Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review
Abstract
1. Introduction
2. Methodology
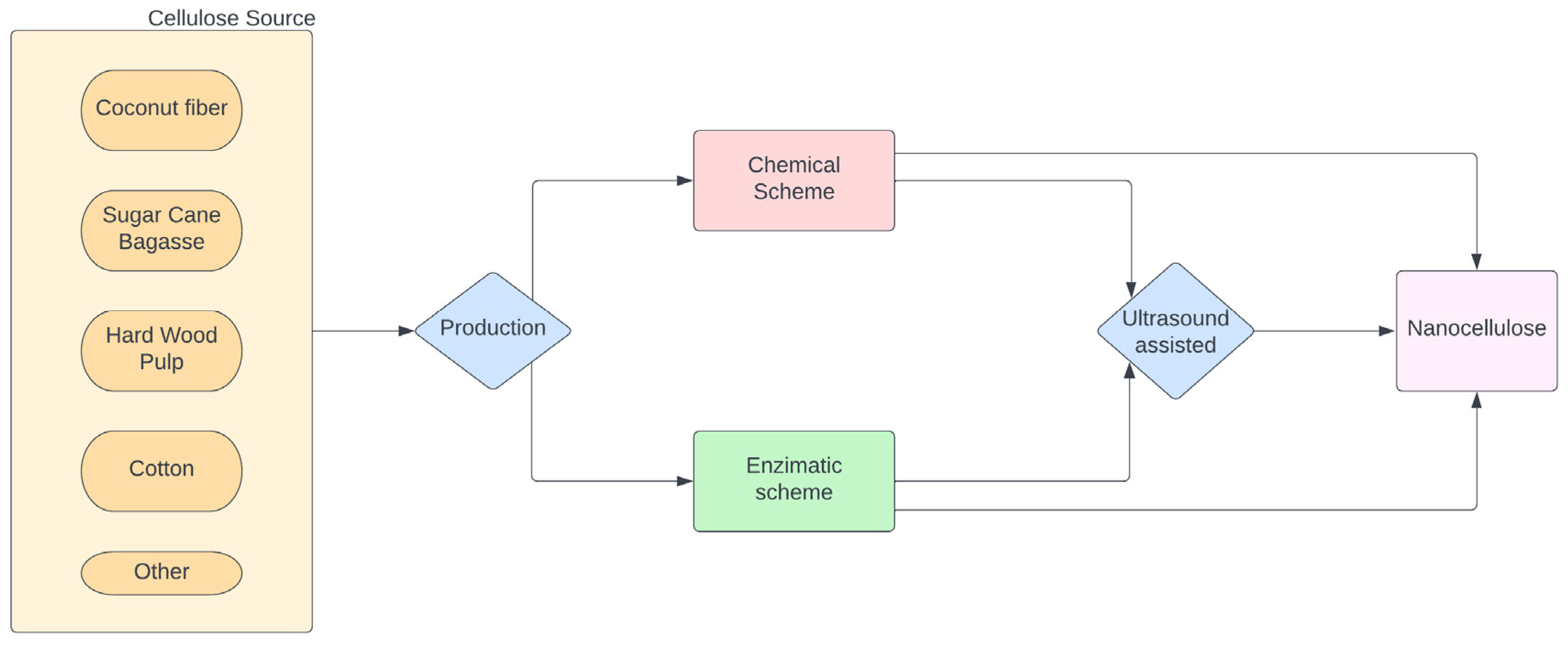
3. Processing of Nanocellulose Biobased Composites
3.1. Processing Techniques: Solvent Casting and Melt Processing
| Reinforcement (Source) | Matrix | Solvent Casting Conditions | Mechanical Properties | ||
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation (%) | |||
| Polysaccharide matrix | |||||
| NC (rice straw) [20] | Corn starch | Solvent: water NC content: 0.25–1 wt.% Plasticizer: 25 wt.% glycerol Temperature: 85 °C Sonication for 5 min | 3.0–5.2 | 82.3–200.8 | 19.4–45.4 |
| CNC (biomass) [21] | Chitosan | Solvent: 1 % v/v glacial acetic solution NC content: 0, 1.5, 2.0, 2.5 wt.% Plasticizer: 30 wt.% glycerol Agitation for 5 min at 15.000 rpm Sonication for 15 min | 34.0–43.8 | 1415.4–2221.8 | 10.9–27.9 |
| CNF (Orange bagasse) [48] | Starch | Solvent: deionized water NC content: 5 wt.% Plasticizers: glycerol Temperature: 70 °C and 90 °C Agitation for 2 h at 1200 rpm Ultrasonication at 42 kHz for 10 min | - | - | - |
| CNC (pea pod) [45] | Chitosan | Solvent: 2 wt.% acetic acid solution. NC content: 0–10 wt.% Mechanical stirring for 15 min at room temperature | 52.2–73.4 | 880.0–1692.0 | 11.2–17.9 |
| BC and CNF (rice husk) [49] | Yeast Biomass | Solvent: water. NC content: 0, 1, 3, and 5 wt.% Plasticizer: 25 wt.% glycerol pH: 6 and 11 Mechanical stirring for 1 min Homogenization at 125 MPa for 9 min. | 1.0–3.5 | 20.0–36.0 | 11.0–44.0 |
| CNC (pomegranate peel) [47] | Chitosan | Solvent: 1% v/v acetic acid glacial. NC content: 0–6 wt.% Plasticizer: 30 wt.% glycerol Mechanical stirring for 2 h at room temperature Ultrasonication for 5 min | 28.9–41.3 | 59.0–96.7 | 88.9–95.8 |
| CNF (açaí) [46] | Chitosan | Solvent: 0.5% v/v acetic acid solution. NC content: 5–20 wt.% Plasticizer: 30 wt.% glycerol Mechanical stirring for 30 min at room temperature | 6.5–9.7 | 458.6–1119.9 | - |
| CNC [50] | Chitosan-corn starch | Solvent: 1% w/v in acetic acid solution. NC content: 0–10 wt.% Plasticizer: 30 wt.% glycerol Mechanical stirring for 10 min at 90 °C. | 2.9–13.6 | - | 5.3–145.1 |
| CNF (Bamboo fibers) [51] | Babul gum | Solvent: distilled water. NC content: 0–10 wt.% Plasticizer: 10 wt.% sorbitol Mechanical stirring for 30 min at 85 °C. | 0.4–3.7 | 4.8–71.7 | 17.5–46.0 |
| CNF (banana peel) [25] | Corn starch | Solvent: distilled water. NC content: 0–15 wt.% Plasticizer: 25 wt.% glycerol Mechanical stirring for 30 min at 81 °C Sonication for 10 min | 7.0–9.0 | - | 20.0–25.0 |
| PVA matrix | |||||
| CNF (agave) [22] | PVA | Solvent: distilled water NC content: 0–10 wt.% Mechanical stirring for 30 min at 50 °C and 350 rpm. Ultrasonication for 2 min | 26.6–47.0 | - | 33.0–112.0 |
| NC [52] | PVA-banana pseudo fiber | Solvent: distilled water NC content: 0–5 wt.% Plasticizer: 10 wt.% sorbitol Mechanical stirring for 40 min Ultrasonication | 28.7–43.8 | - | 87.4–183.0 |
| CNF (sugar cane bagasse) [43] | PVA | Solvent: distilled water NC content: 0–50 wt.% Plasticizer: 70 wt.% PEG Mechanical stirring for 60 min Ultrasonication for 30 min | 26.5–85.4 | 954.0–2846.0 | 7.8–91.1 |
| CNF (olive tree pruning) [24] | PVA | Solvent: distilled water NC content: 0, 2.5, 5.0, and 7.5 wt.% Mechanical stirring for 4 h at room temperature. | 52.5–69.8 | 3587.0–4263.0 | 100.0–143.0 |
| CNC (sugar cane bagasse and coir fiber) [26] | PVA | Solvent: distilled water NC Content: 0.5 and 2.0 wt.% Mixing Sonication for 5 min | 19.4–38.2 | 11.6–26.4 | 105.8–187.0 |
| CNF (Coconut shell) [44] | PVA | Solvent: distilled water NC content: 2 wt.% Mixing at a warm temperature | 2.6–6.7 | - | 36.2–102.44 |
| PLA matrix | |||||
| CNC (office wastepaper) [23] | PLA | Solvent: N, N-dimethyl formamide solution NC content: 1–5 wt.% Mechanical stirring for 4 h at 60 °C. Ultrasonication | 61.0–69.0 | - | 5.0–6.0 |
3.2. Effect of Plasticizers and Additives
4. Effect of Nanocellulose Content in the Morphology and Properties of Biobased Composites
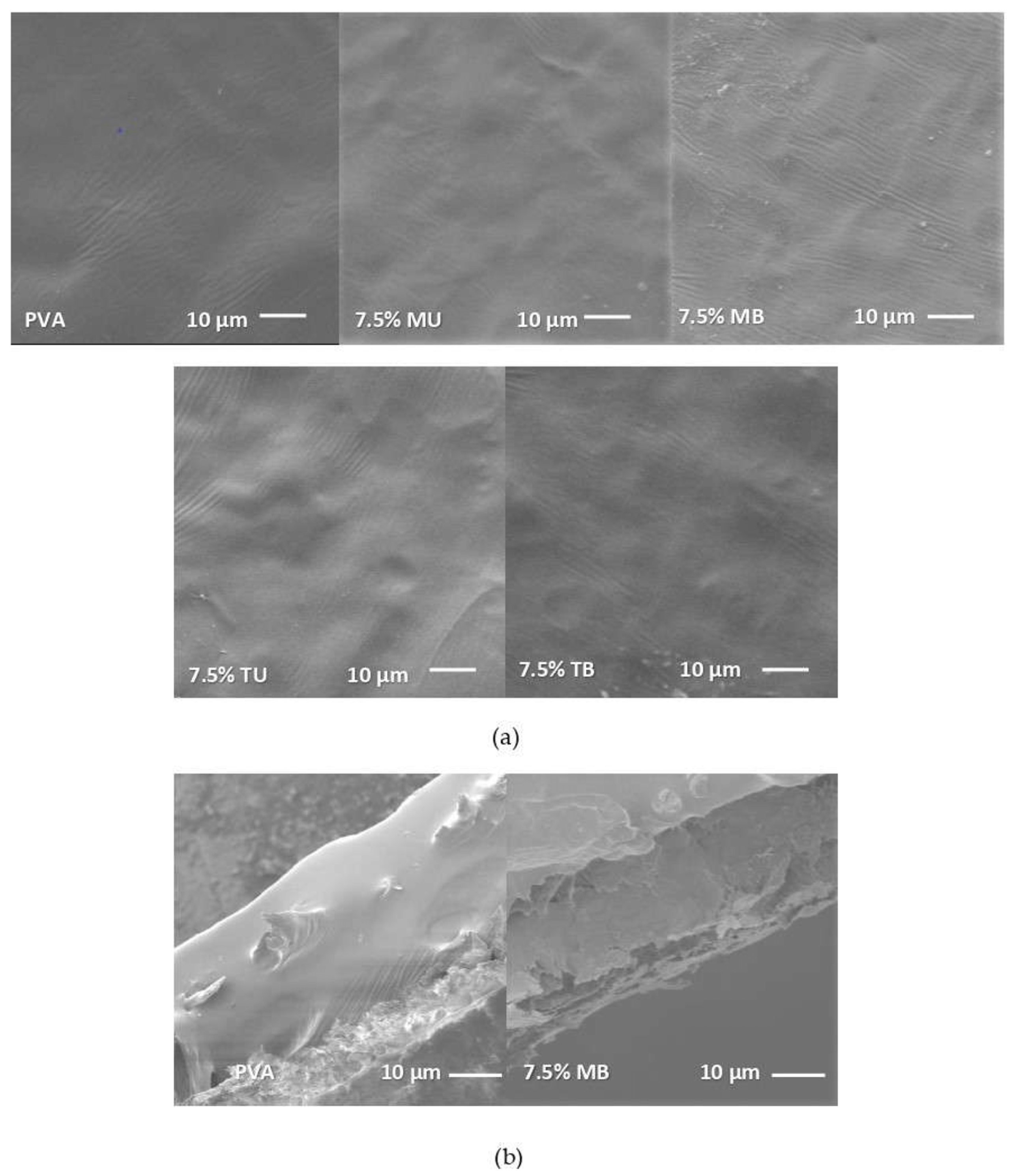
4.1. Morphological and Structural Properties
4.2. Mechanical Properties
4.3. Thermal Behavior
4.4. Thermomechanical and Rheology Analysis
4.5. Barrier and Wettability Properties
4.6. Optical Properties
4.7. Degradability
5. Surface Modification of Nanocellulose to Prepare Biobased Composites
6. Environmental Impact on the Preparation of Nanocellulose and Composites
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| A | Adhesion factor |
| AcCNF | Acetylated cellulose nanofibrils |
| ACNs | acetylated cellulose nanocrystals |
| AFM | Atomic force microscopy |
