Cytotoxicity Analysis for the Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites in Oral Squamous Carcinoma (KB) Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. MWCNT and PMMA
2.2. Functionalization of Carbon Nanotubes
- Superior Conductivity
- Chemical Inertness
- Enhancement of Flexibility
- Biocompatibility
- Water Dissolution rate
- Heat Resistance
- Large Surface
2.3. Synthesis, Testing and Characterization
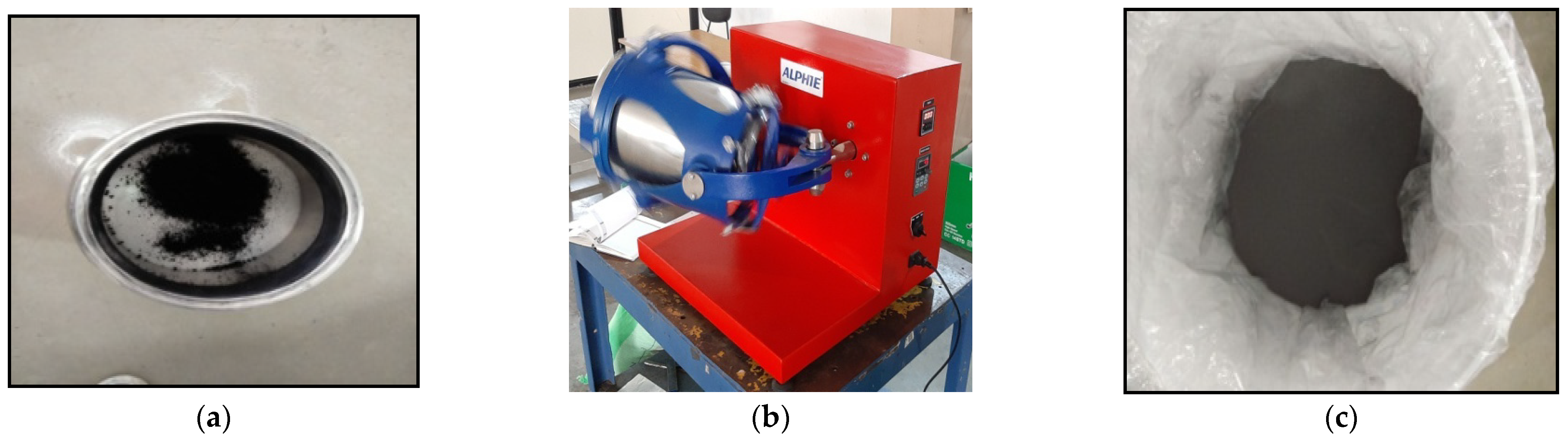
2.3.1. 3D Mixture
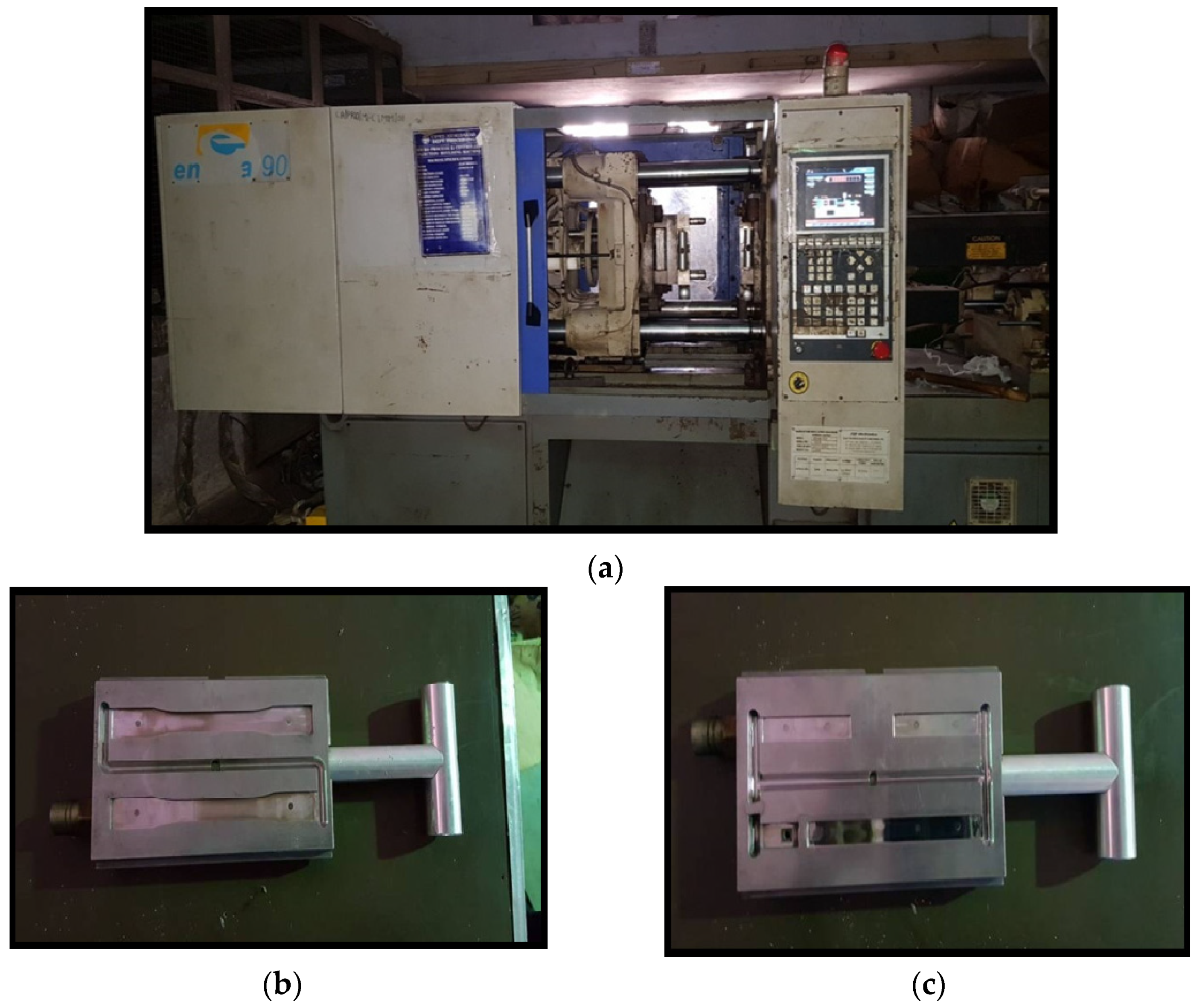
2.3.2. Injection Molding Machine
2.3.3. Universal Testing Machine/Tensile Testing
2.3.4. Three Point Bending Test/Flextural Testing
2.3.5. Scanning Electron Microscopy
2.4. Cell Culture and Maintenance
2.5. In Vitro Uptake Study
2.6. Reactive Oxygen Species Generation through DCHF-DA Assay
2.7. Cellular Reactive Oxygen Species Generation upon Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites
2.8. Apoptosis Detection through Dual Staining with Hoechst and PI Staining
3. Results and Discussion
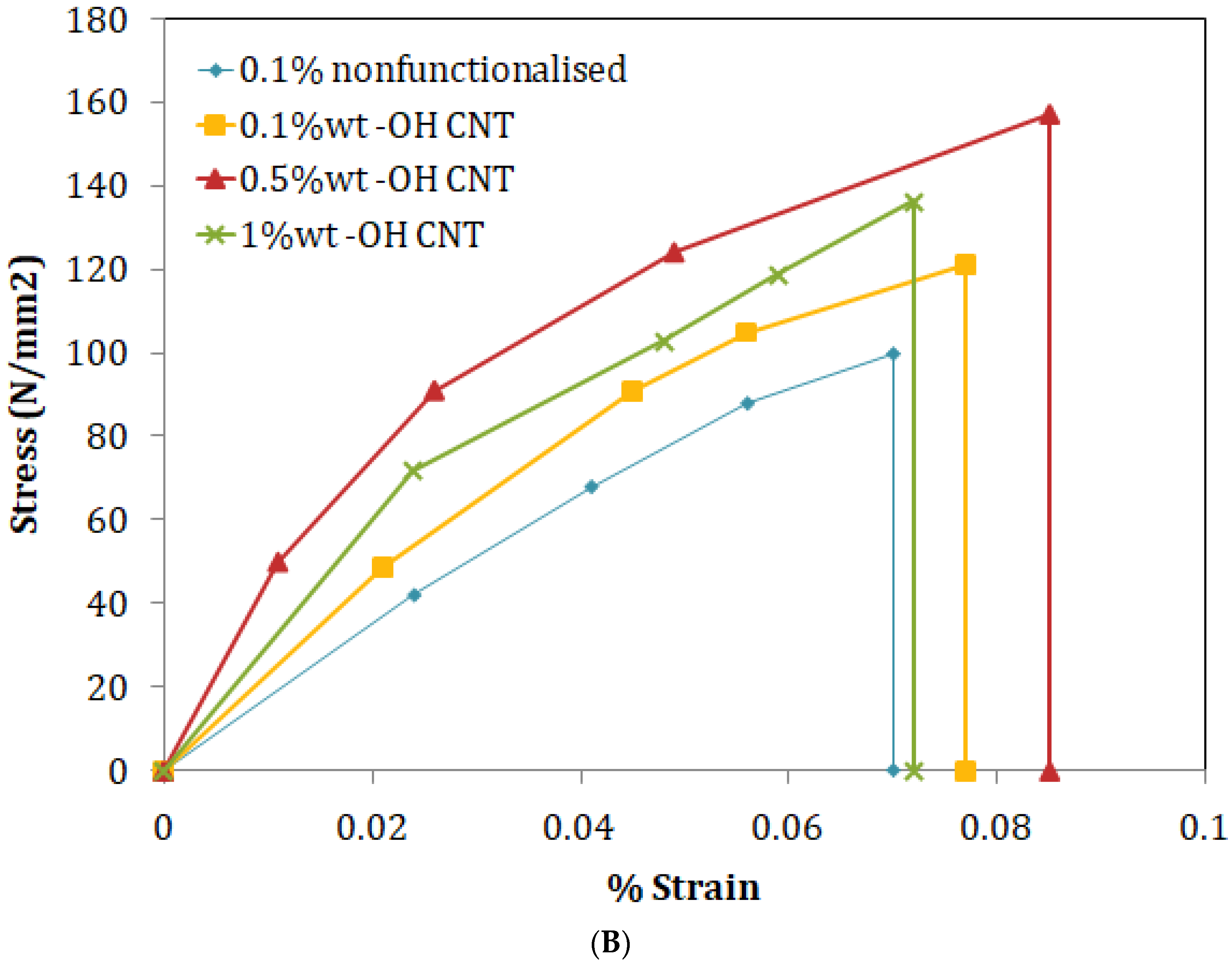
3.1. Mechanical Characterization
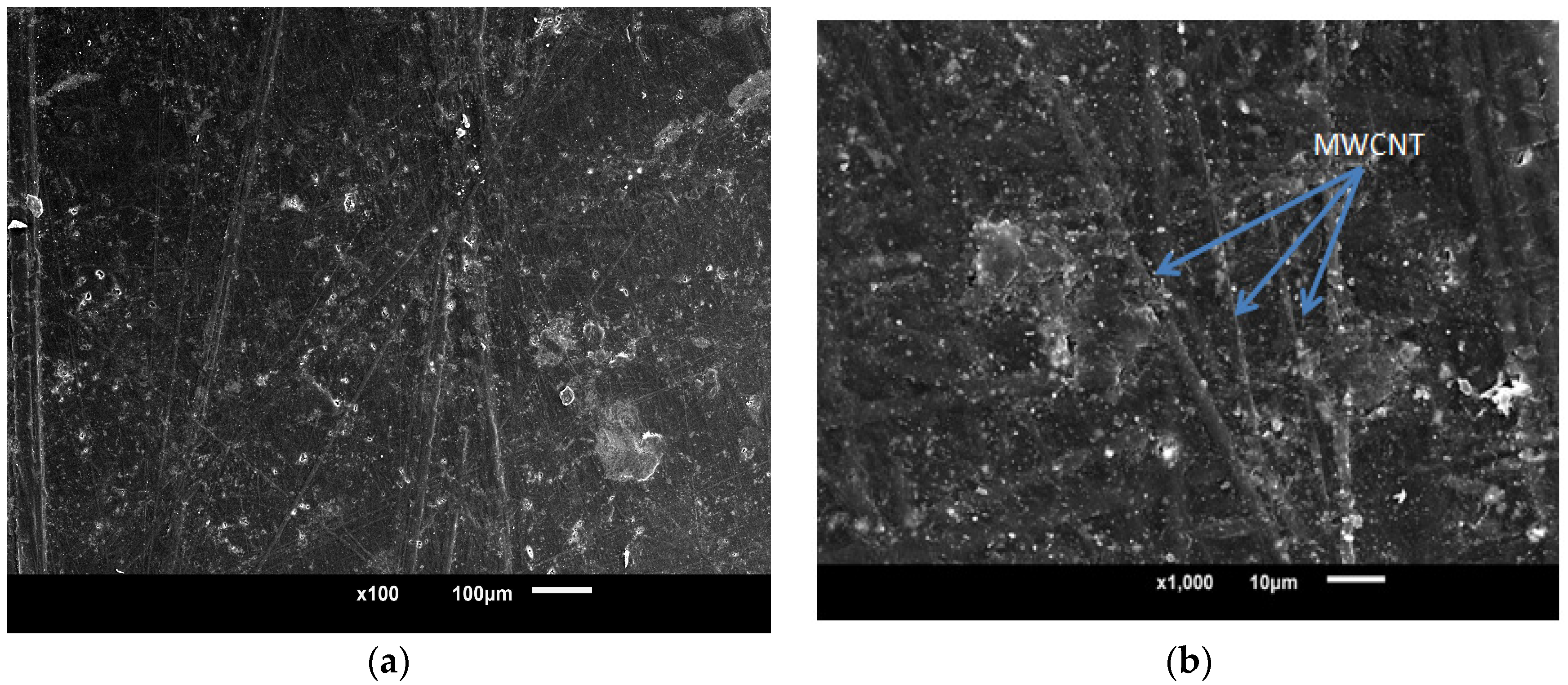
3.2. Morphological Characteristics
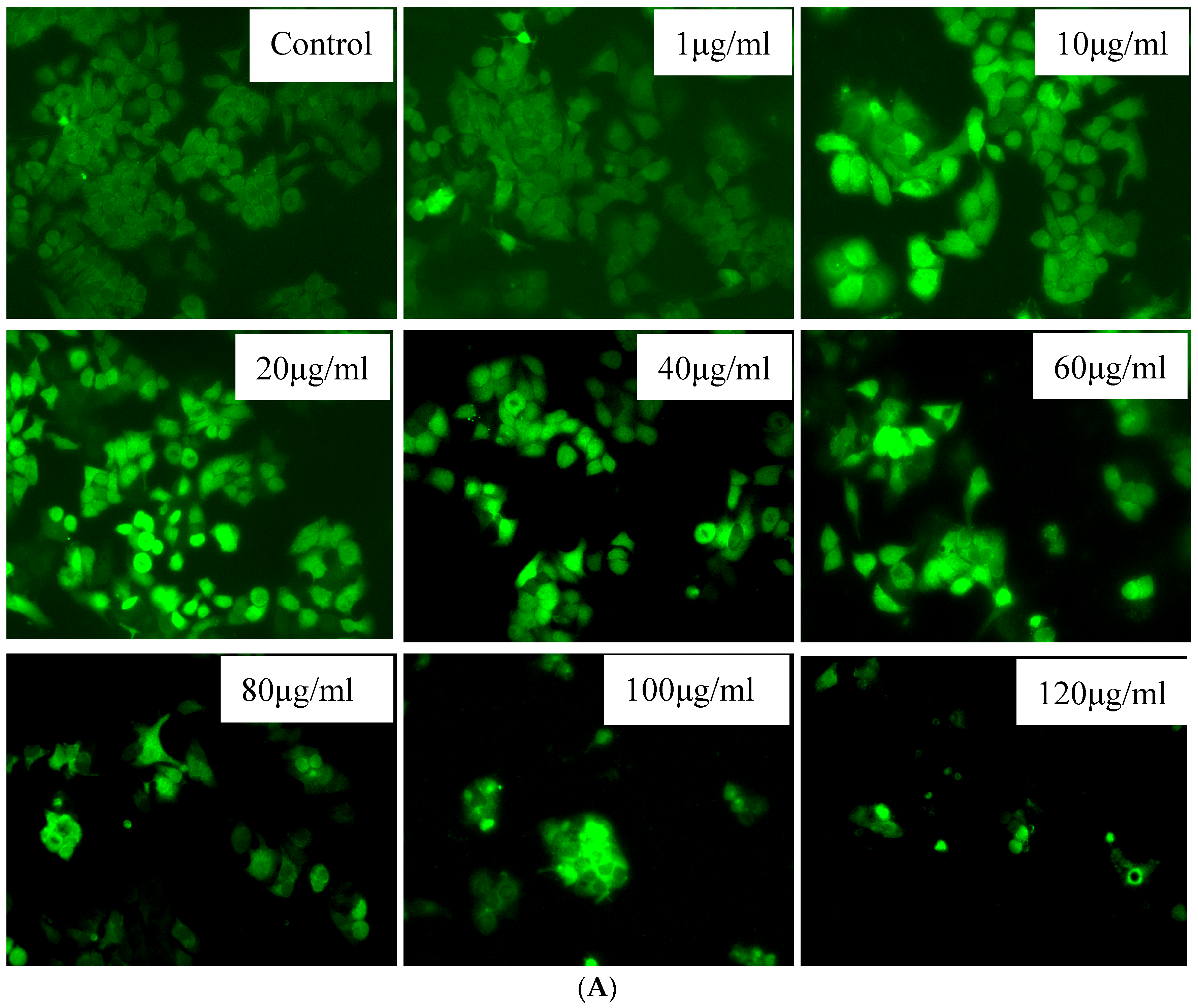
3.3. In Vitro Phagocytic Uptake Studies
