Binary Double Network-like Structure: An Effective Energy-Dissipation System for Strong Tough Hydrogel Design
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Hydrogels with Different Initial Feeding Composition
2.3. Characterizations
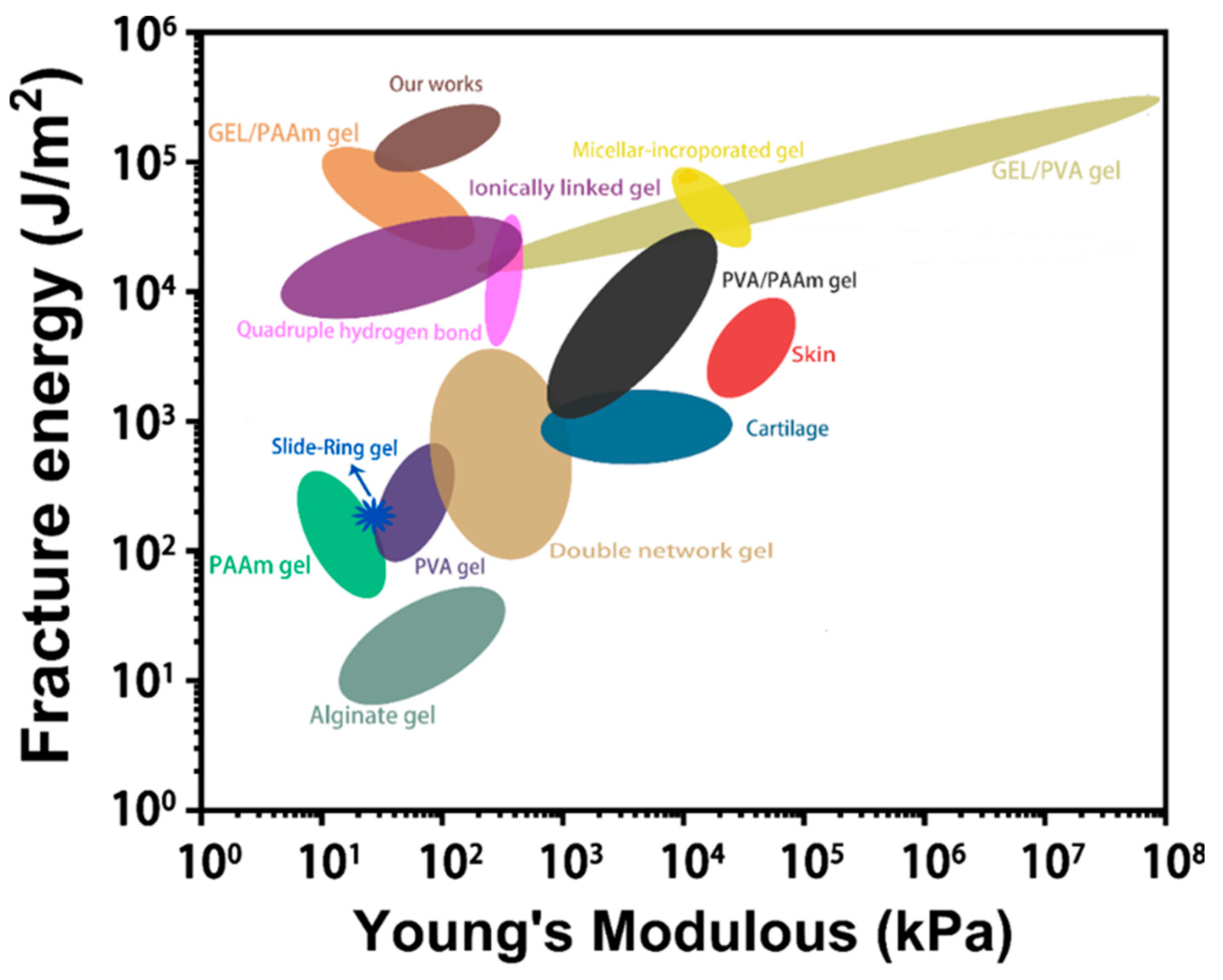
3. Results and Discussions
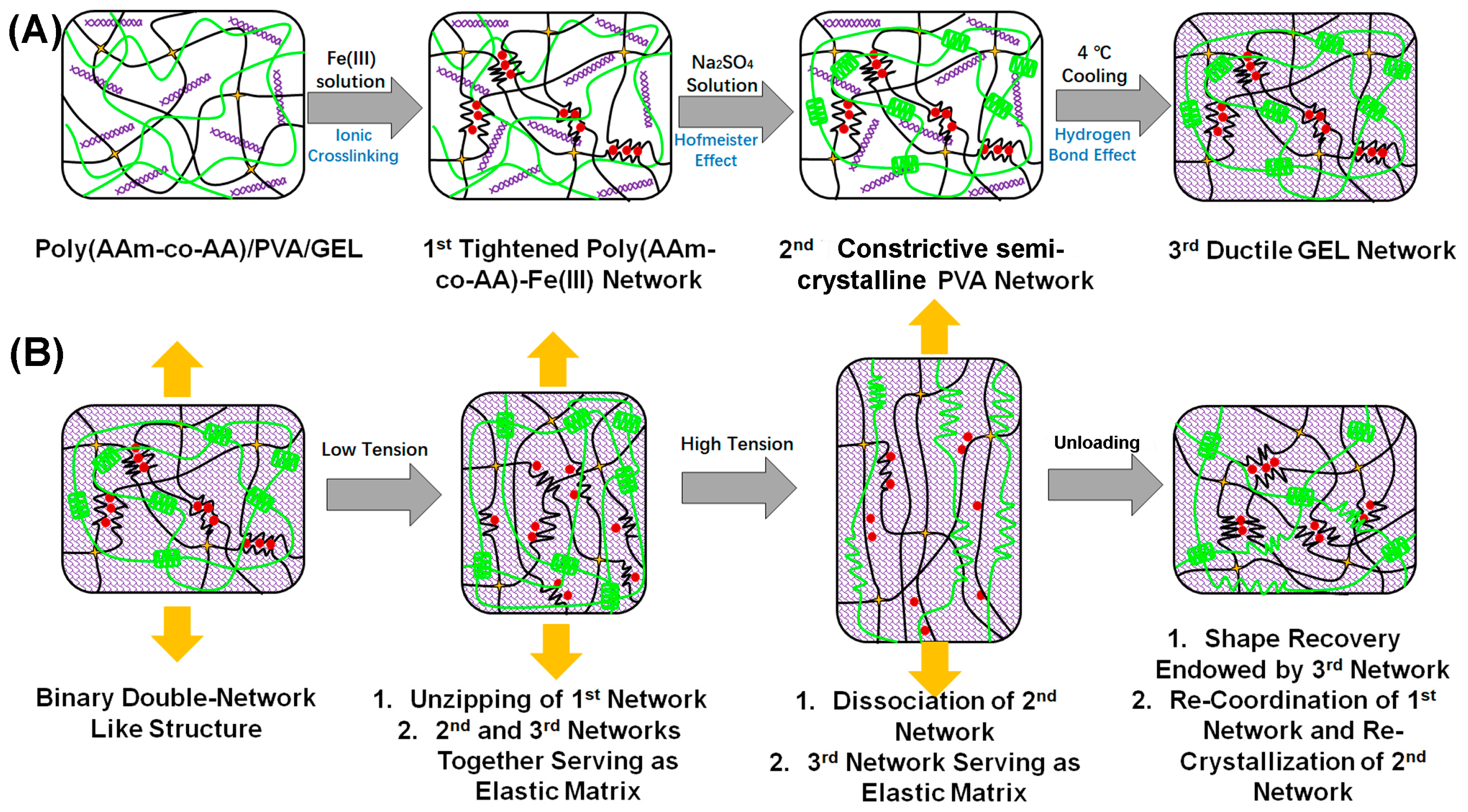
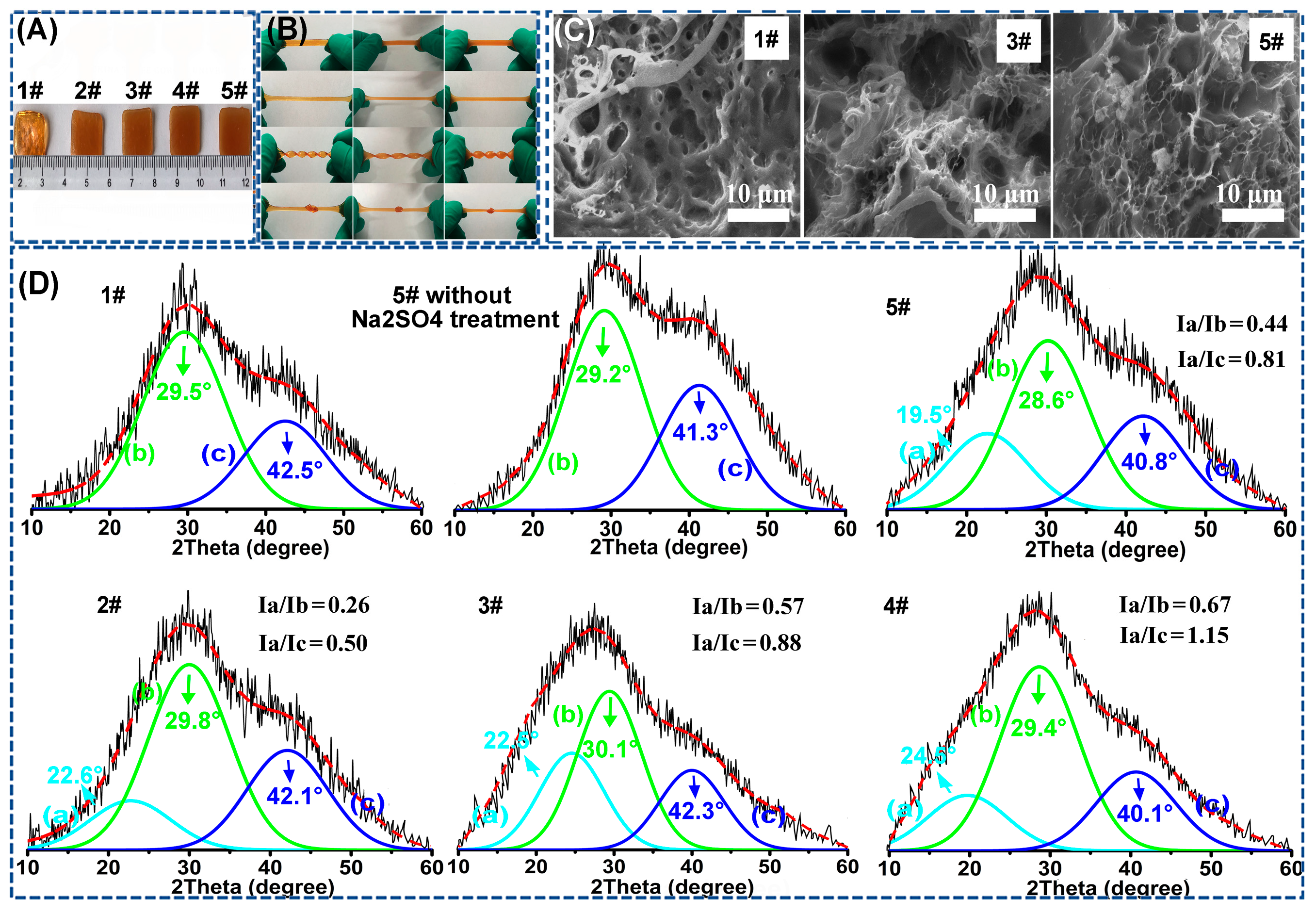
3.1. Identification of the “Binary DN-like” Structure of the As-Fabricated Poly(AAm-co-AA)/PVA/GEL-Fe(III) Hydrogel
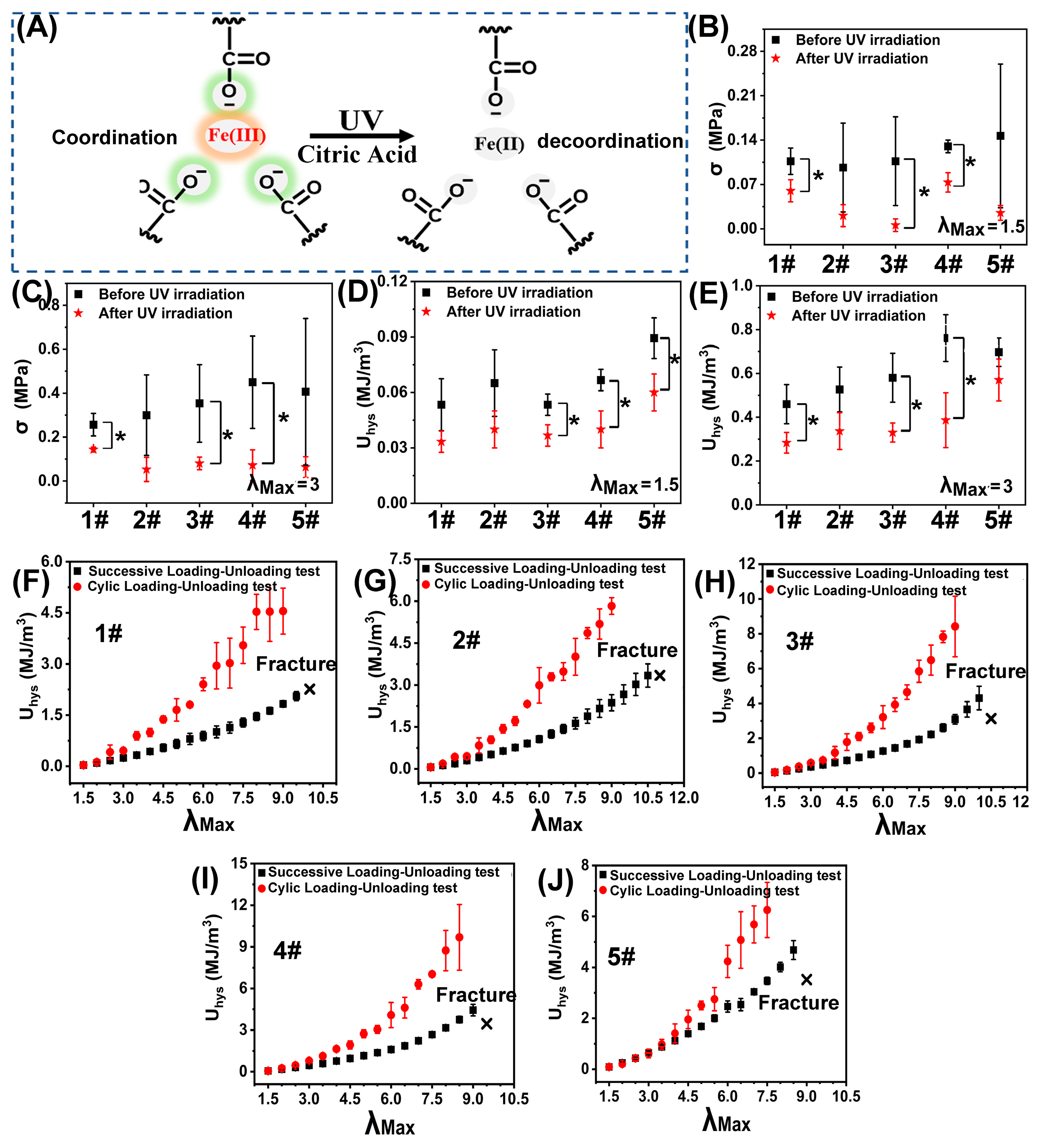
3.2. Mechanical Properties of the “Binary DN-like” Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Zhang, Q.; Ma, L.; Zhao, Y.; Ran, J. Rational design of a high-strength tough hydrogel from fundamental principle. Macromol. Chem. Phys. 2021, 222, 2100064. [Google Scholar] [CrossRef]
- Sun, W.; Xue, B.; Fan, Q.; Tao, R.; Wang, C.; Wang, X.; Li, Y.; Qin, M.; Wang, W.; Chen, B. Molecular engineering of metal coordination interactions for strong, tough, and fast-recovery hydrogels. Sci. Adv. 2020, 6, eaaz9531. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P. Materials both tough and soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Designing toughness and strength for soft materials. Proc. Natl. Acad. Sci. USA 2017, 114, 8138–8140. