Intelligent Eucommia ulmoides Rubber/Ionomer Blends with Thermally Activated Shape Memory and Self-Healing Properties
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of the Blends
2.3. Curing Characteristics
2.4. Mechanical Characterization
2.5. Differential Scanning Calorimetry (DSC)
2.6. Shape Memory Effect
2.7. Self-Healing Effect
3. Results and Discussion
3.1. Curing and Mechanical Properties
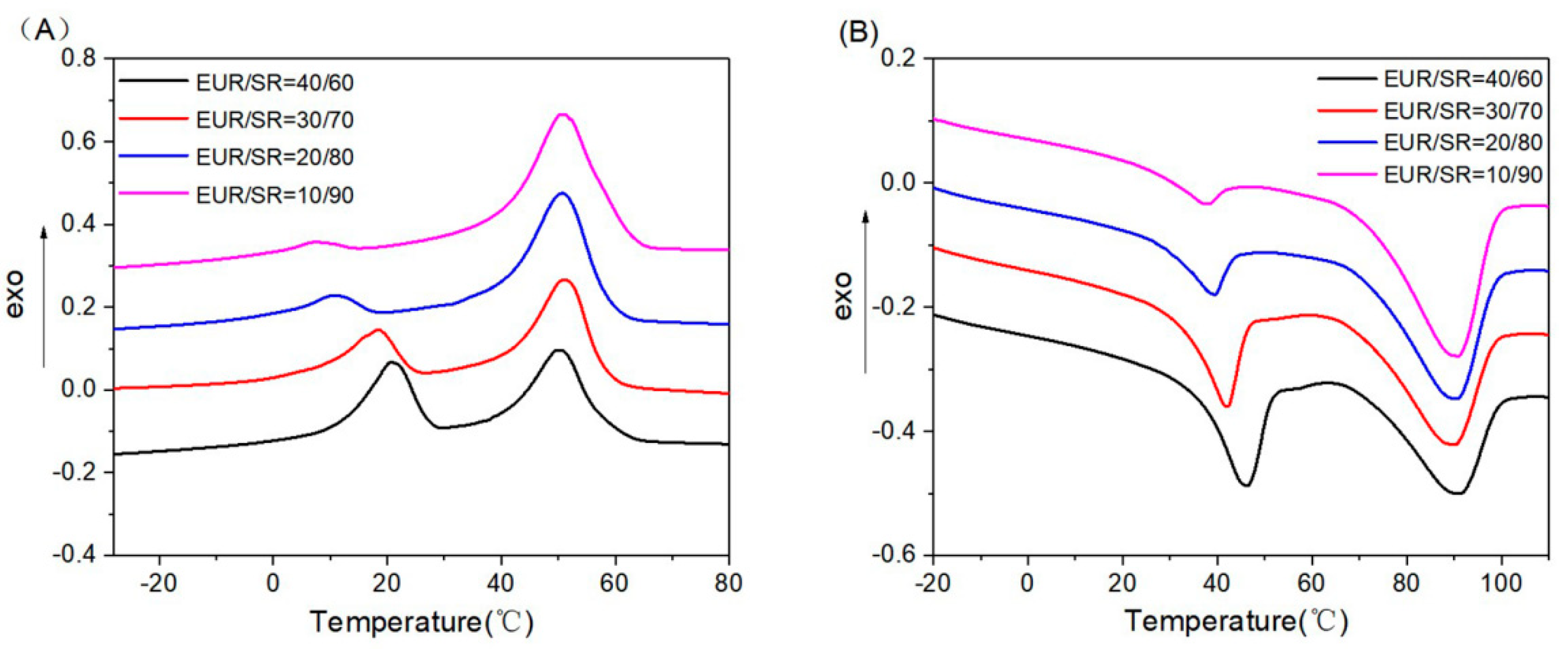
3.2. DSC Analysis
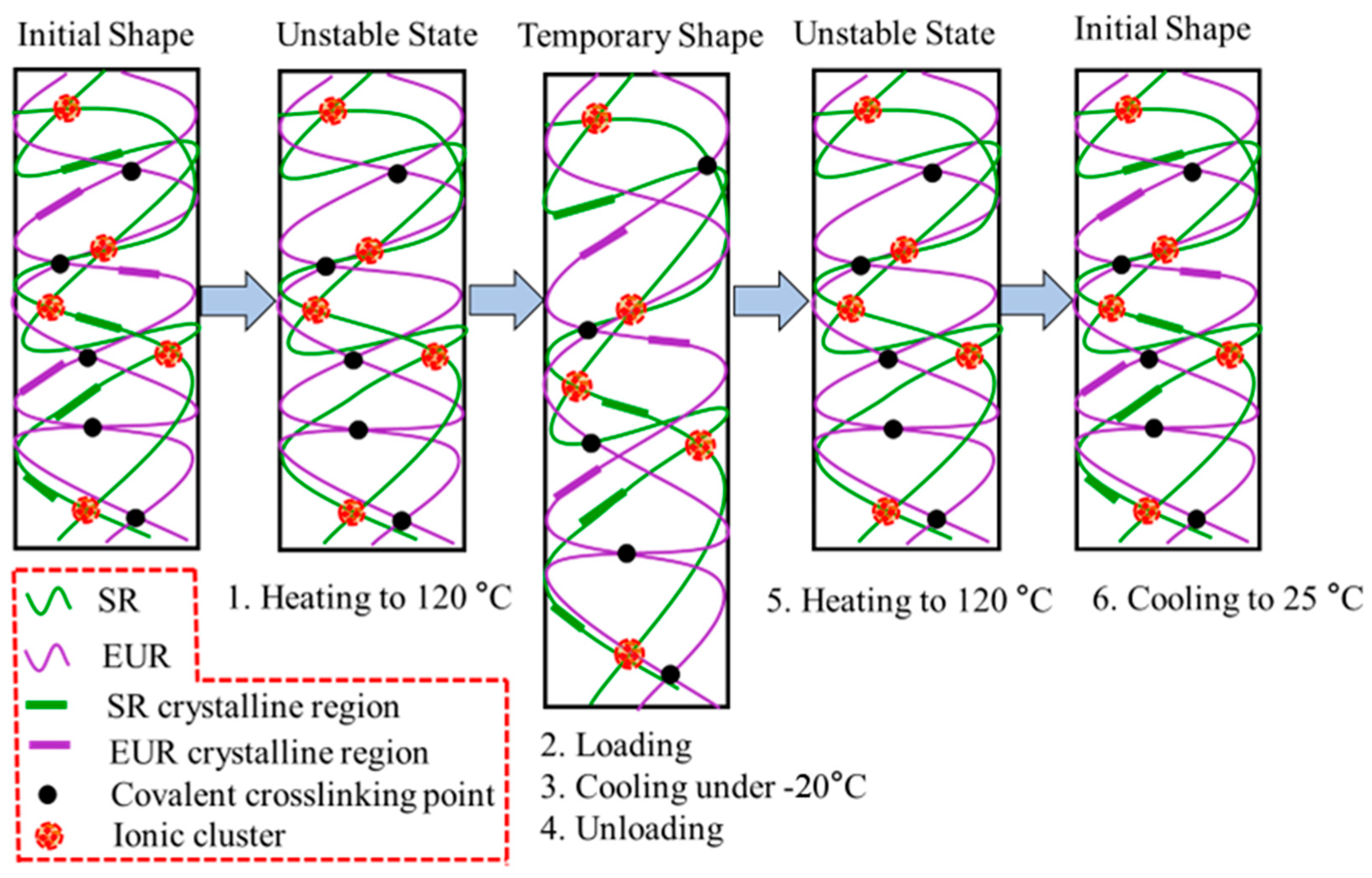
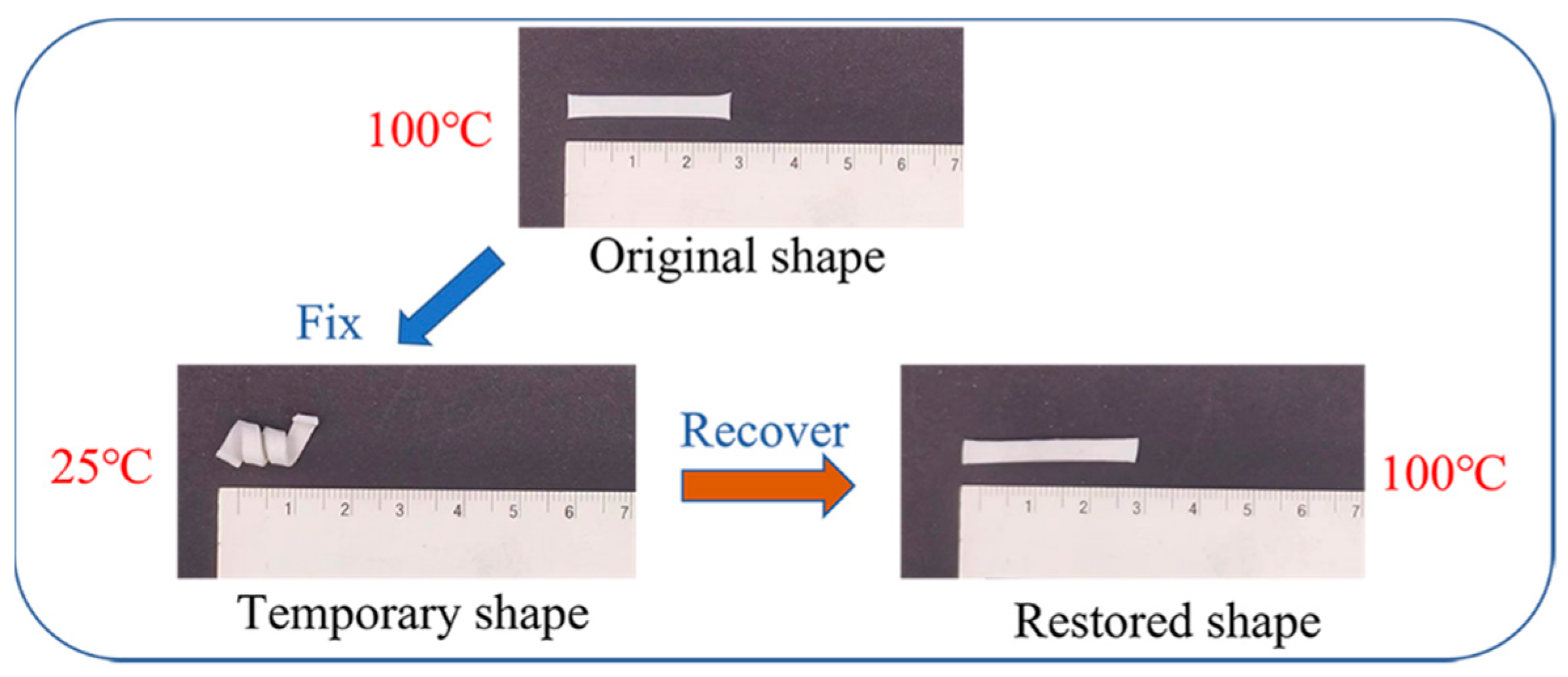
3.3. Shape Memory Effect Analysis
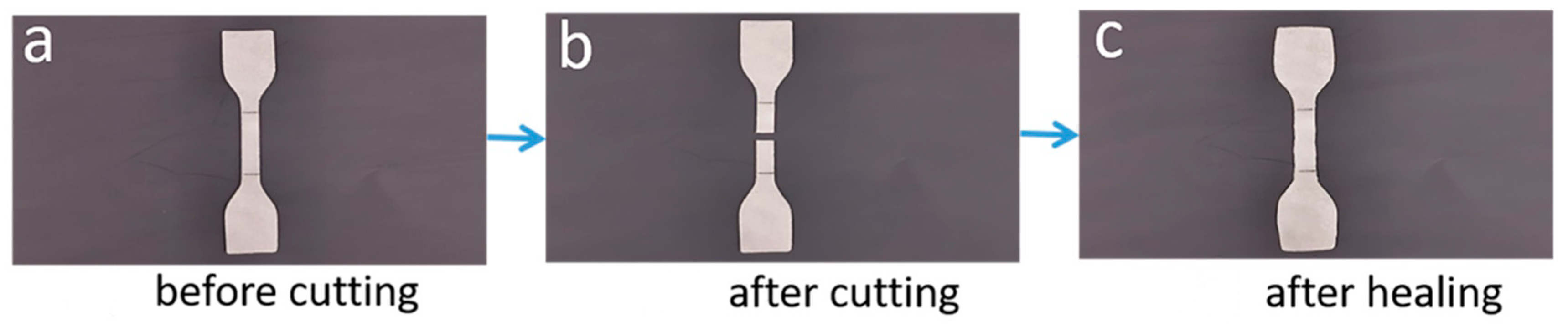
3.4. Self-Healing Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mather, P.T.; Luo, X.; Rousseau, I.A. Shape memory polymer research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Su, M.; Song, Y. Printable smart materials and devices: Strategies and applications. Chem. Rev. 2021, 122, 5144–5164. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, F.; Zhu, X.; Yong, K.; Gu, Z. Stimuli-responsive functional materials for soft robotics. J. Mater. Chem. B 2020, 8, 8972–8991. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, Y.; Rong, H.; Wang, C. SEBS-based composite phase change material with thermal shape memory for thermal management applications. Energy 2021, 221, 119900. [Google Scholar] [CrossRef]
- Zhang, Z.; Qi, X.; Li, S.; Yang, J.; Zhang, N.; Huang, T.; Wang, Y. Water-actuated shape-memory and mechanically-adaptive poly(ethylene vinyl acetate) achieved by adding hydrophilic poly (vinyl alcohol). Eur. Polym. J. 2018, 8, 237–245. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, J.; Ju, J.; Chen, X. Novel near-infrared light-induced triple-shape memory composite based on poly (ethylene-co-vinyl alcohol) and Iron Tannate. ACS Appl. Mater. Inter. 2021, 13, 23011–23019. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Q.; Yang, S.; Wang, T.; Sun, W.; Tong, Z. Unique self-reinforcing and rapid self-healing polyampholyte hydrogels with a pH-induced shape memory effect. Macromolecules 2021, 54, 5218–5228. [Google Scholar] [CrossRef]