| ALH | Alkaline/Lime Juice/High-Pressure treatment |
| ASA | Alkenyl succinic anhydride |
| ATH | Alkaline/TEMPO/High-Pressure treatment |
| BC | Bacterial nanocellulose |
| CMC | Carboxymethyl cellulose |
| CML | Centrum voor Milieukunde Leiden |
| CNC | Cellulose nanocrystals |
| CNF | Cellulose nanofibrils |
| CNF-g-PLA | PLA grafted onto the nanocellulose fibers |
| CS | Chitosan |
| CT | X-ray computed tomography |
| DANC | Dialdehyde nanocrystalline cellulose |
| DCP | Dicumyl peroxide |
| DMA | Dynamic Mechanical Analysis |
| DOE | Department of Energy |
| DSC | Differential scanning calorimetry |
| DTG | Derivative thermogravimetric analysis |
| E’ | Tensile storage modulus |
| EA | Ethane dioic acid |
| FE | Freshwater eutrophication category |
| FITR | Fourier Transform Infrared Spectroscopy |
| GWP | Global warming potential category |
| HPLC | High-Performance Liquid Chromatography |
| HT | Human toxicity category |
| ILCD | International Reference Life Cycle Data System |
| IPCC | Intergovernmental Panel on Climate Change |
| LCA | Life cycle assessment |
| LCNF | Lignin-containing cellulose fibrils |
| MA | Maleic anhydride |
| MCNF | Mechanical cellulose nanofiber |
| ME | Marine eutrophication category |
| MFI | Melt flow index |
| NaOCl | Sodium hypochlorite |
| NaOH | Sodium hydroxide |
| NC | Nanocellulose |
| ODA | Octadecyl amine |
| OLH | Organo-solvent/Lime Juice/High-Pressure treatment |
| OP | Oxygen permeation |
| OTH | Organo-solvent/TEMPO/High-Pressure treatment |
| OTR | Oxygen transmission rate |
| OWP | Orange waste powder |
| PBS | Poly (butylene succinate) |
| PEG | Polyethylene glycol |
| PHB | Poly(β-hydroxybutyrate) |
| PHBH | Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| PHMB | Polyhexamethylene biguanide |
| phr | parts per hundred rubbers |
| PLA | Polylactic acid |
| PLA–g–MA | PLA grafted with maleic anhydride |
| PVA | Polyvinyl alcohol |
| SA | Succinic anhydride |
| SEM | Scanning electron microscope |
| TA | Terrestrial acidification category |
| TCNF | TEMPO–cellulose nanofiber |
| TEM | Transmission electron microscopy |
| TEMPO | (2,2,6,6-Tetramethylpiperidinyloxy) mediated oxidation |
| TGA | Thermogravimetric analysis |
| TPS | Thermoplastic starch |
| TRACI | Tool for Reduction and Assessment of Chemicals and Other Environmental Impacts |
| UV-Vis | Ultraviolet-visible spectrophotometer |
| WD | Water consumption category |
| WVP | Water vapor permeability |
| WVTR | Water vapor transmission rate |
| XRD | X-ray Diffraction |
| tanδ | Loss tangent |
| Tc | Temperature of crystallization |
| Tg | Glass transition point |
| Tm | Melting temperature |
| Tmax, | Temperature of maximum thermal degradation |
| Tonset | Temperature of the initial thermal degradation |
| Xc | Degree of crystallinity |
| ΔH | Heat capacity |
References
- Gowthaman, N.S.K.; Lim, H.N.; Sreeraj, T.R.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers: Social, Economic, and Environmental Aspects. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; Han, X.; Chen, Z.; Wang, S.; Pu, J. MXene/Wood-Derived Hierarchical Cellulose Scaffold Composite with Superior Electromagnetic Shielding. Carbohydr. Polym. 2021, 254, 117033. [Google Scholar] [CrossRef]
- Han, X.; Wu, W.; Wang, J.; Tian, Z.; Jiang, S. Hydrogen-Bonding-Aided Fabrication of Wood Derived Cellulose Scaffold/Aramid Nanofiber into High-Performance Bulk Material. Materials 2021, 14, 5444. [Google Scholar] [CrossRef]
- Wang, Z.X.; Han, X.S.; Zhou, Z.J.; Meng, W.Y.; Han, X.W.; Wang, S.J.; Pu, J.W. Lightweight and Elastic Wood-Derived Composites for Pressure Sensing and Electromagnetic Interference Shielding. Compos. Sci. Technol. 2021, 213, 108931. [Google Scholar] [CrossRef]
- Han, X.; Han, X.; Wang, Z.; Wang, S.; Meng, W.; Lv, H.; Zhou, Z.; Pu, J. High Mechanical Properties and Excellent Anisotropy of Dually Synergistic Network Wood Fiber Gel for Human–Computer Interactive Sensors. Cellulose 2022, 29, 4495–4508. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiyah, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Carvajal-Barriga, E.J.; Putaux, J.-L.; Martín-Ramos, P.; Simbaña, J.; Portero-Barahona, P.; Martín-Gil, J. Opportunities for Ivory Nut Residue Valorization as a Source of Nanocellulose Colloidal Suspensions. Gels 2022, 9, 32. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Wang, J.; Ding, L.; Zhang, K.; Han, J.; Jiang, S. Micro- and Nano-Fibrils of Manau Rattan and Solvent-Exchange-Induced High-Haze Transparent Holocellulose Nanofibril Film. Carbohydr. Polym. 2022, 298, 120075. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, B.S.; Kim, H.S.; Lee, Y.J.; Min, S.K.; Jang, D.; Abas, Z.; Kim, J. Review of Nanocellulose for Sustainable Future Materials. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Zielińska, D.; Skrzypczak, A.; Peplińska, B.; Borysiak, S. Nanocellulose-Based Polymer Composites Functionalized with New Gemini Ionic Liquids. Int. J. Mol. Sci. 2022, 23, 15807. [Google Scholar] [CrossRef] [PubMed]
