Polymer Membrane Modified with Photocatalytic and Plasmonic Nanoparticles for Self-Cleaning Filters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Porous Polymer Membrane Modified with TiO2 and Ag Nanoparticles
2.2.1. Formation and Porosification of Polymer Membrane
2.2.2. Fabrication of Titanium Dioxide Nanoparticles
2.2.3. Colloidal Synthesis of the Ag Nanoparticles
2.2.4. Combination of the Porous Polymer Membrane, TiO2 and Ag Nanoparticles
2.2.5. Bacteria Culturing and Suspension Preparation
2.3. Characteriazation of the Porous Polymer Membrane Modified with TiO2 and Ag Nanoparticles
2.3.1. Morphology Characterization
2.3.2. Study of Photocatalytic Activity of TiO2 Nanoparticles and Thermal Properties of Ag Nanoparticles
2.3.3. Study of the Membrane Permeability and Disinfecting Effectiveness
3. Results and Discussion
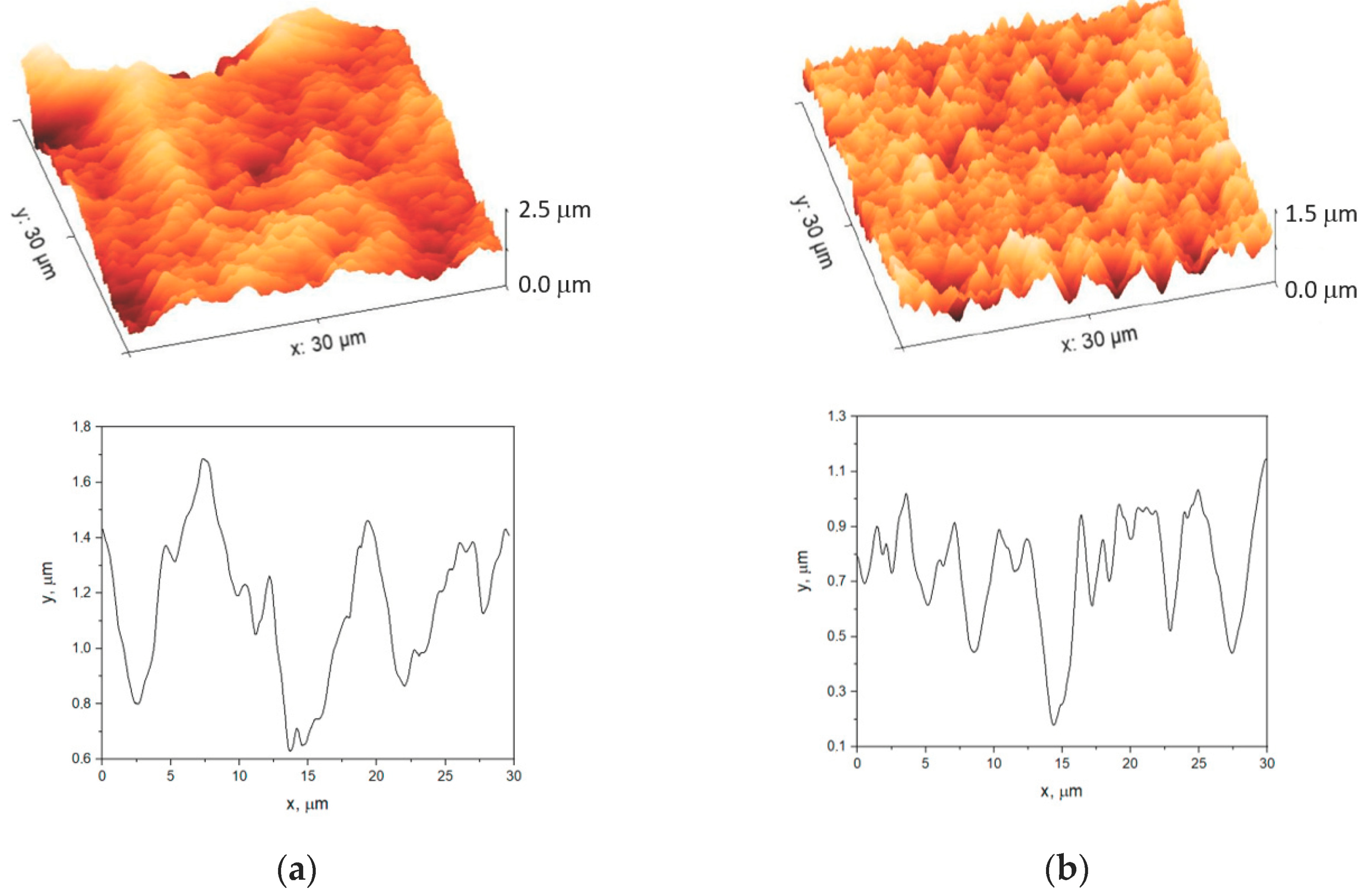
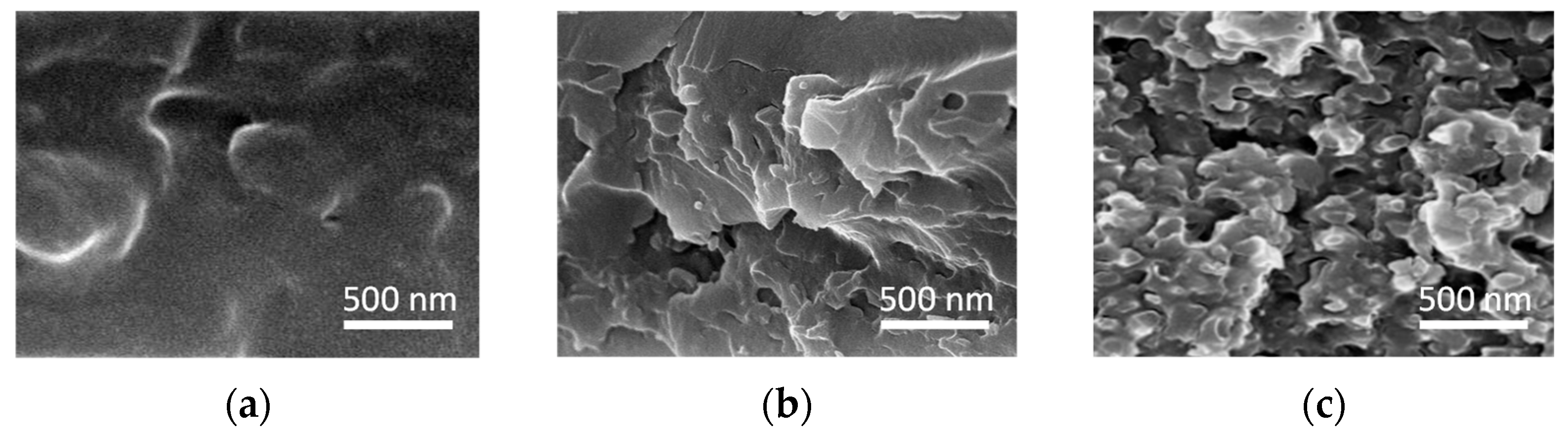
3.1. Morphology of the Polymer Membrane
3.2. Characterization of Photocatalytical and Plasmonic Nanoparticles
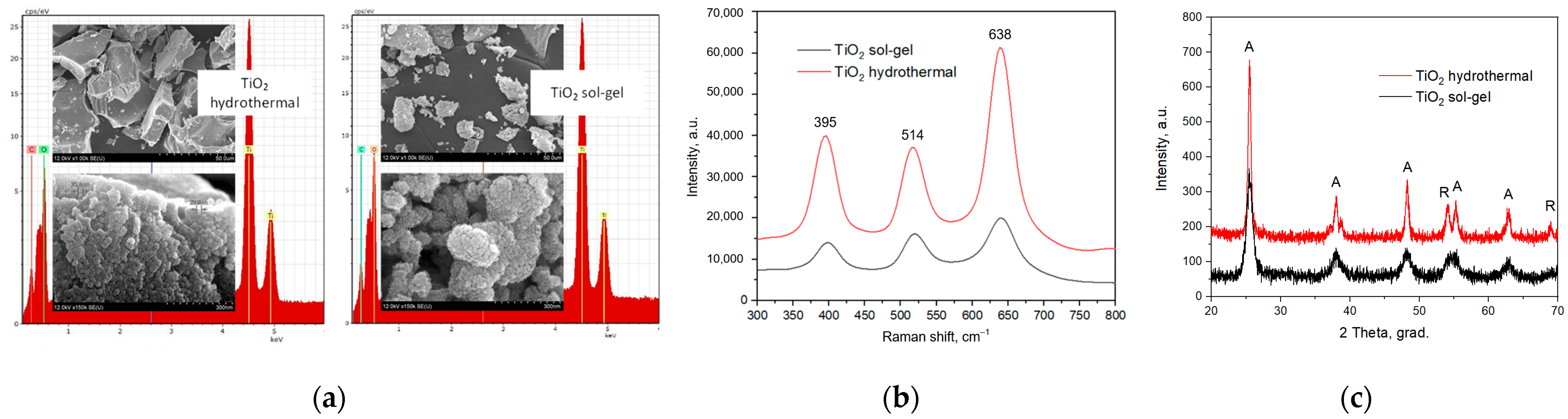
3.2.1. Morphology of Titanium Dioxide Nanoparticles
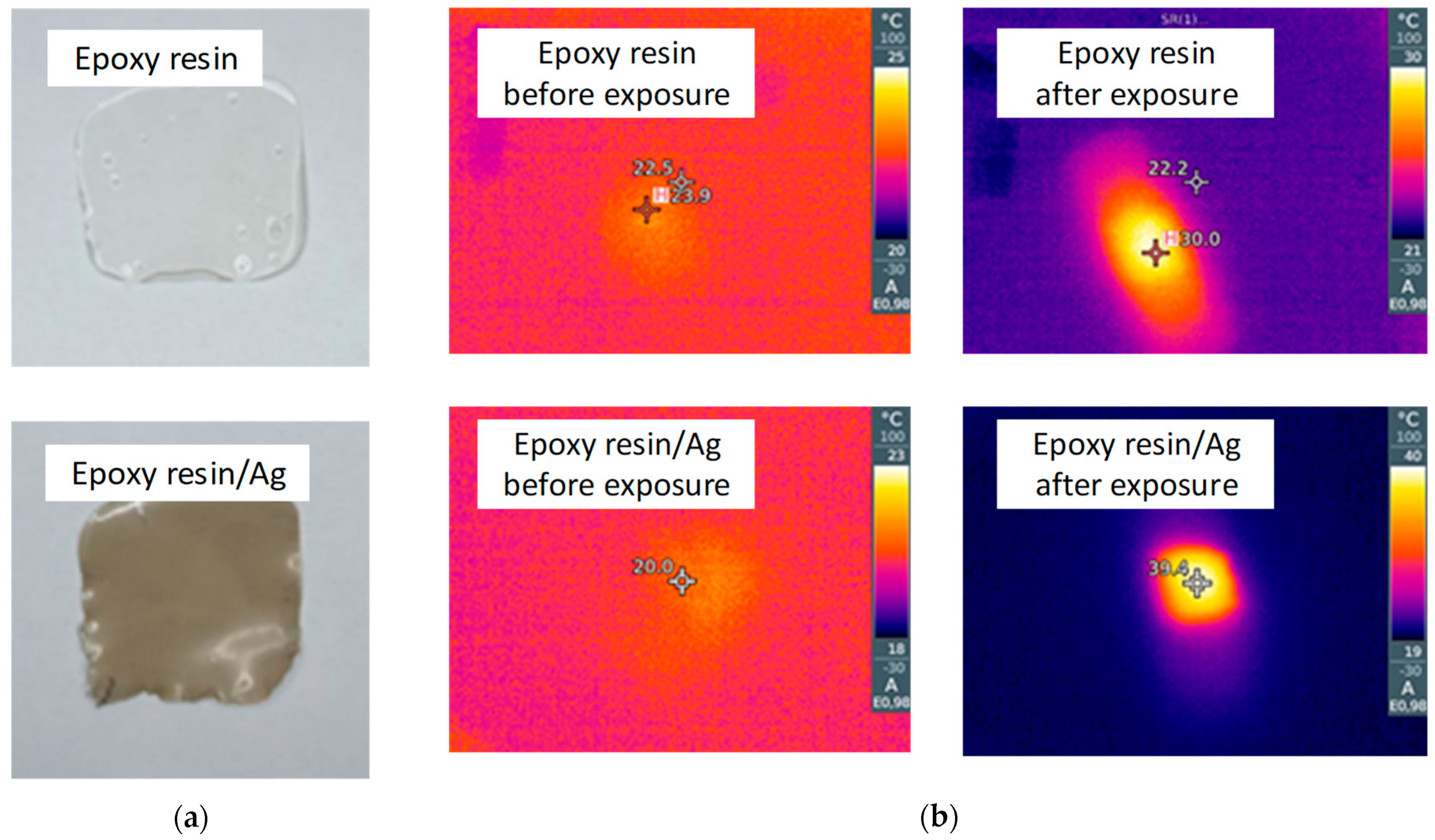
3.2.2. Morphology, Optical and Thermal Properties of Silver Nanoparticles
3.2.3. Decomposition of Organic Dye Molecules