- Russo, C.; Ramírez, J.; Fernández-Francos, X.; Flor, S. Electro-responsive shape-memory composites obtained via dual-curing processing. Polym. Adv. Technol. 2022, 33, 1715–1726. [Google Scholar] [CrossRef]
- Schmidt, A.M. Electromagnetic activation of shape memory polymer networks containing magnetic nanoparticles. Macromol. Rapid Comm. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Arun, D.; Kumar, K.S.; Kumar, B.S.; Chakravarthy, P.; Dona, M.; Santhosh, B. High glass-transition polyurethane-carbon black electro-active shape memory nanocomposite for aerospace systems. Mater. Sci. Technol. 2019, 35, 596–605. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Liu, Y.; Gong, T.; Wang, L.; Zhou, S. Highly pH-sensitive polyurethane exhibiting shape memory and drug release. Polym. Chem. 2014, 5, 5168–5174. [Google Scholar] [CrossRef]
- Lendlein, A. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, G.; Singh, S.; Jhaa, K.; Prakash, C. 3D printed biodegradable functional temperature-stimuli shape memory polymer for customized scaffoldings. J. Mech. Behav. Biomed. Mater. 2020, 108, 103781. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Lee, J.; Kim, D.; Kim, T.; Alexander, G.; Shin, Y.; Park, J.; Baek, S.; Yoon, J.; Lee, Y.; et al. Development of a shape-memory tube to prevent vascular stenosis. Adv. Mater. 2019, 31, 1904476. [Google Scholar] [CrossRef]