- Oprică, M.G.; Uşurelu, C.D.; Frone, A.N.; Gabor, A.R.; Nicolae, C.A.; Vasile, V.; Panaitescu, D.M. Opposite Roles of Bacterial Cellulose Nanofibers and Foaming Agent in Polyhydroxyalkanoate-Based Materials. Polymers 2022, 14, 5358. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; bin Abdullah, M.F.; Omar, M.F. Thermal Properties of Nanocellulose-Reinforced Composites: A Review. J. Appl. Polym. Sci. 2020, 137, 48544. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from Jute Fibers and Their Nanocomposites with Natural Rubber: Preparation and Characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Hawkins, K.; Lewis, A.; Maffeis, T.; Charbonneau, C.; Gazze, A.; Francis, L.W.; Iakovlev, M.; et al. Characterization of Pulp Derived Nanocellulose Hydrogels Using AVAP® Technology. Carbohydr. Polym. 2018, 198, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Sabaruddin, F.A.; Paridah, M.T. Effect of Lignin on the Thermal Properties of Nanocrystalline Prepared from Kenaf Core. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Selangor, Malaysia, 2018; Volume 368. [Google Scholar]
- Qian, S.; Zhang, H.; Yao, W.; Sheng, K. Effects of Bamboo Cellulose Nanowhisker Content on the Morphology, Crystallization, Mechanical, and Thermal Properties of PLA Matrix Biocomposites. Compos. Part B Eng. 2018, 133, 203–209. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S.; Gupta, S. Innovative Strategy for Rice Straw Valorization into Nanocellulose and Nanohemicellulose and Its Application. Ind. Crops Prod. 2022, 179, 114695. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Gomes, L.A.; Coelhoso, I.M.; Godinho, M.H.; Fernando, A.L. Micro and Nanocellulose Extracted from Energy Crops as Reinforcement Agents in Chitosan Films. Ind. Crops Prod. 2022, 186, 115247. [Google Scholar] [CrossRef]
- Yudhanto, F.; Jamasri, J.; Rochardjo, H.S.B.; Kusumaatmaja, A. Experimental Study of Polyvinyl Alcohol Nanocomposite Film Reinforced by Cellulose Nanofibers from Agave Cantala. Int. J. Eng. Trans. A Basics 2021, 34, 987–998. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, J.; Fu, B.; Di, J.; Xu, L.; Zhu, X. Reinforcing Effect of Nanocrystalline Cellulose and Office Waste Paper Fibers on Mechanical and Thermal Properties of Poly (Lactic Acid) Composites. J. Appl. Polym. Sci. 2021, 138, 50462. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Espinosa, E.; Carrasco, E.; Pérez-Rodríguez, F.; Rodríguez, A. Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film. Foods 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Din, Z.; Ullah, H.; Ouyang, Q.; Rani, S.; Jan, I.; Alam, M.; Rahman, Z.; Kamal, T.; Ali, S.; et al. Preparation and Characterization of Bio-Based Nanocomposites Packaging Films Reinforced with Cellulose Nanofibers from Unripe Banana Peels. Starch/Staerke 2022, 74, 2100283. [Google Scholar] [CrossRef]
- Pavalaydon, K.; Ramasawmy, H.; Surroop, D. Comparative Evaluation of Cellulose Nanocrystals from Bagasse and Coir Agro-Wastes for Reinforcing PVA-Based Composites. Environ. Dev. Sustain. 2022, 24, 9963–9984. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Lu, X.; Wang, J.; Wu, D. Cellulosic Nanofibers Filled Poly(β-Hydroxybutyrate): Relations between Viscoelasticity of Composites and Aspect Ratios of Nanofibers. Carbohydr. Polym. 2021, 265, 118093. [Google Scholar] [CrossRef]
- Ghalehno, M.D.; Yousefi, H. Green Nanocomposite Made from Carboxymethyl Cellulose Reinforced with Four Types of Cellulose Nanomaterials of Wheat Straw. J. Appl. Polym. Sci. 2022, 139, e52802. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management–Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- Bataineh, K.M. Life-Cycle Assessment of Recycling Postconsumer High-Density Polyethylene and Polyethylene Terephthalate. Adv. Civ. Eng. 2020, 2020, 8905431. [Google Scholar] [CrossRef]
- Zargar, S.; Jiang, J.; Jiang, F.; Tu, Q. Isolation of Lignin-Containing Cellulose Nanocrystals: Life-Cycle Environmental Impacts and Opportunities for Improvement. Biofuels Bioprod. Biorefin. 2022, 16, 68–80. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life Cycle Assessments of Biodegradable, Commercial Biopolymers—A Critical Review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Jadhav, H.; Jadhav, A.; Takkalkar, P.; Hossain, N.; Nizammudin, S.; Zahoor, M.; Jamal, M.; Mubarak, N.M.; Griffin, G.; Kao, N. Potential of Polylactide Based Nanocomposites-Nanopolysaccharide Filler for Reinforcement Purpose: A Comprehensive Review. J. Polym. Res. 2020, 27, 330. [Google Scholar] [CrossRef]
