Solid State Polymerization of Biodegradable Poly(butylene sebacate-co-terephthalate): A Rapid, Facile Method for Property Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
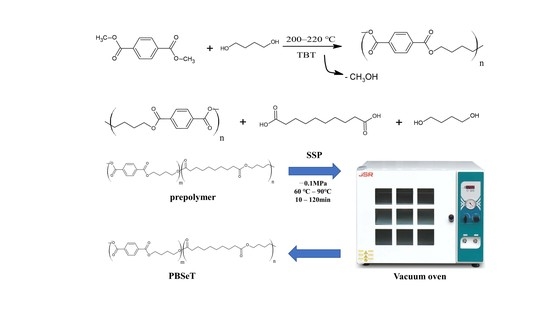
2.2. Synthesis of PBSeT
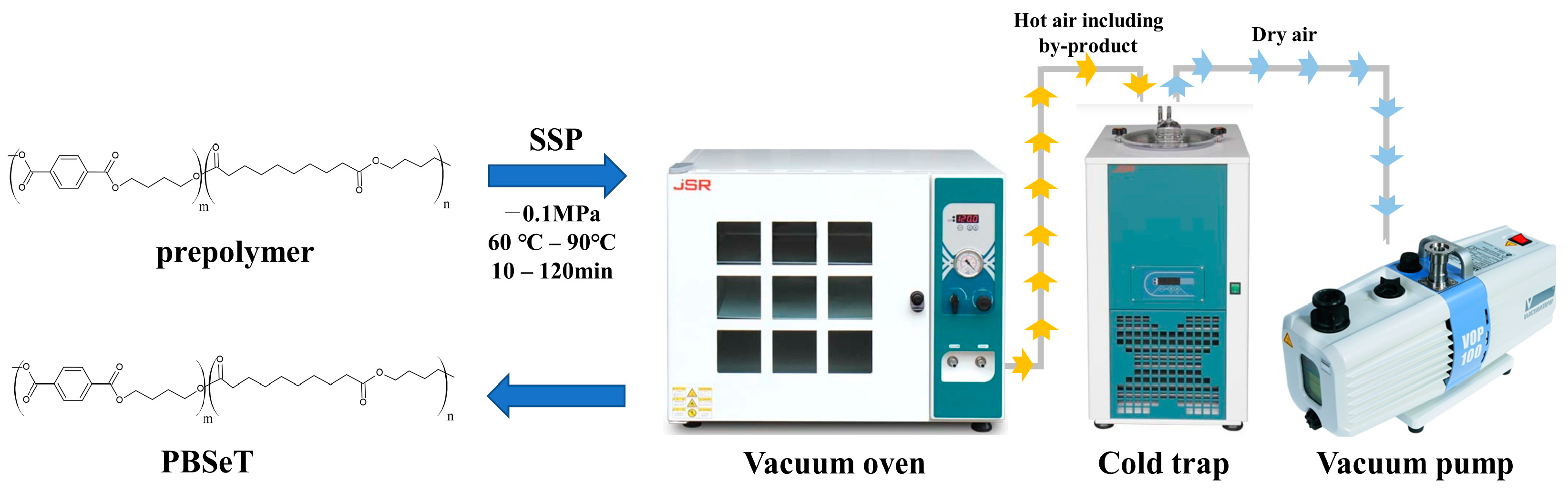
2.3. Solid State Polymerization (SSP)
2.4. Characterization
3. Results
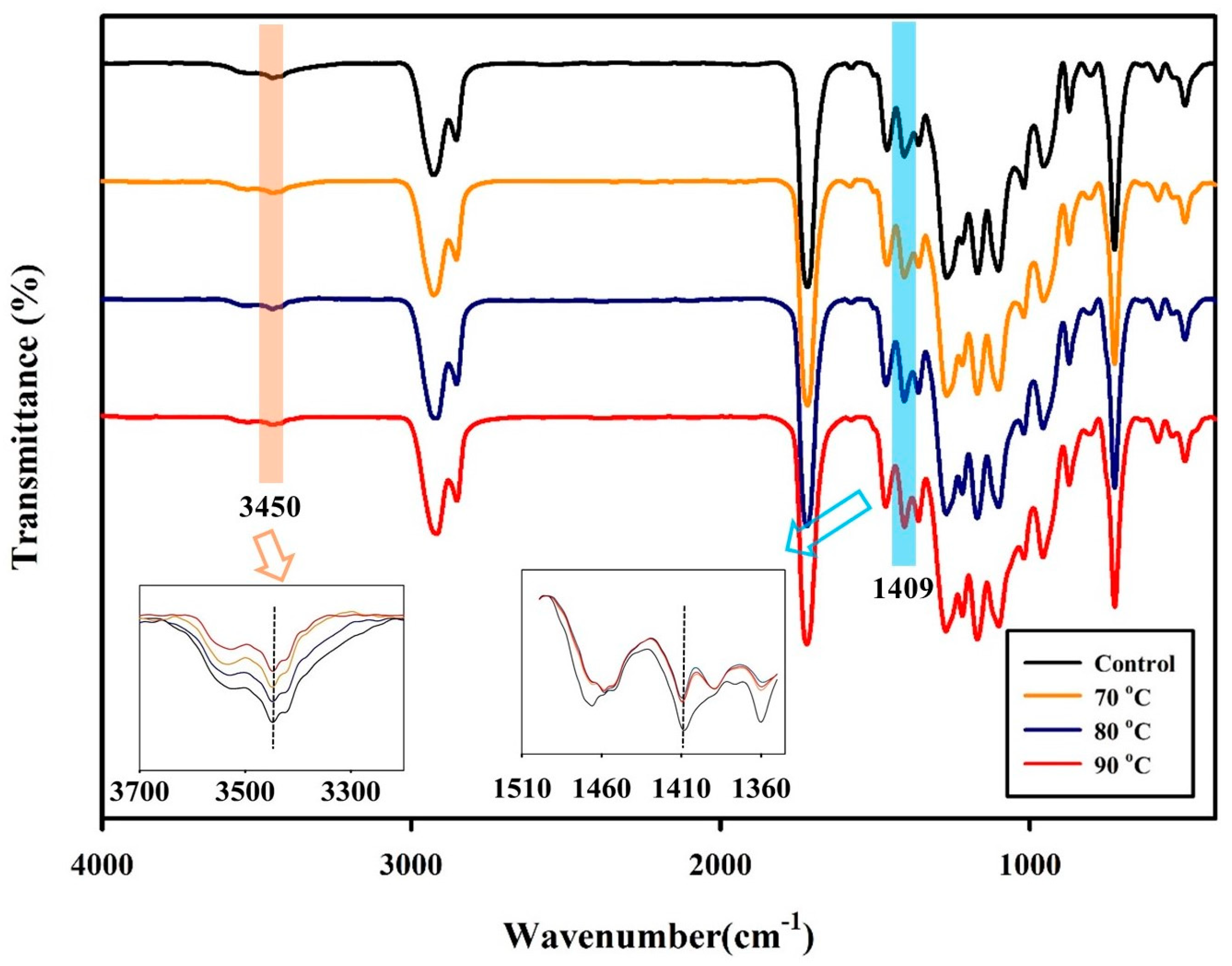
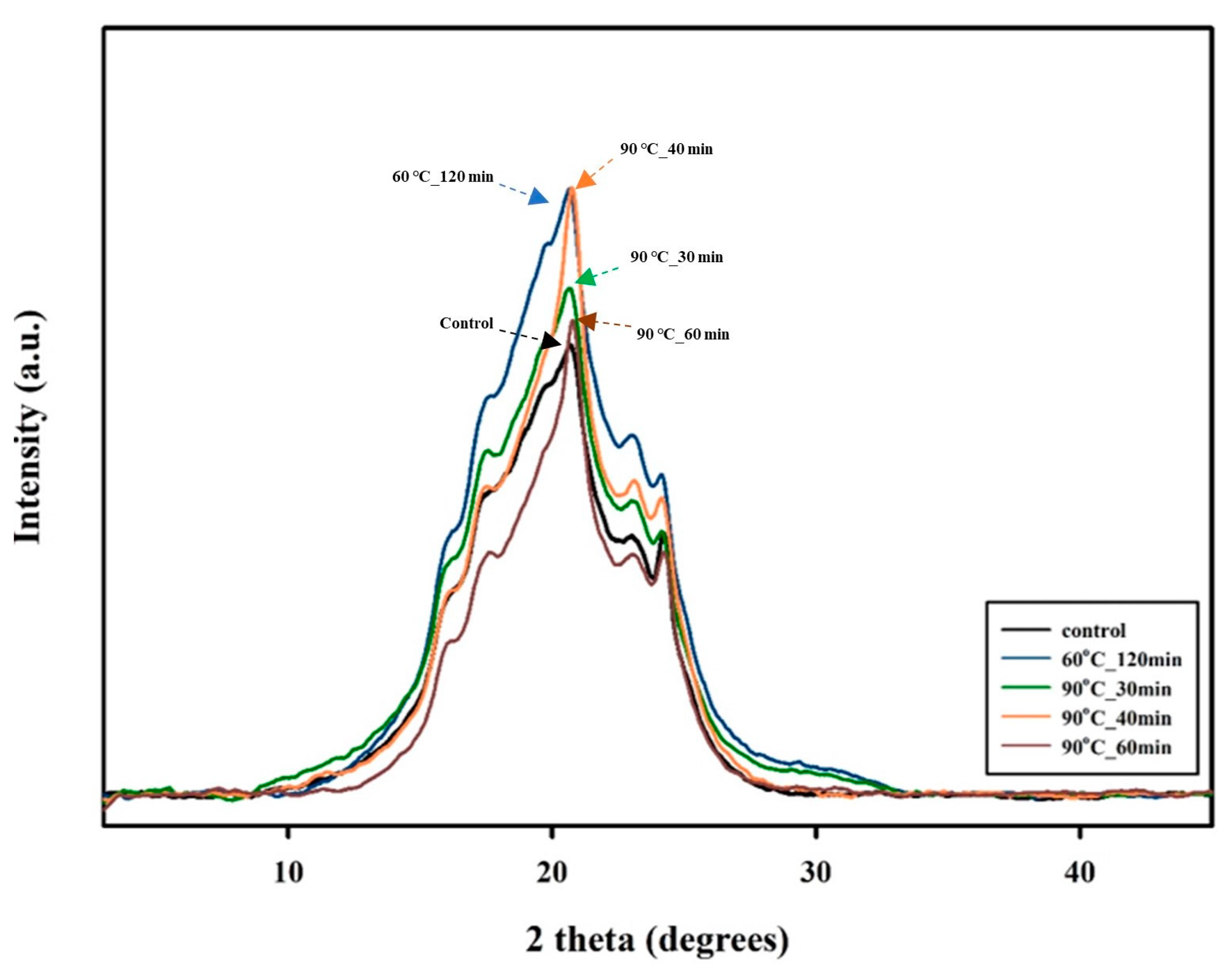
3.1. Effect of the SSP Process on PBSeT
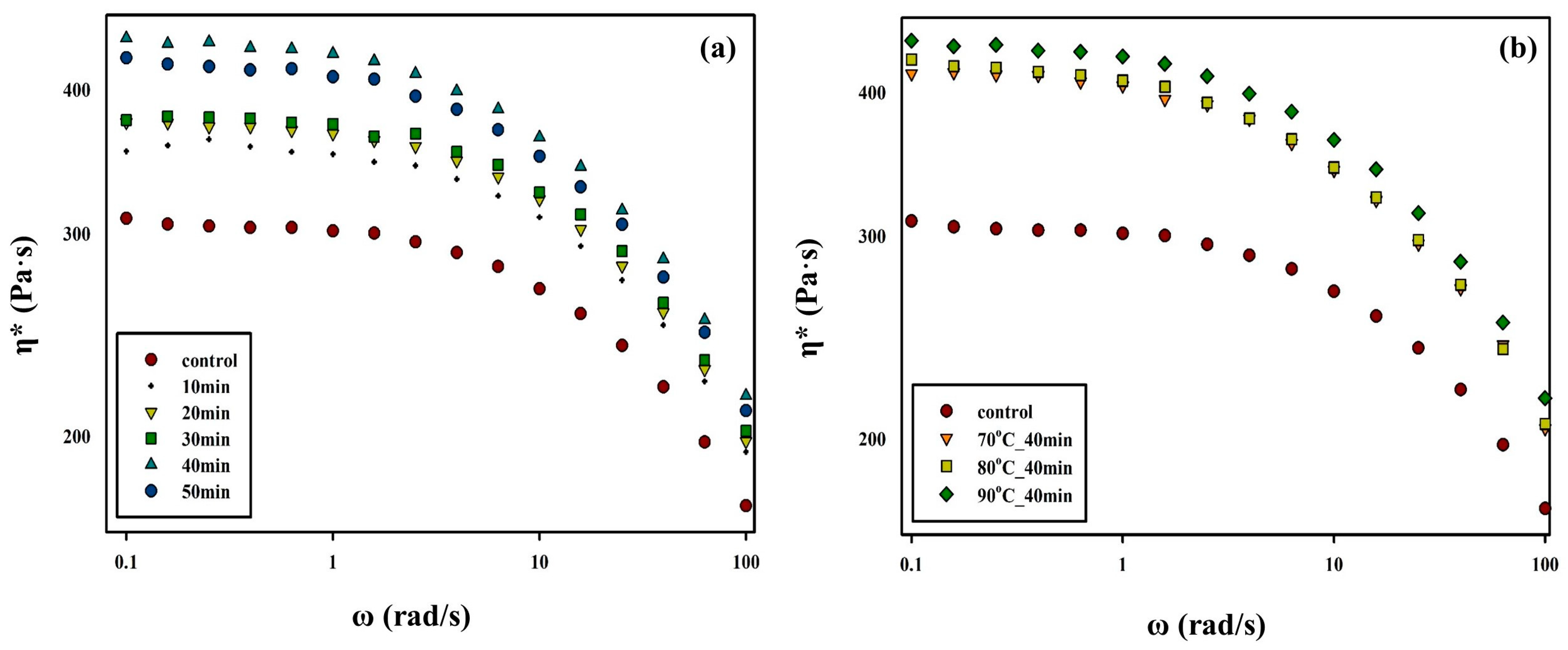
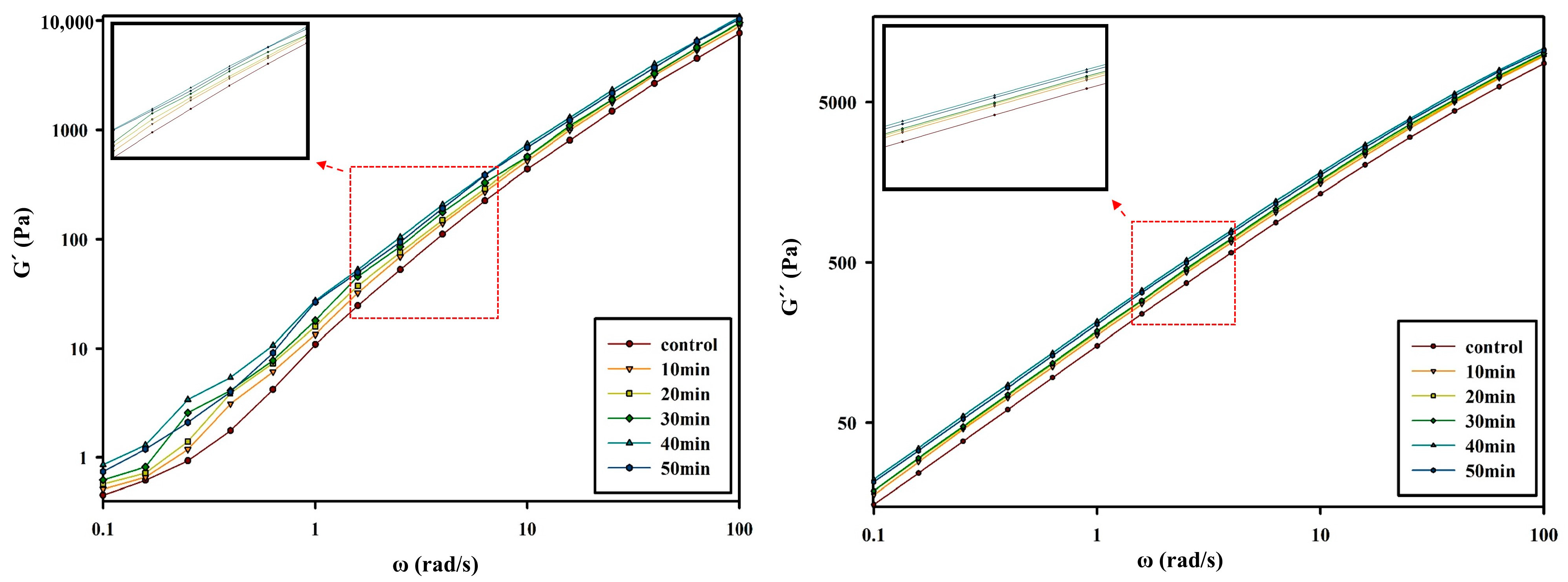
3.2. Changes in Rheological Properties of PBSeT after the SSP Process
3.3. Melting and Crystallization Behaviors of PBSeT after SSP Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esen, G.; AteÅŸ, M. Architectural Vantage Point to Bioplastics in the Circular Economy. J. Archit. Res. Dev. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Wang, S.; Daelemans, L.; D’Hooge, D.R.; Couck, L.; Broeck, W.V.D.; Cornillie, P.; Gou, M.; De Clerck, K.; Cardon, L. Lifting the quality of fused filament fabrication of polylactic acid based composites. Compos. Part B Eng. 2021, 210, 108613. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, R.; Cai, J.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and Thermal Properties of PHBV Produced from Levulinic Acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef]
- Tan, G.-Y.A.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Sharma, V.; Misra, S.; Srivastava, A.K. Developing a green and sustainable process for enhanced PHB production by Azohydromonas australica. Biocatal. Agric. Biotechnol. 2017, 10, 122–129. [Google Scholar] [CrossRef]