3.4. In Vitro Cell Viability Assay
Cell Viability
3.5. Cellular Reactive Oxygen Species Generation upon Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites
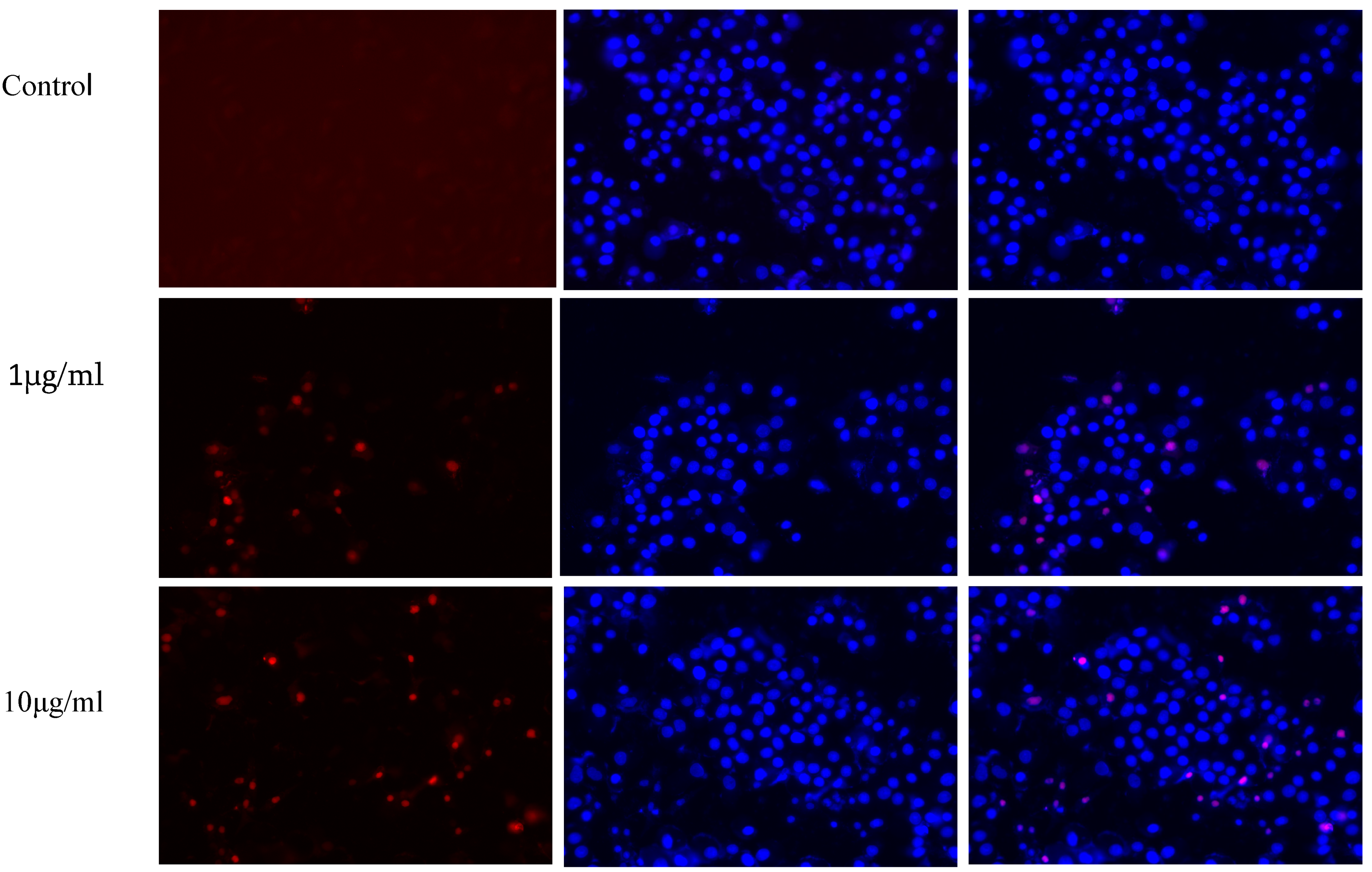
3.6. Hoechst and PI Staining Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Garces, J.; Moll, D.J.; Bicerano, J.; Fibiger, R.; Mc Leod, D.G. Polymeric nanocomposites for automotive applications. Adv. Mater. 2000, 12, 1835–1839. [Google Scholar] [CrossRef]
- Ghosh, S.; Haldar, S.; Gupta, S.; Bisht, A.; Chauhan, S.; Kumar, V.; Roy, P.; Lahiri, D. Anisotropically conductive biodegradable scaffold with coaxially aligned carbon nanotubes for directional regeneration of peripheral nerves. ACS Appl. Bio Mater. 2020, 3, 5796–5812. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Mansur, A.; Ciminelli, V.; Mansur, H. Nanocomposites of poly (vinyl alcohol)/functionalized- multiwall carbon nanotubes conjugated with glucose oxidase for potential application as scaffolds in skin wound healing. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 185–196. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Grasley, Z.; Tyson, B.; Al-RubR, K. Distribution of Carbon Nano fibers and Nano tubes in Cementitious Composites. Transp. Res. Rec. 2010, 2142, 89–95. [Google Scholar] [CrossRef]