- Keneth, E.; Scalet, G.; Layani, M.; Tibi, G.; Degani, A.; Auricchio, F.; Magdassi, S. Pre-programmed tri-layer electro-thermal actuators composed of shape memory polymer and carbon nanotubes. Soft Robot. 2020, 7, 123–129. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-write fabrication of 4D active shape-changing behavior based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Inter. 2016, 9, 876–883. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.; Luo, J.; Liu, R.; Sun, G.; Liu, X. Preparation of dual-chamber microcapsule by Pickering emulsion for self-healing application with ultra-high healing efficiency. J. Colloid Interface Sci. 2021, 600, 660–669. [Google Scholar] [CrossRef]
- Pittala, R.; Dhanaraju, G.; Ben, B.; Ben, B. Self-healing of matrix cracking and delamination damage assessment in microcapsules reinforced carbon fibre epoxy composite under flexural loading. Compos. Struct. 2022, 291, 115691. [Google Scholar] [CrossRef]
- Mohammadi, M.; Eslami-Farsani, R.; Ebrahimnezhad-Khaljiri, H. Experimental investigation of the healing properties of the microvascular channels-based self-healing glass fibers/epoxy composites containing the three-part healant. Polym. Test. 2020, 91, 106862. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Chang, Z.; Wei, W.; Wu, G.; Wu, R.; Li, J. Recent achievements in self-healing materials based on ionic liquids: A review. J. Mater. Sci. 2020, 55, 13543–13558. [Google Scholar] [CrossRef]
- Stein, S.; Mordvinkin, A.; Voit, B.; Komber, H.; Saalwächter, K.; Böhme, F. Self-healing and reprocessable bromo butylrubber based on combined ionic cluster formation and hydrogen bonding. Polym. Chem. 2020, 11, 1188–1197. [Google Scholar] [CrossRef]
- Oehlenschlaeger, K.; Mueller, J.; Brandt, J.; Hilf, S.; Lederer, A.; Wilhelm, M.; Graf, R.; Coote, M.; Schmidt, F.; Barner-Kowollik, C. Adaptable hetero Diels–Alder networks for fast self-healing under mild conditions. Adv. Mater. 