- Huang, F.; Wu, L.; Li, B.-G. Sulfonated biodegradable PBAT copolyesters with improved gas barrier properties and excellent water dispersibility: From synthesis to structure-property. Polym. Degrad. Stab. 2020, 182, 109391. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly (butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2012, 21, 108–114. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Characterization and degradation behavior of poly(butylene adipate-co-terephthalate)s. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Li, G.; Shankar, S.; Rhim, J.-W.; Oh, B.-Y. Effects of preparation method on properties of poly(butylene adipate-co-terephthalate) films. Food Sci. Biotechnol. 2015, 24, 1679–1685. [Google Scholar] [CrossRef]
- Castellan, A.; Bart, J.C.J.; Cavallaro, S. ChemInform Abstract: Industrial Production and Use of Adipic Acid. Cheminform 1991, 22, 237–254. [Google Scholar] [CrossRef]
- Qin, P.; Wu, L.; Li, B.; Li, N.; Pan, X.; Dai, J. Superior Gas Barrier Properties of Biodegradable PBST vs. PBAT Copolyesters: A Comparative Study. Polymers 2021, 13, 3449. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Rafizadeh, M.; Taromi, F.A.; del Valle, L.J.; Franco, L.; Puiggalí, J. Biodegradability and biocompatibility of copoly(butylene sebacate-co-terephthalate)s. Polym. Degrad. Stab. 2017, 135, 18–30. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Jiang, T.; Xi, S.; Li, Y.; Guo, J.; Huang, M.; Algadi, H.; Ye, X.; Jiang, Q. Improvement of thermodynamic properties of poly(butanediol sebacate-butanediol terephthalate) (PBSeT) composites based on the dispersion of PCaCO3@tannic acid formed by complexation of tannic acid and Ti. Adv. Compos. Hybrid Mater. 2022, 5, 2787–2800. [Google Scholar] [CrossRef]