- Khan, W.; Sharma, R.; Saini, P. Carbon Nanotube-Based Polymer Composites: Synthesis, Properties and Applications. In Carbon Nanotubes-Current Progress of Their Polymer Composites; Berber, M.R., Hafez, I.H., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Choudhary, V.; Gupta, A. Polymer/Carbon Nanotube Nanocomposites. In Carbon Nanotubes-Polymer Nanocomposites; Yellampalli, S., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Hallad, S.A.; Banapurmath, N.R.; Khan, T.M.Y.; Umarfarooq, M.A.; Soudagar, M.E.M.; Hunashyal, A.M.; Gujjar, S.V.; Yaradoddi, J.S.; Ganachari, S.V.; Elfasakhany, A.; et al. Statistical Analysis of Polymer Nanocomposites for Mechanical Properties. Molecules 2021, 26, 4135. [Google Scholar] [CrossRef]
- Quadrini, F.; Denise, B.; Loredana, S.; Felicia, S.; Fetecau, C. Compression Moulding of Thermoplastic Nanocomposites Filled with MWCNT. Polym. Polym. Compos. 2017, 25, 611–620. [Google Scholar] [CrossRef]
- Liang, Q.; Kaili, Z.; Hongsheng, T.; Xu, Y.; Lihang, Z.; Shuhua, D. Effect of carbon nanotubes on the electrical, thermal, mechanical properties and crystallization behavior of continuous carbon fiber reinforced polyether-ether-ketone composites. Mater. Res. Express 2021, 8, 045312. [Google Scholar]