2014, 26, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Li, L.; Torkelson, J. Recyclable crosslinked polymer networks via one-step controlled radical polymerization. Adv. Mater. 2016, 28, 6746–6750. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Grande, A.; Dierkes, W.; Bijleveld, J.; Zwaag, S.; García, S. Turning vulcanized natural rubber into a self-healing polymer: Effect of the disulfide/polysulfide ratio. ACS Sustain. Chem. Eng. 2016, 4, 5776–5784. [Google Scholar] [CrossRef]
- Chino, K.; Ashiura, M. Themoreversible cross-linking rubber using supramolecular hydrogen-bonding networks. Macromolecules 2001, 34, 9201–9204. [Google Scholar] [CrossRef]
- Li, Z.; Shan, Y.; Wang, X.; Li, H.; Yang, K.; Cui, Y. Self-healing flexible sensor based on metal-ligand coordination. Chem. Eng. J. 2020, 394, 124932. [Google Scholar] [CrossRef]
- Burattini, S.; Colquhoun, H.; Fox, J.; Friedmann, D.; Greenland, B.; Harris, P.; Hayes, W.; Mackay, M.; Rowan, S. A self-repairing, supramolecular polymer system: Healability as a consequence of donor-acceptor π-π stacking interactions. Chem. Commun. 2009, 44, 6717–6719. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Wool, R.P. Self-healing materials: A review. Soft Matter. 2008, 4, 400–418. [Google Scholar] [CrossRef]
- Rahman, M.; Sartore, L.; Bignotti, F.; Landro, L. Autonomic self-healing in epoxidized natural rubber. ACS Appl. Mater. Inter. 2013, 5, 1494–1502. [Google Scholar] [CrossRef]
- Das, A.; Sallat, A.; Böhme, F.; Suckow, M.; Basu, D.; Wießner, S.; Stöckelhuber, K.; Voit, B.; Heinrich, G. Ionic modification turns commercial rubber into a self-healing material. ACS Appl. Mater. Inter. 2015, 7, 20623–20630. [Google Scholar] [CrossRef] [PubMed]
- Utrera-Barrios, S.; Araujo-Morera, J.; Reyes, L.; Manzanares, R.; Verdejo, R.; López-Manchado, M.; Santana, M. An effective and sustainable approach for achieving self-healing in nitrile rubber. Eur. Polym. J. 2020, 139, 110032. [Google Scholar] [CrossRef]