- Calvino, C.; Macke, N.; Kato, R.; Rowan, S.J. Development, Processing and Applications of Bio-Sourced Cellulose Nanocrystal Composites. Prog. Polym. Sci. 2020, 103, 101221. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-Cellulose Reinforced Starch Bio Composite Films—A Review on Green Composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Gomri, C.; Cretin, M.; Semsarilar, M. Recent Progress on Chemical Modification of Cellulose Nanocrystal (CNC) and Its Application in Nanocomposite Films and Membranes—A Comprehensive Review. Carbohydr. Polym. 2022, 294, 119790. [Google Scholar] [CrossRef]
- Zhang, S.; Vanessa, C.; Khan, A.; Ali, N.; Malik, S.; Shah, S.; Bilal, M.; Yang, Y.; Akhter, M.S.; Iqbal, H.M. Prospecting Cellulose Fibre-Reinforced Composite Membranes for Sustainable Remediation and Mitigation of Emerging Contaminants. Chemosphere 2022, 305, 135291. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Pu, J.; Luo, H.; Wu, C.; Duan, G. Nanocellulose-Based Adsorbents for Heavy Metal Ion. Polymers 2022, 14, 5479. [Google Scholar] [CrossRef] [PubMed]
- Gholamali, I.; Yadollahi, M. Bio-Nanocomposite Polymer Hydrogels Containing Nanoparticles for Drug Delivery: A Review. Regen. Eng. Transl. Med. 2021, 7, 129–146. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Anjana; Raturi, G.; Shree, S.; Sharma, A.; Panesar, P.S.; Goswami, S. Recent Approaches for Enhanced Production of Microbial Polyhydroxybutyrate: Preparation of Biocomposites and Applications. Int. J. Biol. Macromol. 2021, 182, 1650–1669. [Google Scholar] [CrossRef]
- Somvanshi, K.S.; Gope, P.C. Effect of Ultrasonication and Fiber Treatment on Mechanical and Thermal Properties of Polyvinyl Alcohol/Cellulose Fiber Nano-Biocomposite Film. Polym. Compos. 2021, 42, 5310–5322. [Google Scholar] [CrossRef]
- Arun, R.; Shruthy, R.; Preetha, R.; Sreejit, V. Biodegradable Nano Composite Reinforced with Cellulose Nano Fiber from Coconut Industry Waste for Replacing Synthetic Plastic Food Packaging. Chemosphere 2022, 291, 132786. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.H.; Abdellaoui, Y.; Ait Benhamou, A.; Ablouh, E.H.; El Achaby, M.; Kassab, Z. Influence of Cellulose Nanocrystals from Pea Pod Waste on Mechanical, Thermal, Biodegradability, and Barrier Properties of Chitosan-Based Films. Cellulose 2022, 29, 5117–5135. [Google Scholar] [CrossRef]
- Braga, D.G.; Bezerra, P.G.F.; Lima, A.B.F.D.; Pinheiro, H.A.; Gomes, L.G.; Fonseca, A.S.; Bufalino, L. Chitosan-Based Films Reinforced with Cellulose Nanofibrils Isolated from Euterpe oleraceae MART. Polym. Renew. Resour. 2021, 12, 46–59. [Google Scholar] [CrossRef]
- Zeng, J.; Ren, X.; Zhu, S.; Gao, Y. Cellulose Nanocrystals from Pomegranate Peel: Isolation, Characterization, and Its Reinforcement for Chitosan Film. J. Mater. Sci. 2022, 57, 11062–11076. [Google Scholar] [CrossRef]
- Menezes, D.B.; Diz, F.M.; Romanholo Ferreira, L.F.; Corrales, Y.; Baudrit, J.R.V.; Costa, L.P.; Hernández-Macedo, M.L. Starch-Based Biocomposite Membrane Reinforced by Orange Bagasse Cellulose Nanofibers Extracted from Ionic Liquid Treatment. Cellulose 2021, 28, 4137–4149. [Google Scholar] [CrossRef]
- Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Cavallo, E.; Cerrutti, P.; Foresti, M.L.; Peltzer, M.A. Reinforcement of Yeast Biomass Films with Bacterial Cellulose and Rice Husk Cellulose Nanofibres. J. Polym. Environ. 2021, 29, 3242–3251. [Google Scholar] [CrossRef]
- Díaz-Cruz, C.A.; Caicedo, C.; Jiménez-Regalado, E.J.; Díaz de León, R.; López-González, R.; Aguirre-Loredo, R.Y. Evaluation of the Antimicrobial, Thermal, Mechanical, and Barrier Properties of Corn Starch–Chitosan Biodegradable Films Reinforced with Cellulose Nanocrystals. Polymers 2022, 14, 2166. [Google Scholar] [CrossRef]
- Joseph, M.R.; Nizam, P.A.; Gopakumar, S.; Maria, H.J.; Vishnu, R.; Kalarikkal, N.; Thomas, S.; Vidyasagaran, K.; Anoop, E.V. Development and Characterization of Cellulose Nanofibre Reinforced Acacia Nilotica Gum Nanocomposite. Ind. Crops Prod. 2021, 161, 113180. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Dixit, S.; Pal, D.B.; Mishra, P.K.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of Nanocellulose on Mechanical and Barrier Properties of PVA–Banana Pseudostem Fiber Composite Films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Niinivaara, E.; Desmaisons, J.; Dufresne, A.; Bras, J.; Cranston, E.D. Thick Polyvinyl Alcohol Films Reinforced with Cellulose Nanocrystals for Coating Applications. ACS Appl. Nano Mater. 2021, 4, 8015–8025. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Flammability and Physical Stability of Sugar Palm Crystalline Nanocellulose Reinforced Thermoplastic Sugar Palm Starch/Poly(Lactic Acid) Blend Bionanocomposites. Nanotechnol. Rev. 2022, 11, 86–95. [Google Scholar] [CrossRef]