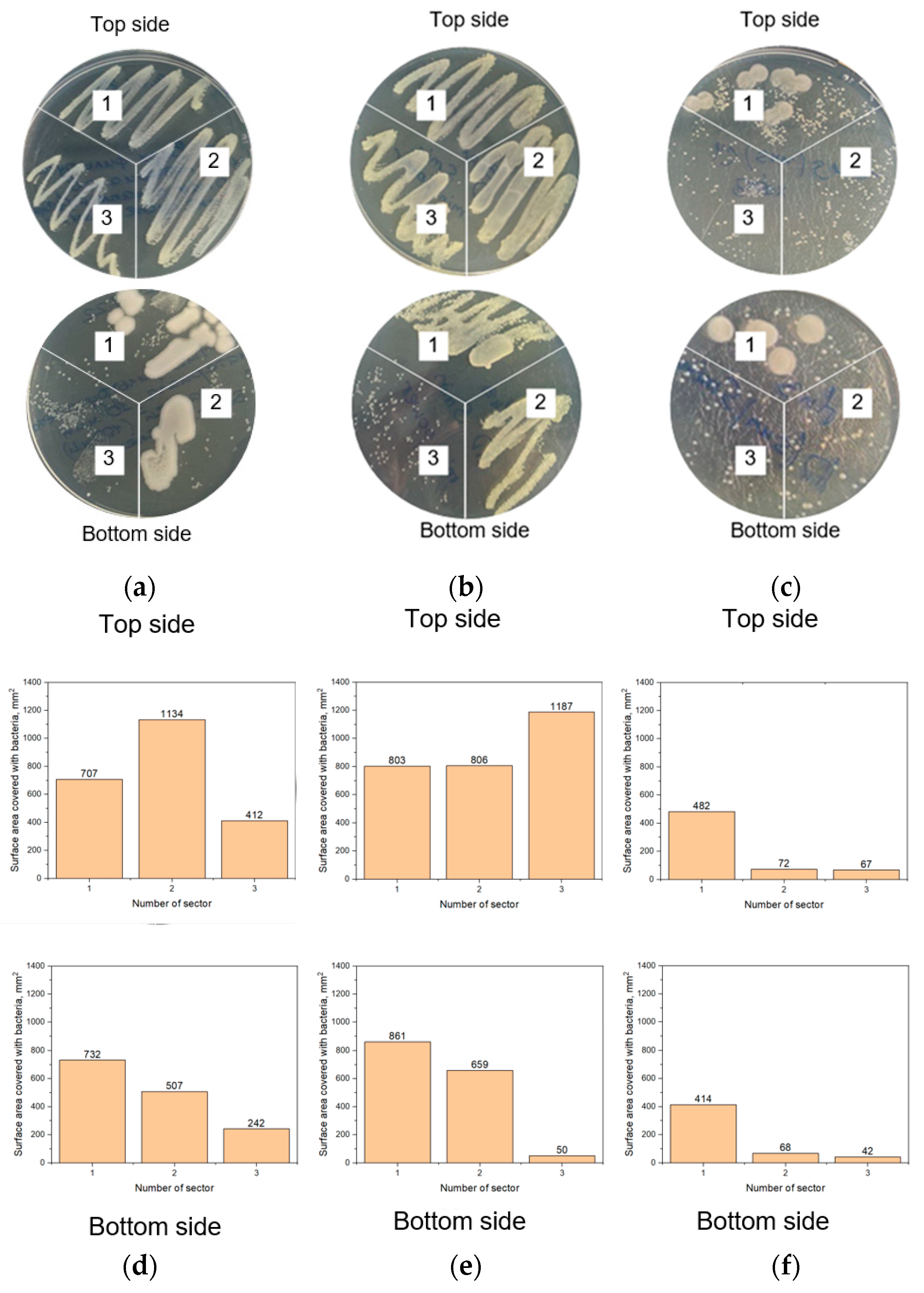
3.3. Study of Disinfecting Effectiveness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in Different Environmental Conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS Coronaviruses and Influenza Virus in Healthcare Settings: The Possible Role of Dry Surface Contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular Immune Pathogenesis and Diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Allison, T. Doctor Blades. Gravure 2007, 21, 32–35. [Google Scholar] [CrossRef]
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N.B. Studies of Spin-Coated Polymer Films. Annu. Reports Prog. Chem.-Sect. C 2005, 101, 174–201. [Google Scholar] [CrossRef]
- Salpavaara, T.; Joki, T.; Skogberg, A.; Calejo, M.T.; Lekkala, J.; Narkilahti, S.; Kallio, P. Microfabricated Porous SU-8 Membranes as Innervation Interfaces for HiPSC-Neurons in Microfluidic Devices. J. Phys. Commun. 2021, 5, 115003. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Asadi, R. Storage of Ag Nanoparticles in Pore-Arrays of SU-8 Matrix for Antibacterial Applications. J. Phys. D. Appl. Phys. 2009, 42, 135416. [Google Scholar] [CrossRef]