- Murugan, C.; Venkatesan, S.; Kannan, S. Cancer Therapeutic Proficiency of Dual-Targeted Mesoporous Silica Nanocomposite Endorses Combination Drug Delivery. ACS Omega 2017, 2, 7959–7975. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef]
- Xue, M.; Zhong, X.; Shaposhnik, Z.; Qu, Y.; Tamanoi, F.; Duan, X.; Zink, J.I. pH-Operated Mechanized Porous Silicon Nanoparticles. J. Am. Chem. Soc. 2011, 133, 8798–8801. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Megan, A.M.; Mostafa, A.E. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J. Am. Chem. Soc. 2010, 132, 1517. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Sci. Rep. 2016, 6, 34053. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Venkatesan, J.; Kiml, S. Polymer Functionalized Single Walled Carbon Nanotubes Mediated Drug Delivery of Gliotoxin in Cancer Cells. J. Biomed. Nanotechnol. 2014, 10, 120–130. [Google Scholar] [CrossRef]
- Tan, J.M.; Bullo, S.; Fakurazi, S.; Hussein, M.Z. Preparation, characterization and biological evaluation of biopolymer-coated multi-walled carbon nanotubes for sustained-delivery of silibinin. Sci. Rep. 2020, 10, 16941. [Google Scholar] [CrossRef]
- Hosseini, S.; Adeli, M.; Pourjavadi, A. Polymer-functionalized carbon nanotubes in cancer therapy: A review. Iran. Polym. J. 2014, 23, 387–403. [Google Scholar]