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Dong, L.; Li, Y.; Zhang, J.; Chen, H.; Wu, J.; Zhang, Y.; Fan, Q.; Xue, B.; Qin, M. Stretchable hydrogels with low hysteresis and anti-fatigue fracture based on polyprotein cross-linkers. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Liu, J.; Yuk, H.; Loh, H.-C.; Parada, G.A.; Settens, C.; Song, J.; Masic, A.; McKinley, G.H. Anti-fatigue-fracture hydrogels. Sci. Adv. 2019, 5, eaau8528. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, J.; Liu, X.; Zhao, X. Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. USA 2019, 116, 10244–10249. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Q.; Zhu, L.; Tang, Z.; Li, Q.; Qin, G.; Yang, J.; Zhang, Y.; Ren, B.; Zheng, J. General strategy to fabricate strong and tough low-molecular-weight gelator-based supramolecular hydrogels with double network structure. Chem. Mater. 2018, 30, 1743–1754. [Google Scholar] [CrossRef]
- Nakajima, T.; Ozaki, Y.; Namba, R.; Ota, K.; Maida, Y.; Matsuda, T.; Kurokawa, T.; Gong, J.P. Tough double-network gels and elastomers from the nonprestretched first network. ACS Macro Lett. 2019, 8, 1407–1412. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, X.; Zhu, L.; Chen, H.; Jiang, B.; Wei, D.; Huang, L.; Yang, J.; Liu, B.; Zheng, J. Improvement of mechanical strength and fatigue resistance of double network hydrogels by ionic coordination interactions. Chem. Mater. 2016, 28, 5710–5720. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef]
- Kimura, T.; Urayama, K. Multiaxial stress relaxation of dual-cross-link Poly(vinyl alcohol) hydrogels. ACS Macro Lett. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-responsive gel− sol/sol− gel transition in poly (acrylic acid) aqueous solution containing Fe (III) ions switched by light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Zheng, Y.; Matsuda, T.; Nakajima, T.; Cui, W.; Zhang, Y.; Hui, C.Y.; Kurokawa, T.; Gong, J.P. How chain dynamics affects crack initiation in double-network gels. Proc. Natl. Acad. Sci. USA 2021, 118, e2111880118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, X.; Ouchi, T.; Kouznetsova, T.B.; Beech, H.K.; Av-Ron, S.; Matsuda, T.; Bowser, B.H.; Wang, S.; Johnson, J.A.; et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 2021, 374, 193–196. [Google Scholar] [CrossRef]
- Zheng, D.; Lin, S.; Ni, J.; Zhao, X. Fracture and fatigue of entangled and unentangled polymer networks. Extrem. Mech. Lett. 2022, 51, 101608. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Q.; Yao, X.; Bai, R.; Hong, W.; Yang, C. Fatigue of amorphous hydrogels with dynamic covalent bonds. Extrem. Mech. Lett. 2022, 53, 101679. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Zhang, D.; Xu, Z.; Liu, W. A highly tough and stiff supramolecular polymer double network hydrogel. Polymer 2018, 153, 193–200. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Z.; Chen, A.; He, H.; He, C.; Shuai, X.; Li, X.; Chen, S.; Zhang, Y.; Ren, B.; et al. Sulfated zwitterionic poly(sulfobetaine methacrylate) hydrogels promote complete skin regeneration. Acta Biomater. 2018, 71, 293–305. [Google Scholar] [CrossRef]
- Lin, P.; Ma, S.; Wang, X.; Zhou, F. Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater. 2015, 27, 2054–2059. [Google Scholar] [CrossRef]
- Peng, K.; Yu, H.; Yang, H.; Hao, X.; Yasin, A.; Zhang, X. A mechanically robust hydrogel with thermally induced plasticity and a shape memory effect. Soft Matter. 2017, 13, 2135–2140. [Google Scholar] [CrossRef]
- Yang, F.; Ren, B.; Cai, Y.; Tang, J.; Li, D.; Wang, T.; Feng, Z.; Chang, Y.; Xu, L.; Zheng, J. Mechanically tough and recoverable hydrogels via dual physical crosslinkings. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1294–1305. [Google Scholar] [CrossRef]