- Vu, D.T.; Pham, T.N.; Hsu, C.C.; Benisty, H.; Lai, N.D. Elaboration and Characterization of Nanoporous SU-8 Template Using PMMA as Porogen. J. Porous Mater. 2021, 28, 813–823. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Sergeyeva, N.V.; Arinichev, V.N.; Shevkun, N.A.; Ovsiannikova, E.A.; Chistova, Y.S. Economic Evaluation of Innovative Engineering Solutions in Animal Husbandry. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 022036. [Google Scholar] [CrossRef]
- Shtyka, O.; Ciesielski, R.; Kedziora, A.; Dubkov, S.; Gromov, D.; Zakrzewski, M.; Maniecki, T. Catalytic Activity of Semiconductors under the Influence of Electric Fields. Appl. Catal. A Gen. 2022, 635, 118541. [Google Scholar] [CrossRef]
- Sorokina, L.; Savitskiy, A.; Shtyka, O.; Maniecki, T.; Szynkowska-Jozwik, M.; Trifonov, A.; Pershina, E.; Mikhaylov, I.; Dubkov, S.; Gromov, D. Formation of Cu-Rh Alloy Nanoislands on TiO2 for Photoreduction of Carbon Dioxide. J. Alloys Compd. 2022, 904, 164012. [Google Scholar] [CrossRef]
- Shtyka, O.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Dubkov, S.; Gromov, D.; Maniecki, T. Photocatalytic Reduction of CO2 Over Me (Pt, Pd, Ni, Cu)/TiO2 Catalysts. Top. Catal. 2020, 63, 113–120. [Google Scholar] [CrossRef]
- Shtyka, O.; Shatsila, V.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Dubkov, S.; Gromov, D.; Tarasov, A.; Rogowski, J.; Stadnichenko, A.; et al. Adsorption and Photocatalytic Reduction of Carbon Dioxide on TiO2. Catalysts 2021, 11, 47. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Ouyang, K.; Dai, K.; Walker, S.L.; Huang, Q.; Yin, X.; Cai, P. Efficient Photocatalytic Disinfection of Escherichia coli O157:H7 Using C70-TiO2 Hybrid under Visible Light Irradiation. Sci. Rep. 2016, 6, 25702. [Google Scholar] [CrossRef]
- Leyland, N.S.; Podporska-Carroll, J.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly Efficient F, Cu Doped TiO2 Anti-Bacterial Visible Light Active Photocatalytic Coatings to Combat Hospital-Acquired Infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef]
- Sophee, S.S.; Prasad, R.G.S.V.; Srinivas, J.V.; Aparna, R.S.L.; Phani, A.R. Antibacterial Activity of TiO2 and ZnO Microparticles Combination on Water Polluting Bacteria. J. Green Sci. Technol. 2014, 1, 20–26. [Google Scholar] [CrossRef]
- Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Kusiak-Nejman, E.; Morawski, A.W. Photocatalytic Water Disinfection under the Artificial Solar Light by Fructose-Modified TiO2. Chem. Eng. J. 2019, 372, 203–215. [Google Scholar] [CrossRef]
- Chu, X.; Mao, L.; Johnson, O.; Li, K.; Phan, J.; Yin, Q.; Li, L.; Zhang, J.; Chen, W.; Zhang, Y. Exploration of TiO2 Nanoparticle Mediated Microdynamic Therapy on Cancer Treatment. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 272–281. [Google Scholar] [CrossRef]