- Nouruzi, N.; Dinari, M.; Gholipour, B.; Mokhtari, N.; Farajzadeh, M.; Rostamnia, S.; Shokouhimehr, M. Photocatalytic hydrogen generation using colloidal covalent organic polymers decorated bimetallic Au-Pd nanoalloy (COPs/Pd-Au). Mol. Catal. 2021, 518, 112058. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Narayanan, R.P.; George, S.J.; Maji, T.K. Luminescent Microporous Metal–Organic Framework with Functional Lewis Basic Sites on the Pore Surface: Specific Sensing and Removal of Metal Ions. Inorg. Chem. 2012, 51, 10089–10091. [Google Scholar] [CrossRef]
- Eshghi, F.; Mehrabadi, Z.; Farsadrooh, M.; Hayati, P.; Javadian, H.; Karimi, M.; Karimi-Maleh, H.; Rostamnia, S.; Karaman, C.; Aghababaei, F. Photocatalytic degradation of remdesivir nucleotide pro-drug using [Cu (1-methylimidazole) 4 (SCN) 2] nanocomplex synthesized by sonochemical process: Theoretical, hirshfeld surface analysis, degradation kinetic, and thermodynamic studies. Environ. Res. 2023, 22, 115321. [Google Scholar] [CrossRef]
- Jaisankar, V.; Nanthini, R.; Karunanidhi, M.; Ravi, A. Study on biodegradable random copolyesters derived from 1, 4-butane diol, terephthalic acid and adipic acid/sebacic acid. Asian J. Chem. 2010, 22, 5077. [Google Scholar]
- Kim, S.J.; Kwak, H.W.; Kwon, S.; Jang, H.; Park, S.-I. Synthesis, Characterization and Properties of Biodegradable Poly(Butylene Sebacate-Co-terephthalate). Polymers 2020, 12, 2389. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Dong, X.; Wang, W.; Zhu, Y.-C.; Murugadoss, V.; Song, G.; Naik, N.; Pan, D.; Guo, Z. Synthesis, characterization and properties of poly(butanediol sebacate–butanediol terephthalate) (PBSeT) copolyesters using glycerol as cross-linking agent. Mater. Today Commun. 