- Yu, J.; Xu, K.; Chen, X.; Zhao, X.; Yang, Y.; Chu, D.; Xu, Y.; Zhang, Q.; Zhang, Y.; Cheng, Y. Highly Stretchable, Tough, Resilient, and Antifatigue Hydrogels Based on Multiple Hydrogen Bonding Interactions Formed by Phenylalanine Derivatives. Biomacromolecules 2021, 22, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Jin, Z. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer–Tannic Acid Multiple Hydrogen Bonds. Macromol. 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Q.; Zhu, L.; Chen, H.; Wei, D.; Chen, F.; Tang, Z.; Yang, J.; Zheng, J. High strength and self-healable gelatin/polyacrylamide double network hydrogels. J. Mater. Chem. B 2017, 5, 7683–7691. [Google Scholar] [CrossRef]
- Sun, X.; Ye, L.; Liang, H. Extremely stretchable and tough hybrid hydrogels based on gelatin, kappa-carrageenan and polyacrylamide. Soft Matter 2021, 17, 9708–9715. [Google Scholar] [CrossRef]
- Sun, Y.N.; Gao, G.R.; Du, G.L.; Cheng, Y.J.; Fu, J. Super Tough, Ultrastretchable, and Thermoresponsive Hydrogels with Functionalized Triblock Copolymer Micelles as Macro-Cross-Linkers. ACS Macro. Lett. 2014, 3, 496–500. [Google Scholar] [CrossRef]