- Xia, L.; Wu, H.; Qiu, G. Shape memory behavior of carbon nanotube-reinforced trans-1,4-polyisoprene and low density polyethylene composites. Polym. Advan. Technol. 2019, 31, 107–113. [Google Scholar] [CrossRef]
- Xia, L.; Xian, J.; Geng, J.; Wang, Y.; Shi, F.; Zhou, Z.; Lu, N.; Du, A.; Xin, Z. Multiple shape memory effects of trans-1,4-polyisoprene and low-density polyethylene blends. Polym. Int. 2017, 66, 1382–1388. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Zhang, L.; Yue, D. Bio-based, robust, shape memory, self-healing and recyclable elastomers based on a semi-interpenetrating dynamic network. J. Mater. Chem. 2021, 45, 25399–25407. [Google Scholar] [CrossRef]
- Kalista, S.J.; Ward, T. Thermal characteristics of the self-healing response in poly(ethylene-co-methacrylic acid) copolymers. J. R. Soc. Interface 2007, 4, 405–411. [Google Scholar] [CrossRef]
- Kalista, S.J.; Ward, T.; Oyetunji, Z. Self-healing of poly(ethylene-co-methacrylic acid) copolymers following projectile puncture. Mech. Adv. Mater. Struc. 2007, 14, 391–397. [Google Scholar] [CrossRef]
- Rahman, M.; Penco, M.; Peroni, I.; Ramorino, G.; Janszen, G.; Landro, L. Autonomous healing materials based on epoxidized natural rubber and ethylene methacrylic acid ionomers. Smart Mater. Struct. 2012, 21, 035014. [Google Scholar] [CrossRef]
- Zhan, S.; Wang, X.; Sun, J. Rediscovering Surlyn: A supramolecular thermoset capable of healing and recycling. Macromol. Rapid Comm. 2020, 41, 2000097. [Google Scholar] [CrossRef]
- Dolog, R.; Weiss, R. Shape memory behavior of a polyethylene-based carboxylate ionomer. Macromolecules 2013, 46, 7845–7852. [Google Scholar] [CrossRef]
- Lu, L.; Li, G. One-way multishape-memory effect and tunable two-way shape memory effect of ionomer poly(ethylene-co-methacrylic acid). ACS Appl. Mater. Inter. 2016, 8, 14812–14823. [Google Scholar] [CrossRef] [PubMed]