- Shazleen, S.S.; Yasim-Anuar, T.A.T.; Ibrahim, N.A.; Hassan, M.A.; Ariffin, H. Functionality of Cellulose Nanofiber as Bio-Based Nucleating Agent and Nano-Reinforcement Material to Enhance Crystallization and Mechanical Properties of Polylactic Acid Nanocomposite. Polymers 2021, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver, K.; Li, K.; Lamm, M.E.; Walker, C.; Johnson, D.; Han, Y.; Wang, L.; Zhao, X.; Pu, Y.; Hinton, H.; et al. Recycled Cardboard Containers as a Low Energy Source for Cellulose Nanofibrils and Their Use in Poly(l-Lactide) Nanocomposites. ACS Sustain. Chem. Eng. 2021, 9, 13460–13470. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Ker, D.; Zhao, X.; Hinton, H.E.; Copenhaver, K.; Tekinalp, H.; Ozcan, S. Exploiting Chitosan to Improve the Interface of Nanocellulose Reinforced Polymer Composites. Cellulose 2022, 29, 3859–3870. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Water Barrier and Mechanical Properties of Sugar Palm Crystalline Nanocellulose Reinforced Thermoplastic Sugar Palm Starch (TPS)/Poly(Lactic Acid) (PLA) Blend Bionanocomposites. Nanotechnol. Rev. 2021, 10, 431–442. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Parveez, B. Preparation, Characterization and Properties of Biodegradable Composites from Bamboo Fibers—Mechanical and Morphological Study. J. Polym. Environ. 2021, 29, 4120–4126. [Google Scholar] [CrossRef]
- Chihaoui, B.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Lignin-Containing Cellulose Fibrils as Reinforcement of Plasticized PLA Biocomposites Produced by Melt Processing Using PEG as a Carrier. Ind. Crops Prod. 2022, 175, 114287. [Google Scholar] [CrossRef]
- Zhou, P.; Luo, Y.; Lv, Z.; Sun, X.; Tian, Y.; Zhang, X. Melt-Processed Poly (Vinyl Alcohol)/Corn Starch/Nanocellulose Composites with Improved Mechanical Properties. Int. J. Biol. Macromol. 2021, 183, 1903–1910. [Google Scholar] [CrossRef]
- Kesari, A.K.; Mandava, S.; Munagala, C.K.; Nagar, H.; Aniya, V. DES-Ultrasonication Processing for Cellulose Nanofiber and Its Compounding in Biodegradable Starch Based Packaging Films through Extrusion. Ind. Crops Prod. 2022, 188, 115566. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Nadanathangam, V.; Dhakane-Lad, J.; Mahapatra, A.; Jagajanantha, P.; Saxena, S. Nanocellulose Reinforced Corn Starch-Based Biocomposite Films: Composite Optimization, Characterization and Storage Studies. Food Packag. Shelf Life 2022, 33, 100860. [Google Scholar] [CrossRef]
- Csiszár, E.; Kun, D.; Fekete, E. The Role of Structure and Interactions in Thermoplastic Starch–Nanocellulose Composites. Polymers 2021, 13, 3186. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.A.; de Oliveira, A.C.S.; Lago, A.M.T.; Yoshida, M.I.; Dias, M.V.; Borges, S.V. Properties of Chitosan–Papain Biopolymers Reinforced with Cellulose Nanofibers. J. Food Process. Preserv. 2021, 45, e15740. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Dunno, K.D.; Cavender, G.A.; Dawson, P. Pearl Millet Starch-Based Nanocomposite Films Reinforced with Kudzu Cellulose Nanocrystals and Essential Oil: Effect on Functionality and Biodegradability. Food Res. Int. 2022, 157, 111384. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, H. Preparation and Characterization of a Biodegradable Starch-Based Antibacterial Film Containing Nanocellulose and Polyhexamethylene Biguanide. Food Packag. Shelf Life 2021, 30, 100718. [Google Scholar] [CrossRef]
- Zhao, Y.; Troedsson, C.; Bouquet, J.M.; Thompson, E.M.; Zheng, B.; Wang, M. Mechanically Reinforced, Flexible, Hydrophobic and Uv Impermeable Starch-Cellulose Nanofibers (Cnf)-Lignin Composites with Good Barrier and Thermal Properties. Polymers 2021, 13, 4346. [Google Scholar] [CrossRef] [PubMed]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and Degradability Properties of Polyvinyl Alcohol/Gelatin Nanocomposite Films Filled Water Hyacinth Cellulose Nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Chin, K.M.; Sam, S.T.; Ong, H.L.; Wong, Y.S.; Tan, W.K.; Vannaladsaysy, V. Bioinspired Crosslinked Nanocomposites of Polyvinyl Alcohol-Reinforced Cellulose Nanocrystals Extracted from Rice Straw with Ethanedioic Acid. J. Nanomater. 2022, 2022, 3225211. [Google Scholar] [CrossRef]