- Rizwan, W.; Khan, F.; Maqsood, A.; Javed, A.; Quaiser, S.; Abdulaziz, A. Cytotoxic assessment of liver cancer cells (HepG2) with raw, functionalized multiwalled carbon nanotubes and their comparison with nano hydroxyapatite. J. King Saud Univ.-Sci. 2021, 33, 101444. [Google Scholar]
- Nafiseh, N.; Yahya, R.; Mansour, A.; Yousef, M. Cellular Toxicity of Multi-walled Carbon Nanotubes on Human Lung Cells. J. Chem. Health Risks 2020, 10, 135–144. [Google Scholar]
- González, E.; Valdivia, L.; García, A.; González, F.; Pesquera, C.; Valiente, R.; Fanarraga, M. Multi-walled carbon nanotubes complement the anti-tumoral effect of 5-Fluorouracil. Oncotarget 2019, 10, 2022–2029. [Google Scholar] [CrossRef]
- Bellucci, S.; Simona, D.; Pierpaolo, C.; Mariano, B.; Angela, C.; Federico, M.; Immacolata, S.; Giulia, R.; Alessandra, C. Multiwalled carbon nanotubes-induced cytotoxic effects on human breast adenocarcinoma cell line. In Proceedings of the CAS 2012 (International Semiconductor Conference), Sinaia, Romania, 15–17 October 2012; pp. 37–42. [Google Scholar]
- Vittorio, O.; Raffa, V.; Cuschieri, A. Influence of purity and surface oxidation on cytotoxicity of multi-wall carbon nanotubes with human neuroblastoma cells. Nano Med. Nanotechnol. Biol. Med. 2009, 5, 424–431. [Google Scholar] [CrossRef]