2021, 28, 102557. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.; Jang, H.; Kim, S.J.; Park, S.I. Poly (ethylene oxide)(PEO) influence on mechanical, thermal, and degradation properties of PLA/PBSeT blends. J. Appl. Polym. Sci. 2023, 140, e53299. [Google Scholar] [CrossRef]
- Jang, H.; Kwon, S.; Kim, S.J.; Park, S.-I. Maleic Anhydride-Grafted PLA Preparation and Characteristics of Compatibilized PLA/PBSeT Blend Films. Int. J. Mol. Sci. 2022, 23, 7166. [Google Scholar] [CrossRef]
- Erber, M.; Boye, S.; Hartmann, T.; Voit, B.I.; Lederer, A. A convenient room temperature polycondensation toward hyperbranched AB2 -type all-aromatic polyesters with phenol terminal groups. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5158–5168. [Google Scholar] [CrossRef]
- Vouyiouka, S.; Theodoulou, P.; Symeonidou, A.; Papaspyrides, C.D.; Pfaendner, R. Solid state polymerization of poly(lactic acid): Some fundamental parameters. Polym. Degrad. Stab. 2013, 98, 2473–2481. [Google Scholar] [CrossRef]
- Li, H.; Shang, Y.; Huang, W.; Xue, B.; Zhang, X.; Cui, Z.; Fu, P.; Pang, X.; Zhao, Q.; Liu, M. Synthesis of succinic acid-based polyamide through direct solid-state polymerization method: Avoiding cyclization of succinic acid. J. Appl. Polym. Sci. 2021, 138, 51017. [Google Scholar] [CrossRef]
- Cruz, S.A.; Zanin, M. PET recycling: Evaluation of the solid state polymerization process. J. Appl. Polym. Sci. 2005, 99, 2117–2123. [Google Scholar] [CrossRef]
- Medellin-Rodriguez, F.J.; Lopez-Guillen, R.; Waldo-Mendoza, M.A. Solid-state polymerization and bulk crystallization behavior of poly (ethylene terephthalate)(PET). J. Appl. Polym. Sci. 2000, 75, 78–86. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Mhaisgawali, V.T. Post-extrusion solid-state polymerization of fully drawn polyester yarns. J. Appl. Polym. Sci. 2006, 102, 5113–5122. [Google Scholar] [CrossRef]