- Mousavi Kalajahi, S.E.; Alizadeh, A.; Hamishehkar, H.; Almasi, H.; Asefi, N. Orange Juice Processing Waste as a Biopolymer Base for Biodegradable Film Formation Reinforced with Cellulose Nanofiber and Activated with Nettle Essential Oil. J. Polym. Environ. 2022, 30, 258–269. [Google Scholar] [CrossRef]
- Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. Available online: https://www.astm.org/d1238-10.html (accessed on 18 December 2022).
- Chou, C.T.; Shi, S.C.; Chen, C.K. Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material. Polymers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Available online: https://www.astm.org/d0882-18.html (accessed on 18 December 2022).
- George, T.S.; Muhammadaly, S.A.; Parambath Kanoth, B.; Joseph, T.; Chemmarickal Dominic, M.D.; George, N.; Balachandrakurupp, V.; John, H. Isolation of High Crystalline Nanocellulose from Mimosa Pudica Plant Fibres with Potential in Packaging Applications. Packag. Technol. Sci. 2022, 35, 163–174. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Fully Biodegradable Poly(Butylene Succinate-Co-1,2-Decylene Succinate)/Cellulose Nanocrystals Composites with Significantly Enhanced Crystallization and Mechanical Property*. Polymer 2022, 252, 124946. [Google Scholar] [CrossRef]
- Kurokawa, N.; Matsumoto, K.; Hotta, A. Highly-Toughened and Dimensionally-Stable TEMPO Cellulose Nanofiber/Bio-PBSA Nanocomposites Fabricated via Pickering Emulsion Process. Compos. Sci. Technol. 2022, 223, 109402. [Google Scholar] [CrossRef]
- Standard Test Method for Water Vapor Transmission of Materials. Available online: https://www.astm.org/e0096-95.html (accessed on 18 December 2022).
- ISO 846:2019; Plastics—Evaluation of the Action of Microorganisms. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/74599.html (accessed on 18 December 2022).
- Szefer, E.; Leszczyńska, A.; Hebda, E.; Pielichowski, K. The Application of Cellulose Nanocrystals Modified with Succinic Anhydride under the Microwave Irradiation for Preparation of Polylactic Acid Nanocomposites. J. Renew. Mater. 2021, 9, 1127–1142. [Google Scholar] [CrossRef]
- Wang, M.; Miao, X.; Li, H.; Chen, C. Effect of Length of Cellulose Nanofibers on Mechanical Reinforcement of Polyvinyl Alcohol. Polymers 2022, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, X.; Li, D.; Dong, J. Toward Sustainable Biocomposites Based on MMT and PHBH Reinforced with Acetylated Cellulose Nanocrystals. Cellulose 2021, 28, 2981–2993. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Saharudin, N.I.; Hazwan, C.M.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Abdullah, C.K.; Alfatah, T.; Gopakumar, D.A.; Pasquini, D. Improved Hydrophobicity of Macroalgae Biopolymer Film Incorporated with Kenaf Derived Cnf Using Silane Coupling Agent. Molecules 2021, 26, 2254. [Google Scholar] [CrossRef]
- Gao, C.; Wang, S.; Liu, B.; Yao, S.; Dai, Y.; Zhou, L.; Qin, C.; Fatehi, P. Sustainable Chitosan-Dialdehyde Cellulose Nanocrystal Film. Materials 2021, 14, 5851. [Google Scholar] [CrossRef]
- Li, F.J.; Yu, X.T.; Huang, Z.; Liu, D.F. Interfacial Improvements in Cellulose Nanofibers Reinforced Polylactide Bionanocomposites Prepared by in Situ Reactive Extrusion. Polym. Adv. Technol. 2021, 32, 2352–2366. [Google Scholar] [CrossRef]
- Nasution, H.; Olaiya, N.G.; Haafiz, M.K.M.; Abdullah, C.K.; Bakar, S.A.; Olaiya, F.G.; Mohamed, A.; Abdul, A.K. The Role of Amphiphilic Chitosan in Hybrid Nanocellulose–Reinforced Polylactic Acid Biocomposite. Polym. Adv. Technol. 2021, 32, 3446–3457. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Sabo, R.; Bissessur, R.; Acharya, B. Polylactic Acid Cellulose Nanocomposite Films Comprised of Wood and Tunicate CNCs Modified with Tannic Acid and Octadecylamine. Polymers 2021, 13, 3661. [Google Scholar] [CrossRef]
- Ren, Q.; Wu, M.; Wang, L.; Zheng, W.; Hikima, Y.; Semba, T.; Ohshima, M. Cellulose Nanofiber Reinforced Poly (Lactic Acid) with Enhanced Rheology, Crystallization and Foaming Ability. Carbohydr. Polym. 2022, 286, 119320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hikima, Y.; Sekiguchi, T.; Ohshima, M. Thermal, Rheological, and Mechanical Properties of Cellulose Nanofiber (CNF) and Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBH) Biopolymer Nanocomposites. Cellulose 2022, 29, 3901–3913. [Google Scholar] [CrossRef]
- Do Nascimento, D.M.; Dias, A.F.; de Araújo Junior, C.P.; Rosa, M.d.F.; Morais, J.P.S.; de Figueirêdo, M.C.B. A Comprehensive Approach for Obtaining Cellulose Nanocrystal from Coconut Fiber. Part II: Environmental Assessment of Technological Pathways. Ind. Crops Prod. 2016, 93, 58–65. [Google Scholar] [CrossRef]
