrinkage, and Morphology of Chemically Coupled Talc-Filled Polypropylene. J. Vinyl Addit. Technol. 2010, 16, 70–77. [Google Scholar] [CrossRef]
- Syed, S.F.; Chen, J.C.; Guo, G. Optimization of Tensile Strength and Shrinkage of Talc-Filled Polypropylene as a Packaging Material in Injection Molding. J. Packag. Technol. Res. 2020, 4, 69–78. [Google Scholar] [CrossRef]
- Browning, R.; Lim, G.T.; Moyse, A.; Sun, L.; Sue, H.-J. Effects of Slip Agent and Talc Surface-Treatment on the Scratch Behavior of Thermoplastic Olefins. Polym. Eng. Sci. 2006, 46, 601–608. [Google Scholar] [CrossRef]
- Mar’in, A. Additives Solubility in Polymers. Int. J. Polym. Mater Polym. Biomater. 1998, 42, 125–163. [Google Scholar] [CrossRef]
- Saunier, J.; Mazel, V.; Paris, C.; Yagoubi, N. Polymorphism of Irganox 1076®: Discovery of New Forms and Direct Characterization of the Polymorphs on a Medical Device by Raman Microspectroscopy. Eur. J. Pharm. Biopharm. 2010, 75, 443–450. [Google Scholar] [CrossRef]
- Saunier, J.; Herry, J.M.; Yagoubi, N.; Marlière, C. Exploring Complex Transitions between Polymorphs on a Small Scale by Coupling AFM, FTIR and DSC: The Case of Irganox 1076® Antioxidant. RSC Adv. 2017, 7, 3804–3818. [Google Scholar] [CrossRef]
- Saunier, J.; Herry, J.M.; Marlière, C.; Renault, M.; Bellon-Fontaine, M.N.; Yagoubi, N. Modification of the Bacterial Adhesion of Staphylococcus Aureus by Antioxidant Blooming on Polyurethane Films. Mater. Sci. Eng. C 2015, 56, 522–531. [Google Scholar] [CrossRef]
- Viebke, J.; Hedenqvist, M.; Gedde, U.W. Antioxidant Efficiency Loss by Precipitation and Diffusion to Surrounding Media in Polyethylene Hot-Water Pipes. Polym. Eng. Sci. 1996, 36, 2896–2904. [Google Scholar] [CrossRef]
- Tanasescu, C.; Moisin, A.; Mihetiu, A.; Serban, D.; Costache, A.; Bratu, D. The Use of Polypropylene Mesh in Inguinal Hernia Surgery: A Retrospective Study. Exp. Ther. Med. 2021, 22, 1193. [Google Scholar] [CrossRef]
- Chapple, C.R.; Cruz, F.; Deffieux, X.; Milani, A.L.; Arlandis, S.; Artibani, W.; Bauer, R.M.; Burkhard, F.; Cardozo, L.; Castro-Diaz, D.; et al. Consensus Statement of the European Urology Association and the European Urogynaecological Association on the Use of Implanted Materials for Treating Pelvic Organ Prolapse and Stress Urinary Incontinence. Eur. Urol. 2017, 72, 424–431. [Google Scholar] [CrossRef]
- Verhorstert, K.W.J.; Guler, Z.; de Boer, L.; Riool, M.; Roovers, J.P.W.R.; Zaat, S.A.J. In Vitro Bacterial Adhesion and Biofilm Formation on Fully Absorbable Poly-4-Hydroxybutyrate and Nonabsorbable Polypropylene Pelvic Floor Implants. ACS Appl. Mater. Interfaces 2020, 12, 53646–53653. [Google Scholar] [CrossRef]
- Mangir, N.; Roman, S.; Chapple, C.R.; MacNeil, S. Complications Related to Use of Mesh Implants in Surgical Treatment of Stress Urinary Incontinence and Pelvic Organ Prolapse: Infection or Inflammation? World J. Urol. 2020, 38, 73–80. [Google Scholar] [CrossRef]
- Jacombs, A.S.W.; Karatassas, A.; Klosterhalfen, B.; Richter, K.; Patiniott, P.; Hensman, C. Biofilms and Effective Porosity of Hernia Mesh: Are They Silent Assassins? Hernia 2020, 24, 197–204. [Google Scholar] [CrossRef]
- Wang See, C.; Kim, T.; Zhu, D. Hernia Mesh and Hernia Repair: A Review. Eng. Regen. 2020, 1, 19–33. [Google Scholar] [CrossRef]
- Laitala, K.; Mollan Jensen, H. Cleaning Effect of Household Laundry Detergents at Low Temperatures. Tenside Surfactants Deterg. 2010, 47, 413–420. [Google Scholar] [CrossRef]
- Terpstra, P.M.J. Domestic and Institutional Hygiene in Relation to Sustainability. Historical, Social and Environmental Implications. Int. Biodeterior. Biodegradation 1998, 41, 169–175. [Google Scholar] [CrossRef]
- Callewaert, C.; van Nevel, S.; Kerckhof, F.M.; Granitsiotis, M.S.; Boon, N. Bacterial Exchange in Household Washing Machines. Front. Microbiol. 2015, 6, 1381. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Moreno, M.; Avila-Novoa, M.G.; Gutiérrez-Lomelí, M. Resistance of Pathogenic and Spoilage Microorganisms to Disinfectants in the Presence of Organic Matter and Their Residual Effect on Stainless Steel and Polypropylene. J. Glob. Antimicrob. Resist. 2018, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cremer, J.; Kaltschmidt, B.P.; Kiel, A.; Eberhard, J.; Schmidt, S.; Kaltschmidt, C.; Kaltschmidt, B.; Hütten, A.; Anselmetti, D. Aging of Industrial Polypropylene Surfaces in Detergent Solution and Its Consequences for Biofilm Formation. Polymers 2023, 15, 1247. https://doi.org/10.3390/polym15051247
Cremer J, Kaltschmidt BP, Kiel A, Eberhard J, Schmidt S, Kaltschmidt C, Kaltschmidt B, Hütten A, Anselmetti D. Aging of Industrial Polypropylene Surfaces in Detergent Solution and Its Consequences for Biofilm Formation. Polymers. 2023; 15(5):1247. https://doi.org/10.3390/polym15051247
Chicago/Turabian StyleCremer, Julian, Bernhard P. Kaltschmidt, Annika Kiel, Jens Eberhard, Stephan Schmidt, Christian Kaltschmidt, Barbara Kaltschmidt, Andreas Hütten, and Dario Anselmetti. 2023. "Aging of Industrial Polypropylene Surfaces in Detergent Solution and Its Consequences for Biofilm Formation" Polymers 15, no. 5: 1247. https://doi.org/10.3390/polym15051247
APA StyleCremer, J., Kaltschmidt, B. P., Kiel, A., Eberhard, J., Schmidt, S., Kaltschmidt, C., Kaltschmidt, B., Hütten, A., & Anselmetti, D. (2023). Aging of Industrial Polypropylene Surfaces in Detergent Solution and Its Consequences for Biofilm Formation. Polymers, 15(5), 1247. https://doi.org/10.3390/polym15051247