- Xia, D.; Lanxia, L.; Dunwan, Z.; Hailing, Z.; Li, Y.; Xigang, L. Effects of Carboxylated Multiwalled Carbon Nanotubes on the Function of Macrophages. J. Nanomater. 2015, 2015, 4. [Google Scholar]
- Zafar, M. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Asghar, M.; Din, S.U.; Zafar, M.S. Thermoset polymethacrylate-based materials for dental applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Patel, V.; Joshi, U.; Joshi, A.; Oza, A.D.; Prakash, C.; Linul, E.; Campilho, R.D.S.G.; Kumar, S.; Saxena, K.K. Strength Evaluation of Functionalized MWCNT-Reinforced Polymer Nanocomposites Synthesized Using a 3D Mixing Approach. Materials 2022, 15, 7263. [Google Scholar] [CrossRef] [PubMed]
- Zahra, R.; Parvaneh, E.; Hossein, M.; Mehdi, K. Functionalization of carbon nanotubes by combination of controlled radical polymerization and “grafting to” method. Adv. Colloid Interface Sci. 2020, 278, 102–126. [Google Scholar]
- Singh, R.; Kumar, S. Cancer Targeting and Diagnosis: Recent Trends with Carbon Nanotubes. Nanomaterials 2022, 12, 2283. [Google Scholar] [CrossRef] [PubMed]