- Jia, H.; Huang, Z.; Fei, Z.; Dyson, P.J.; Zheng, Z.; Wang, X. Unconventional Tough Double-Network Hydrogels with Rapid Mechanical Recovery, Self-Healing, and Self-Gluing Properties. ACS Appl. Mater. Interfaces 2016, 8, 31339–31347. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Tang, S.; Yan, H.; Zhu, X.; Wang, H.; Ma, L.; Mao, K.; Yang, C.; Ran, J. Binary Double Network-like Structure: An Effective Energy-Dissipation System for Strong Tough Hydrogel Design. Polymers 2023, 15, 724. https://doi.org/10.3390/polym15030724
Chen G, Tang S, Yan H, Zhu X, Wang H, Ma L, Mao K, Yang C, Ran J. Binary Double Network-like Structure: An Effective Energy-Dissipation System for Strong Tough Hydrogel Design. Polymers. 2023; 15(3):724. https://doi.org/10.3390/polym15030724
Chicago/Turabian StyleChen, Genxin, Sijie Tang, Honghan Yan, Xiongbin Zhu, Huimin Wang, Liya Ma, Kang Mao, Changying Yang, and Jiabing Ran. 2023. "Binary Double Network-like Structure: An Effective Energy-Dissipation System for Strong Tough Hydrogel Design" Polymers 15, no. 3: 724. https://doi.org/10.3390/polym15030724
APA StyleChen, G., Tang, S., Yan, H., Zhu, X., Wang, H., Ma, L., Mao, K., Yang, C., & Ran, J. (2023). Binary Double Network-like Structure: An Effective Energy-Dissipation System for Strong Tough Hydrogel Design. Polymers, 15(3), 724. https://doi.org/10.3390/polym15030724