- Chronaki, K.; Korres, D.M.; Papaspyrides, C.D.; Vouyiouka, S. Poly(lactic acid) microcapsules: Tailoring properties via solid state polymerization. Polym. Degrad. Stab. 2020, 179, 109283. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Climent-Pascual, E.; de la Orden, M.U.; Urreaga, J.M. Effect of solid-state polymerization on the structure and properties of mechanically recycled poly(lactic acid). Polym. Degrad. Stab. 2020, 171, 109045. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, H.W.; Kwon, S.; Jang, H.; Park, S.-I. Characterization of PLA/PBSeT Blends Prepared with Various Hexamethylene Diisocyanate Contents. Materials 2021, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, L.; Xanthopoulou, E.; Nikolaidis, G.; Zamboulis, A.; Achilias, D.; Papageorgiou, G.; Bikiaris, D. Towards High Molecular Weight Furan-Based Polyesters: Solid State Polymerization Study of Bio-Based Poly(Propylene Furanoate) and Poly(Butylene Furanoate). Materials 2020, 13, 4880. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, C.; Porfyris, A.; Korres, D.; Vouyiouka, S. Solid-State Polymerization as a Vitrimerization Tool Starting from Available Thermoplastics: The Effect of Reaction Temperature. Materials 2020, 14, 9. [Google Scholar] [CrossRef]
- Xi, Z.; Liu, T.; Si, W.; Bi, F.; Xu, Z.; Zhao, L. High-efficiency acetaldehyde removal during solid-state polycondensation of poly(ethylene terephthalate) assisted by supercritical carbon dioxide. Chin. J. Chem. Eng. 2018, 26, 1285–1291. [Google Scholar] [CrossRef]