- García-Huete, N.; Post, W.; Laza, J.; Vilas, J.; León, L.; García, S. Effect of the blend ratio on the shape memory and self-healing behaviour of ionomer-polycyclooctene crosslinked polymer blends. Eur. Polym. J. 2018, 98, 154–161. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, J.; Ma, Y.; Xia, L. Thermally assisted self-healing and shape memory behaviour of natural rubber based composites. Express Polym. Lett. 2021, 15, 929–939. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Meng, J.; Liu, X.; Xia, L. Thermal-triggered Trans-1, 4-polyisoprene/polyethylene wax shape memory and self-healing composites. Polym. Test. 2022, 111, 107601. [Google Scholar] [CrossRef]
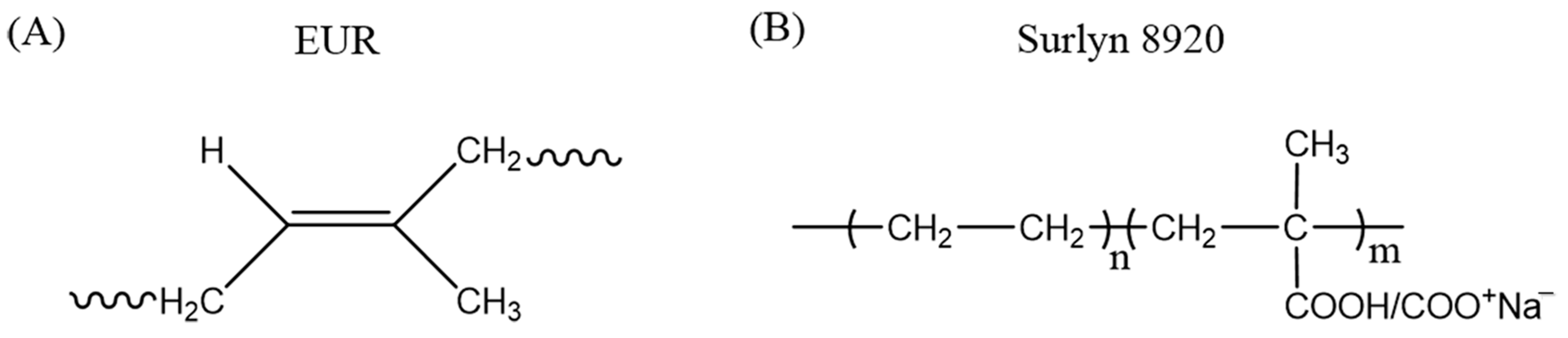



| Formulation | A | B | C | D |
|---|---|---|---|---|
| EUR | 40 | 30 | 20 | 10 |
| Surlyn resin | 60 | 70 | 80 | 90 |
| ENR | 3 | 3 | 3 | 3 |
| DCP | 1 | 1 | 1 | 1 |
| Antioxidant MB | 1.5 | 1.5 | 1.5 | 1.5 |
| Properties | Blends with Different Ratios of EUR/SR | |||
|---|---|---|---|---|
| A | B | C | D | |
| T10 (min) | 1.85 | 2.07 | 2.36 | 2.80 |
| T90 (min) | 21.85 | 24.76 | 28.18 | 30.50 |
| ML (dN·m) | 0.6 | 0.5 | 0.5 | 0.4 |
| MH (dN·m) | 2.5 | 2.2 | 1.8 | 1.5 |
| MH-ML (dN·m) | 1.9 | 1.7 | 1.3 | 1.1 |
| Properties | Blends with Different Ratios of EUR/SR | |||
|---|---|---|---|---|
| A | B | C | D | |
| Tensile strength (MPa) | 13.82 (±0.50) | 14.46 (±0.35) | 14.68 (±0.42) | 16.59 (±0.41) |
| 100% modulus (MPa) | 12.22 (±0.50) | 12.78 (±0.35) | 13.20 (±0.41) | 14.46 (±41) |
| Elongation at break (%) | 175 (±21) | 183 (±11) | 195 (±14) | 206 (±15) |
| Tear strength (KN·m−1) | 91.04 (±0.68) | 94.33 (±0.48) | 102.55 (±0.51) | 110.21 (±0.51) |
| Hardness (Shore A) | 92 | 93 | 93 | 94 |