- Cob, A.; Oliva, A.; Avil, F.; Oliva, A. Influence of concentration, length and orientation of multiwall carbon nanotubes on the electromechanical response of polymer nanocomposites. Mater. Res. Express 2019, 6, 115024. [Google Scholar] [CrossRef]
- Patel, V.; Joshi, U.; Joshi, A. Investigating the Effect of Hydroxyl Functionalized MWCNT on the Mechanical Properties of PMMA-Based Polymer Nanocomposites. Curr. Nanomater. 2022, 8, 162–174. [Google Scholar] [CrossRef]







| PMMA Properties | |
| Properties | Description |
| Color | White |
| Particle Size | 48 μm |
| Tensile Strength | 45 MPa |
| Young Modulus | 2855 MPa |
| Density | 1.18 g/cm3 |
| MWCNT Properties | |
| Color | Black Powder |
| Diameter | 10–20 nm |
| Length | 3–8 μm |
| Purity | >98 wt% (MWCNT) |
| OH Content % | 2–4 wt% |
| Surface Area | 90–350 m2/g |
| Density | 2.6 g/cm3 |
| Young Modulus | 1.1 TPa |
| Tensile Test Results | |
| CNT %wt of MWCNT | Tensile Strength (N/mm2) |
| 0.10 wt% non-functionalized MWCNT | 43.67 |
| 0.1 wt% OH functionalized MWCNT | 57.42 |
| 0.5 wt% OH functionalized MWCNT | 66.75 |
| 1 wt% OH functionalized MWCNT | 61.58 |
| Flexural Test Results | |
| CNT %wt | Flexural Strength (N/mm2) |
| 0.10% non functionalized MWCNT/PMMA | 99.85 |
| 0.10% OH-functionalized MWCNT/PMMA | 121.12 |