- Kim, J.; Roberts, G.W.; Kiserow, D.J. Effect of prepolymer molecular weight on solid state polymerization of poly(bisphenol a carbonate) with nitrogen as a sweep fluid. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4959–4969. [Google Scholar] [CrossRef]
- Gross, S.M.; Roberts, G.W.; Kiserow, D.J.; DeSimone, J.M. Synthesis of High Molecular Weight Polycarbonate by Solid-State Polymerization. Macromolecules 2001, 34, 3916–3920. [Google Scholar] [CrossRef]
- Dawin, T.P.; Ahmadi, Z.; Taromi, F.A. Biocompatible PLA/PHB coatings obtained from controlled solid state polymerization. Prog. Org. Coatings 2019, 132, 41–49. [Google Scholar] [CrossRef]
- Sadiku-Agboola, O.; Sadiku, E.R.; Adegbola, A.T.; Biotidara, O.F. Rheological Properties of Polymers: Structure and Morphology of Molten Polymer Blends. Mater. Sci. Appl. 2011, 2, 30–41. [Google Scholar] [CrossRef]
- Devotta, I.; Mashelkar, R. Modelling of polyethylene terephthalate reactors—X. A comprehensive model for solid-state polycondensation process. Chem. Eng. Sci. 1993, 48, 1859–1867. [Google Scholar] [CrossRef]
- Li, L.-J.; Duan, R.-T.; Zhang, J.-B.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. Phosphorus-Containing Poly(ethylene terephthalate): Solid-State Polymerization and Its Sequential Distribution. Ind. Eng. Chem. Res. 2013, 52, 5326–5333. [Google Scholar] [CrossRef]
- van der Wal, A.; Mulder, J.; Gaymans, R. Fracture of polypropylene: The effect of crystallinity. Polymer 1998, 39, 5477–5481. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Ye, X.; Li, Z.; Wang, W.; Liu, T.; El Azab, I.H.; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, Z.M.; et al. Synthesis and characterization of 2,5-furandicarboxylic acid poly(butanediol sebacate-butanediol) terephthalate (PBSeT) segment copolyesters with excellent water vapor barrier and good mechanical properties. J. Mater. Sci. 2022, 57, 10997–11012. [Google Scholar] [CrossRef]