- Çeşmeli, S.; Biray Avci, C. Application of Titanium Dioxide (TiO2) Nanoparticles in Cancer Therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef]
- Lisovski, O.; Piskunov, S.; Bocharov, D.; Kenmoe, S. 2D Slab Models of TiO2 Nanotubes for Simulation of Water Adsorption: Validation over a Diameter Range. Results Phys. 2020, 19, 103527. [Google Scholar] [CrossRef]
- Lepock, J.R. Measurement of Protein Stability and Protein Denaturation in Cells Using Differential Scanning Calorimetry. Methods 2005, 35, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Dubkov, S.V.; Savitskiy, A.I.; Trifonov, A.Y.; Yeritsyan, G.S.; Shaman, Y.P.; Kitsyuk, E.P.; Tarasov, A.; Shtyka, O.; Ciesielski, R.; Gromov, D.G. SERS in Red Spectrum Region through Array of Ag–Cu Composite Nanoparticles Formed by Vacuum-Thermal Evaporation. Opt. Mater. X 2020, 7, 100055. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.; Tong, H.; Wang, K.; Tao, L.; Zhang, Y.; Zhou, X. Inverted Pyramid Er3+ and Yb3+ Co-Doped TiO2 Nanorod Arrays Based Perovskite Solar Cell: Infrared Response and Improved Current Density. Ceram. Int. 2020, 46, 12073–12079. [Google Scholar] [CrossRef]