- Shazleen, S.S.; Foong Ng, L.Y.; Ibrahim, N.A.; Hassan, M.A.; Ariffin, H. Combined Effects of Cellulose Nanofiber Nucleation and Maleated Polylactic Acid Compatibilization on the Crystallization Kinetic and Mechanical Properties of Polylactic Acid Nanocomposite. Polymers 2021, 13, 3226. [Google Scholar] [CrossRef] [PubMed]
- Haroni, S.; Zaki Dizaji, H.; Bahrami, H.; González Alriols, M. Sustainable Production of Cellulose Nanofiber from Sugarcane Trash: A Quality and Life Cycle Assessment. Ind. Crops Prod. 2021, 173, 114084. [Google Scholar] [CrossRef]
- Arvidsson, R.; Nguyen, D.; Svanström, M. Life Cycle Assessment of Cellulose Nanofibrils Production by Mechanical Treatment and Two Different Pretreatment Processes. Environ. Sci. Technol. 2015, 49, 6881–6890. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Life Cycle Assessment of a New Technology to Extract, Functionalize and Orient Cellulose Nanofibers from Food Waste. ACS Sustain. Chem. Eng. 2015, 3, 1047–1055. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the Environmental Impact of a Future Nanocellulose Production at Industrial Scale: Application of the Life Cycle Assessment Scale-up Framework. J. Clean. Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Hervy, M.; Evangelisti, S.; Lettieri, P.; Lee, K.Y. Life Cycle Assessment of Nanocellulose-Reinforced Advanced Fibre Composites. Compos. Sci. Technol. 2015, 118, 154–162. [Google Scholar] [CrossRef]
- Turk, J.; Oven, P.; Poljanšek, I.; Lešek, A.; Knez, F.; Malovrh Rebec, K. Evaluation of an Environmental Profile Comparison for Nanocellulose Production and Supply Chain by Applying Different Life Cycle Assessment Methods. J. Clean. Prod. 2020, 247, 119107. [Google Scholar] [CrossRef]
- Kanematsu, Y.; Kikuchi, Y.; Yano, H. Life Cycle Greenhouse Gas Emissions of Acetylated Cellulose Nanofiber-Reinforced Polylactic Acid Based on Scale-Up from Lab-Scale Experiments. ACS Sustain Chem. Eng. 2021, 9, 10444–10452. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Batista, G.; De Aguiar, J.; Lorevice, M.V.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Cellulose Nanocrystals from Sugar Cane Bagasse Using Organic and/or Inorganic Acids: Techno-Economic Analysis and Life Cycle Assessment. ACS Sustain Chem. Eng. 2022, 10, 4660–4676. [Google Scholar] [CrossRef]
- Katakojwala, R.; Venkata Mohan, S. Multi-Product Biorefinery with Sugarcane Bagasse: Process Development for Nanocellulose, Lignin and Biohydrogen Production and Lifecycle Analysis. Chem. Eng. J. 2022, 446, 137233. [Google Scholar] [CrossRef]
- Krexner, T.; Bauer, A.; Zollitsch, W.; Weiland, K.; Bismarck, A.; Mautner, A.; Medel-Jiménez, F.; Gronauer, A.; Kral, I. Environmental Life Cycle Assessment of Nano-Cellulose and Biogas Production from Manure. J. Environ. Manag. 2022, 314, 115093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, X.; Ai, Y.; Huang, R.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Greener Production of Cellulose Nanocrystals: An Optimised Design and Life Cycle Assessment. J. Clean. Prod. 2022, 345, 131073. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment. ReCiPe 2016 v1.1. 2017. Available online: https://pre-sustainability.com/legacy/download/Report_ReCiPe_2017.pdf (accessed on 1 December 2022).
- OpenLCA Software 2022. Available online: https://www.openlca.org/ (accessed on 1 December 2022).
- Pré-Sustainability, B.V. SimaPro 2022. Available online: https://pre-sustainability.com/solutions/tools/simapro/ (accessed on 1 December 2022).
- European Commission; Joint Research Centre; Institute for Environment and Sustainability. International Reference Life Cycle Data System (ILCD) Handbook—General Guide for Life Cycle Assessment—Detailed Guidance; Publications Office of the European Union: Luxembourg, 2010; ISBN 9789279190926. [Google Scholar]
- CML—Department of Industrial Ecology CML 2016. Available online: https://www.universiteitleiden.nl/en/science/environmental-sciences/department-of-industrial-ecology (accessed on 1 December 2022).
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC, Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Bulle, C.; Margni, M.; Patouillard, L.; Boulay, A.-M.; Bourgault, G.; De Bruille, V.; Cao, V.; Hauschild, M.; Henderson, A.; Humbert, S.; et al. IMPACT World+: A Globally Regionalized Life Cycle Impact Assessment Method. Int. J. Life Cycle Assess. 2019, 24, 1653–1674. [Google Scholar] [CrossRef]
- PE International AG GaBi Software 2012. Available online: http://www.gabi-software.com (accessed on 1 December 2022).