- Kuran, W.; Dębek, C.; Wielgosz, Z.; Kuczyńska, L.; Sobczak, M. Application of a solid-state postpolycondensation method for synthesis of high molecular weight polycarbonates. J. Appl. Polym. Sci. 2000, 77, 2165–2171. [Google Scholar] [CrossRef]
- Zimmerman, J. Equilibria in solid phase polyamidation. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 955–958. [Google Scholar] [CrossRef]
- Wach, R.A.; Wolszczak, P.; Adamus-Wlodarczyk, A. Enhancement of Mechanical Properties of FDM-PLA Parts via Thermal Annealing. Macromol. Mater. Eng. 2018, 303, 1800169. [Google Scholar] [CrossRef]
- Chen, J.; Deng, C.; Hong, R.; Fu, Q.; Zhang, J. Effect of thermal annealing on crystal structure and properties of PLLA/PCL blend. J. Polym. Res. 2020, 27, 1–11. [Google Scholar] [CrossRef]



| Temperature (°C) | SSP Time (min) | Tg (°C) | Tm1 a (°C) | Tm2 b (°C) | Hm1 c (J/g) | Hm2 d (J/g) | Xc e (%) | Tonset (°C) | Tmax (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | −41.7 | 31.6 | 93.4 | 9.5 | 8.4 | 10.4 | 378.5 | 399.2 |
| 70 | 10 | −41.8 | 31.1 | 91.1 | 9.1 | 9.2 | 10.8 | 377.1 | 405.2 |
| 20 | −42.5 | 31.3 | 95.3 | 9.6 | 9.0 | 10.9 | 375.6 | 401.9 | |
| 30 | −42.9 | 31.5 | 93.9 | 9.4 | 9.2 | 10.9 | 378.3 | 400.5 | |
| 40 | −43.5 | 31.2 | 93.6 | 9.7 | 9.1 | 11.0 | 380.1 | 399.8 | |
| 50 | −41.2 | 32.8 | 98.5 | 10 | 9.4 | 11.4 | 376.4 | 403.9 | |
| 80 | 10 | −43.0 | 31.8 | 93.9 | 9.1 | 8.8 | 10.8 | 379.4 | 400.3 |
| 20 | −42.8 | 31.6 | 94.7 | 9.2 | 9.2 | 11.0 | 380.6 | 399.6 | |