- Gromov, D.G.; Dubkov, S.V.; Savitskiy, A.I.; Shaman, Y.P.; Polokhin, A.A.; Belogorokhov, I.A.; Trifonov, A.Y. Optimization of Nanostructures Based on Au, Ag, Au[Sbnd]Ag Nanoparticles Formed by Thermal Evaporation in Vacuum for SERS Applications. Appl. Surf. Sci. 2019, 489, 701–707. [Google Scholar] [CrossRef]
- Talane, T.E.; Mbule, P.S.; Noto, L.L.; Shingange, K.; Mhlongo, G.H.; Mothudi, B.M.; Dhlamini, M.S. Sol-Gel Preparation and Characterization of Er3+ Doped TiO2 Luminescent Nanoparticles. Mater. Res. Bull. 2018, 108, 234–241. [Google Scholar] [CrossRef]
- Khinevich, N.; Juodėnas, M.; Tamulevičienė, A.; Bandarenka, H.; Tamulevičius, S. Tailoring Mesoporous Silicon Surface to Form a Versatile Template for Nanoparticle Deposition. Coatings 2021, 11, 699. [Google Scholar] [CrossRef]
- Elia, P.; Nativ-Roth, E.; Zeiri, Y.; Porat, Z. Determination of the Average Pore-Size and Total Porosity in Porous Silicon Layers by Image Processing of SEM Micrographs. Microporous Mesoporous Mater. 2016, 225, 465–471. [Google Scholar] [CrossRef]
- Zavatski, S.; Khinevich, N.; Girel, K.; Redko, S.; Kovalchuk, N.; Komissarov, I.; Lukashevich, V.; Semak, I.; Mamatkulov, K.; Vorobyeva, M.; et al. Surface Enhanced Raman Spectroscopy of Lactoferrin Adsorbed on Silvered Porous Silicon Covered with Graphene. Biosensors 2019, 9, 34. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A Rapid Method to Estimate the Concentration of Citrate Capped Silver Nanoparticles from UV-Visible Light Spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating Photodegradation Properties of Anatase and Rutile TiO2 Nanoparticles for Organic Compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- Simonis, F.D.; Neto, A.S.; Schultz, M.J. The Tidal Volume Fix and More… J. Thorac. Dis. 2019, 11, E117–E122. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Cao, C.; Xiang, Y.; Ruan, L.; Qian, G. Age-Specific Transmissibility Change of COVID-19 and Associations with Breathing Air Volume, Preexisting Immunity, and Government Response. Front. Public Health 2022, 10, 487. [Google Scholar] [CrossRef]

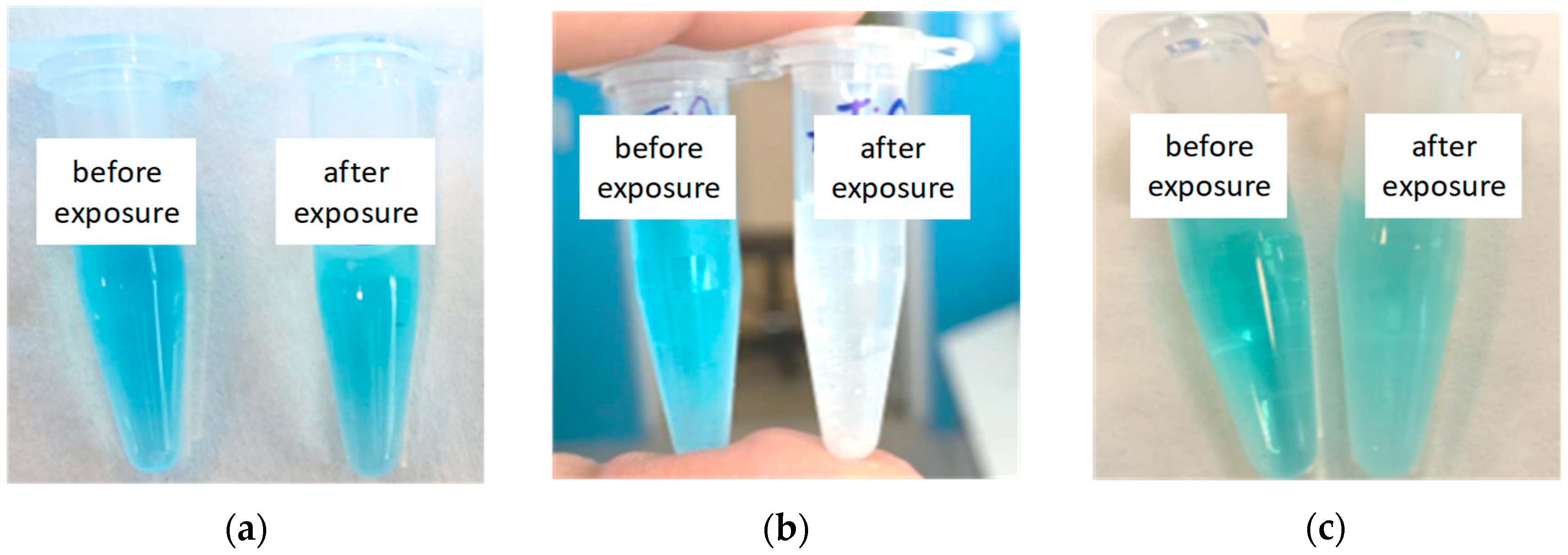

| Sample Type | Absorption, a.u. | Absorption Delta for Fresh and Exposed Samples, % | |||
|---|---|---|---|---|---|
| Fresh | Kept in Dark Place | Exposed | Exposed and Corrected for Control MB | ||
| MB | 22 | 22 | 15 (UV) | - | 32 |
| MB + TiO2 (hydroth.) | 88 | 86 | 43 (UV) | 50 | 43 |
| MB + TiO2 (sol-gel) | 81 | 80 | 36 (UV) | 43 | 47 |
| MB | 22 | 22 | 20 (LED) | - | 9 |
| MB + Ag (100 µg) | 28 | 27 | 18 (LED) | 20 | 29 |
| MB + Ag (200 µg) | 32 | 32 | 22 (LED) | 24 | 25 |