| 0.50% OH-functionalized MWCNT/PMMA | 156.97 |
| 1.00% OH-functionalized MWCNT/PMMA | 136.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, V.; Joshi, U.; Joshi, A.; Upadhyay, T.K.; Al-Keridis, L.A.; Saeed, M. Cytotoxicity Analysis for the Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites in Oral Squamous Carcinoma (KB) Cells. Polymers 2023, 15, 1192. https://doi.org/10.3390/polym15051192
Patel V, Joshi U, Joshi A, Upadhyay TK, Al-Keridis LA, Saeed M. Cytotoxicity Analysis for the Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites in Oral Squamous Carcinoma (KB) Cells. Polymers. 2023; 15(5):1192. https://doi.org/10.3390/polym15051192
Chicago/Turabian StylePatel, Vijay, Unnati Joshi, Anand Joshi, Tarun Kumar Upadhyay, Lamya Ahmed Al-Keridis, and Mohd Saeed. 2023. "Cytotoxicity Analysis for the Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites in Oral Squamous Carcinoma (KB) Cells" Polymers 15, no. 5: 1192. https://doi.org/10.3390/polym15051192
APA StylePatel, V., Joshi, U., Joshi, A., Upadhyay, T. K., Al-Keridis, L. A., & Saeed, M. (2023). Cytotoxicity Analysis for the Hydroxyl Functionalized MWCNT Reinforced PMMA Nanocomposites in Oral Squamous Carcinoma (KB) Cells. Polymers, 15(5), 1192. https://doi.org/10.3390/polym15051192