| 30 | −43.0 | 30.8 | 93.5 | 9.9 | 9.4 | 11.2 | 378.6 | 400.2 | |
| 40 | −43.5 | 32.3 | 95.5 | 10.9 | 9.2 | 11.6 | 380.3 | 398.9 | |
| 50 | −43.7 | 31.3 | 96.3 | 10.8 | 9.5 | 11.8 | 381.7 | 401.4 | |
| 90 | 10 | −43.9 | 31.5 | 95.0 | 8.9 | 9.7 | 11.0 | 375.1 | 403.4 |
| 20 | −42.5 | 31.5 | 93.9 | 9.9 | 9.5 | 11.3 | 378.2 | 401.0 | |
| 30 | −43.5 | 31.4 | 96.2 | 9.9 | 9.7 | 11.5 | 382.1 | 403.7 | |
| 40 | −44.0 | 31.2 | 96.3 | 10.7 | 9.9 | 12.0 | 381.0 | 404.3 | |
| 50 | −43.9 | 31.3 | 95.9 | 10.5 | 10.0 | 11.9 | 379.7 | 400.8 |
| Sample | 2 Theta (Degrees) | FWHM | D (Å) |
|---|---|---|---|
| Control | 20.0 | 7.3 | 11.8 |
| 60_120 | 20.5 | 7.9 | 10.2 |
| 90_30 | 20.0 | 6.8 | 11.0 |
| 90_40 | 20.7 | 7.5 | 10.7 |
| 90_60 | 20.8 | 7.1 | 11.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.; Park, S.-i. Solid State Polymerization of Biodegradable Poly(butylene sebacate-co-terephthalate): A Rapid, Facile Method for Property Enhancement. Polymers 2023, 15, 1133. https://doi.org/10.3390/polym15051133
Lim D, Park S-i. Solid State Polymerization of Biodegradable Poly(butylene sebacate-co-terephthalate): A Rapid, Facile Method for Property Enhancement. Polymers. 2023; 15(5):1133. https://doi.org/10.3390/polym15051133
Chicago/Turabian StyleLim, Daegyu, and Su-il Park. 2023. "Solid State Polymerization of Biodegradable Poly(butylene sebacate-co-terephthalate): A Rapid, Facile Method for Property Enhancement" Polymers 15, no. 5: 1133. https://doi.org/10.3390/polym15051133
APA StyleLim, D., & Park, S.-i. (2023). Solid State Polymerization of Biodegradable Poly(butylene sebacate-co-terephthalate): A Rapid, Facile Method for Property Enhancement. Polymers, 15(5), 1133. https://doi.org/10.3390/polym15051133