| MB + Ag (300 µg) | 34 | 34 | 27 (LED) | 29 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burko, A.; Zavatski, S.; Baturova, A.; Kholiboeva, M.; Kozina, J.; Kravtsunova, K.; Popov, V.; Gudok, A.; Dubkov, S.; Khartov, S.; et al. Polymer Membrane Modified with Photocatalytic and Plasmonic Nanoparticles for Self-Cleaning Filters. Polymers 2023, 15, 726. https://doi.org/10.3390/polym15030726
Burko A, Zavatski S, Baturova A, Kholiboeva M, Kozina J, Kravtsunova K, Popov V, Gudok A, Dubkov S, Khartov S, et al. Polymer Membrane Modified with Photocatalytic and Plasmonic Nanoparticles for Self-Cleaning Filters. Polymers. 2023; 15(3):726. https://doi.org/10.3390/polym15030726
Chicago/Turabian StyleBurko, Aliaksandr, Siarhei Zavatski, Arina Baturova, Makhina Kholiboeva, Julia Kozina, Kseniya Kravtsunova, Vladimir Popov, Artem Gudok, Sergey Dubkov, Stanislav Khartov, and et al. 2023. "Polymer Membrane Modified with Photocatalytic and Plasmonic Nanoparticles for Self-Cleaning Filters" Polymers 15, no. 3: 726. https://doi.org/10.3390/polym15030726
APA StyleBurko, A., Zavatski, S., Baturova, A., Kholiboeva, M., Kozina, J., Kravtsunova, K., Popov, V., Gudok, A., Dubkov, S., Khartov, S., & Bandarenka, H. (2023). Polymer Membrane Modified with Photocatalytic and Plasmonic Nanoparticles for Self-Cleaning Filters. Polymers, 15(3), 726. https://doi.org/10.3390/polym15030726