| Properties | Blends with Different Ratios of EUR/SR | |||
|---|---|---|---|---|
| A | B | C | D | |
| Tm (EUR) (°C) | 45.36 | 41.69 | 38.56 | 36.54 |
| Tm (SR) (°C) | 88.74 | 89.55 | 89.69 | 90.53 |
| Xc (EUR) (%) | 21.54 | 20.09 | 18.90 | 17.51 |
| Xc (SR) (%) | 12.00 | 12.34 | 12.42 | 12.77 |
| Properties | Blends with Different Ratios of EUR/SR | |||
|---|---|---|---|---|
| A | B | C | D | |
| Rf (%) | 90.10 | 92.16 | 95.78 | 96.46 |
| Rr (%) | 75.78 | 78.87 | 78.97 | 82.47 |
| ε1,load − ε0 (%) | ~50 | ~55 | ~85 | ~120 |
| Properties | Blends with Different Ratios of EUR/SR | |||
|---|---|---|---|---|
| A | B | C | D | |
| Tensile strength (MPa) | 6.12 (±0.93) | 12.64 (±0.76) | 12.19 (±0.72) | 10.95 (±0.65) |
| Elongation at break (%) | 1.12 (±0.16) | 18.17 (±2.31) | 13.69 (±1.74) | 2.24 (±0.56) |
| Healing efficiency (%) | 44.28 | 87.41 | 83.04 | 63.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, Y.; Xiao, J.; Xia, L. Intelligent Eucommia ulmoides Rubber/Ionomer Blends with Thermally Activated Shape Memory and Self-Healing Properties. Polymers 2023, 15, 1182. https://doi.org/10.3390/polym15051182
Wang Q, Li Y, Xiao J, Xia L. Intelligent Eucommia ulmoides Rubber/Ionomer Blends with Thermally Activated Shape Memory and Self-Healing Properties. Polymers. 2023; 15(5):1182. https://doi.org/10.3390/polym15051182
Chicago/Turabian StyleWang, Qi, Yutao Li, Jianbin Xiao, and Lin Xia. 2023. "Intelligent Eucommia ulmoides Rubber/Ionomer Blends with Thermally Activated Shape Memory and Self-Healing Properties" Polymers 15, no. 5: 1182. https://doi.org/10.3390/polym15051182
APA StyleWang, Q., Li, Y., Xiao, J., & Xia, L. (2023). Intelligent Eucommia ulmoides Rubber/Ionomer Blends with Thermally Activated Shape Memory and Self-Healing Properties. Polymers, 15(5), 1182. https://doi.org/10.3390/polym15051182