| Reinforcement (Source) | Matrix | Processing Conditions | Mechanical Properties | ||
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation (%) | |||
| CNC (sugar palm) [54] | PLA-Sugar palm starch | Solvent: distilled water NC content: 0.5 wt.% Plasticizer: 15 wt.% glycerol and 15 wt.% sorbitol Mechanical stirring for 30–45 min at 80 °C Melt blending at 170 °C for 13 min and 50 rpm Hot processing | - | - | - |
| CNF (bamboo pulp) and corn starch [61] | PVA | NC content: 0, 10, and 20 wt.% Plasticizer: Formamide and water Melt mixed at 150 °C, 30 rpm for 10 min Compression molding | 19.5–28.1 | 0.7–1.6 | 2.5–11.0 |
| CNF (cardboard) [56] | PLA | NC content: 0–40 wt.% Melt mixed in a Brabender at 170 °C, 60 rpm for 3–5 min Compression molding at 175 °C | 52.0–60.0 | 3.4–5.9 | - |
| CNF [55] | PLA | NC content: 0–6 wt.% Melt blended in a Brabender at 170 °C, 30 rpm for 30 min Compression molding at 160 °C for 10 min. | 68.5–76.1 | 2.9–3.3 | - |
| CNF/CS [57] | PLA | CNF–CS content: 5–30 wt.% Meltblended in a Brabender 175 °C, 60 rpm for 3 min Compression molding at 180 °C | 51.0–58.0 | 3.0–4.2 | - |
| CNC (bamboo) [59] | PLA/PBS | NC content: 0–1.5 wt.% 20 wt.% PBS Melt mixed at 175 °C and 60 rpm for 10 min Compression molding at 180 °C and 150 MPa for 4 min Postcuring at 50 °C for 24 h | 69.0–86.0 | 8.3–10.4 | 12.0–18.0 |
| LCNF (palm waste) [60] | PLA | NC content: 0–10 wt.% Plasticizer: 20 wt.% PEG Mechanical stirring and sonication at 30 °C Melt compounding in a corotating conical twin-screw at 190 °C and 100 rpm for 5 min | 12.5–20.0 | 0.4–1.4 | 2.0–100.0 |
| CNF (bamboo) [62] | Corn starch | NC content: 0–5 wt.% Plasticizer: 30 wt.% glycerol and 1 wt.% stearic acid. Melt processing in a co-rotating twin-screw extruder from 90–130 °C. Compression molding at 70 MPa and 130 °C for 5 min. | 3.0–5.1 | 0.9–2.1 | 22.2–48.6 |
| Research/Type of Nanocellulose | Production Scheme | Source | GWP (kg CO2 eq) | TA (kg SO2 eq) | FE (kg P eq) | ME (kg N eq) | HT (kg 1,4-DB eq) | WD (m3) |
|---|---|---|---|---|---|---|---|---|
| Arvidsson et al. [93]/CNF | Enzymatic pretreatment | Wood Pulp | 0–2 | 0–0.02 | - | - | - | 0.2–0.3 |
| Carboxymethylation pretreatment | 95–105 | 0.17–0.2 | - | - | - | 1–1.1 | ||
| No pretreatment | 0–5 | 0–0.02 | - | - | - | 0.1–0.2 | ||
| Piccino et al. [94,95]/CNF | Enzymatic-Wet Spinning | Carrot waste | 1.9 | - | - | - | - | - |
| Enzymatic-Electro Spinning | 1.6 | - | - | - | - | - | ||
| Hervy et al. [96]/CNF-Epoxy composite | Bacterial Cellulose | Kraft Pulp | 14 | - | - | - | - | - |
| Nanofribillated Cellulose | 8.5 | - | - | - | - | - | ||
| do Nascimento et al. [90]/CNC | Oxidation-Ultrasound-Hydrolysis | Coconut Fiber | 207 | 0.45 | 0.0568 | 0.0303 | 47.7 | 2.3 |
| Turk et al. [97]/CNF | Tempo | Woody biomass | 806.92 | 6.44 | 0.76 | 0.05 | 49.38 | 28.2 |
| Kanematsu et al. [98]/AcCNF-PLA | Acetylation-kneading | Woody chips | 5.20–11.20 | - | - | - | - | - |
| Haroni et al. [92]/CNF | Organo-solvent/TEMPO/High-Pressure (OTH) | Sugarcane trash | 7.89 | 0.07 | - | - | 61.37 | - |
| Organo-solvent/Lime Juice/High-Pressure (OLH) | 9.96 | 0.09 | - | - | 85.23 | - | ||
| Alkaline/TEMPO/High-Pressure (ATH) | 4.08 | 0.03 | - | - | 41.44 | - | ||
| Alkaline/Lime Juice/High-Pressure (ALH) | 2.85 | 0.02 | - | - | 23.52 | - | ||
| Zargar et al. [31]/LCNC-CNC | Deep Eutetic Solvent | Thermomechanical pulp | 31.7 | 0.14 | - | - | - | - |
| Acid Hydrolysis | 12.9 | 0.29 | - | - | - | - | ||
| Bondancia et al. [99]/CNC | Citric acid | Sugar Cane Bagasse | 389 | - | - | - | - | - |
| Sulfuric acid | 879 | - | - | - | - | - | ||
| Citric + sulfuric acid | 894 | - | - | - | - | - | ||
| Katakojwala et al. [100]/NCC | Baseline | Sugar Cane Bagasse | 286 | - | - | - | - | - |
| Biorefinery | 213 | - | - | - | - | - | ||
| Krexner et al. [101]/CNF | Fermentation-Grinding | Manure | 1.4 | 0.01 | 0.0009 | - | 1.9 | - |
| Fermentation-Grinding | Hardwood chips | 1.5 | 0.06 | 0.0008 | - | 2.1 | - | |
| Zhang et al. [102]/CNC | Acid hydrolysis + gravity settling | Cotton | 45–50 | 0.35–0.4 | 0.014–0.016 | - | - | - |
| Acid hydrolysis + gravity settling + disc stack centrifugation | 40–45 | 0.22–0.26 | 0.007–0.009 | - | - | - | ||
| Acid hydrolysis + centrifugation + disc stack centrifugation | 40–45 | 0.25–0.3 | 0.008–0.009 | - | - | - | ||
| Acid hydrolysis + microfiltration + disc stack centrifugation | 28–32 | 0.18–0.2 | 0.006–0.008 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aigaje, E.; Riofrio, A.; Baykara, H. Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review. Polymers 2023, 15, 1219. https://doi.org/10.3390/polym15051219
Aigaje E, Riofrio A, Baykara H. Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review. Polymers. 2023; 15(5):1219. https://doi.org/10.3390/polym15051219
Chicago/Turabian StyleAigaje, Elizabeth, Ariel Riofrio, and Haci Baykara. 2023. "Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review" Polymers 15, no. 5: 1219. https://doi.org/10.3390/polym15051219
APA StyleAigaje, E., Riofrio, A., & Baykara, H. (2023). Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review. Polymers, 15(5), 1219. https://doi.org/10.3390/polym15051219







