Ring-Opening Polymerization of Trimethylene Carbonate with Phosphazene Organocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Polymerization
3. Results and Discussion
3.1. Ring Opening Polymerization of TMC by t-BuP4
3.2. Controlled/Living Manner of the ROP of PTMC by t-BuP4
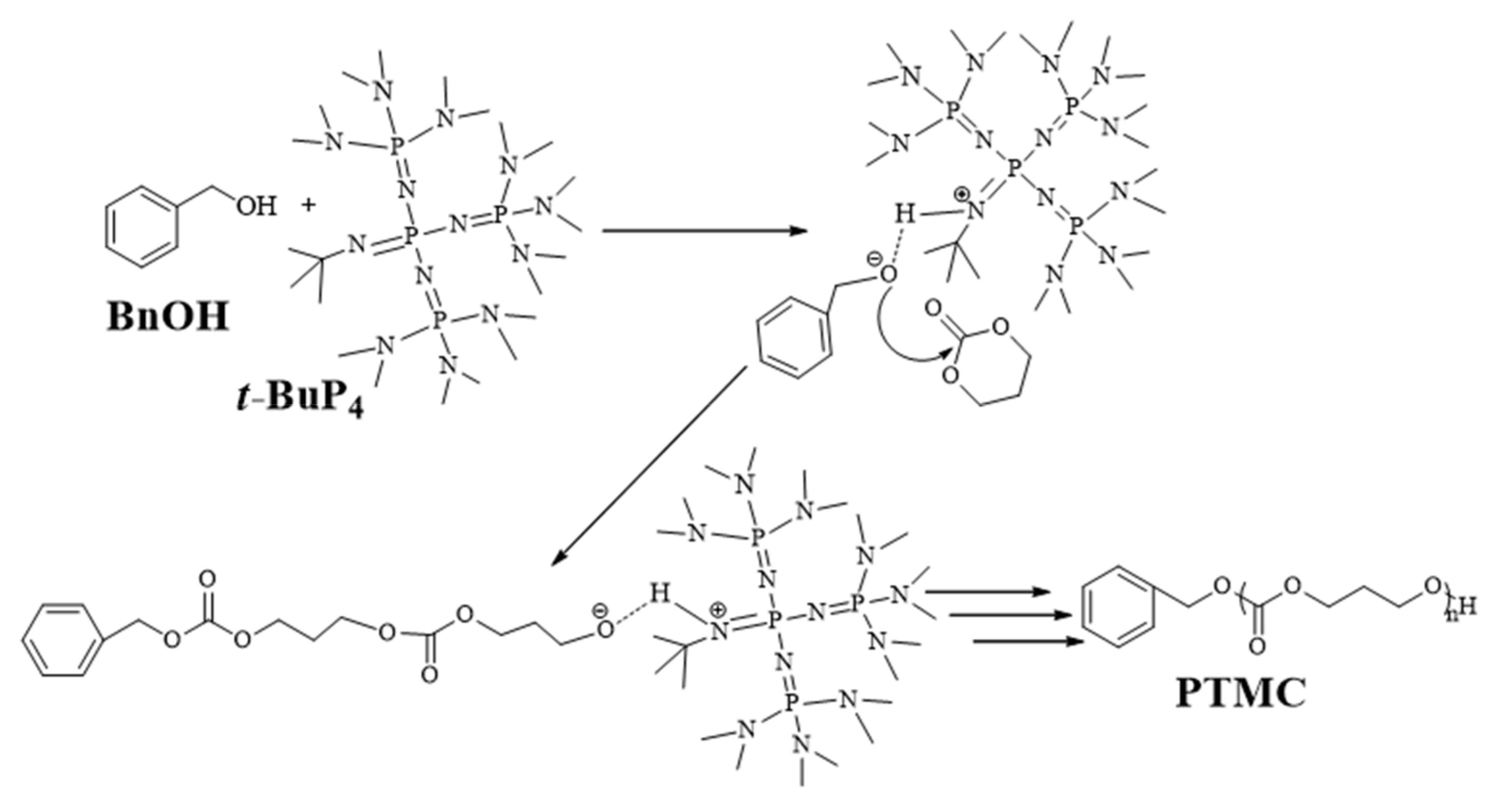
3.3. A Plausible Reaction Mechanism
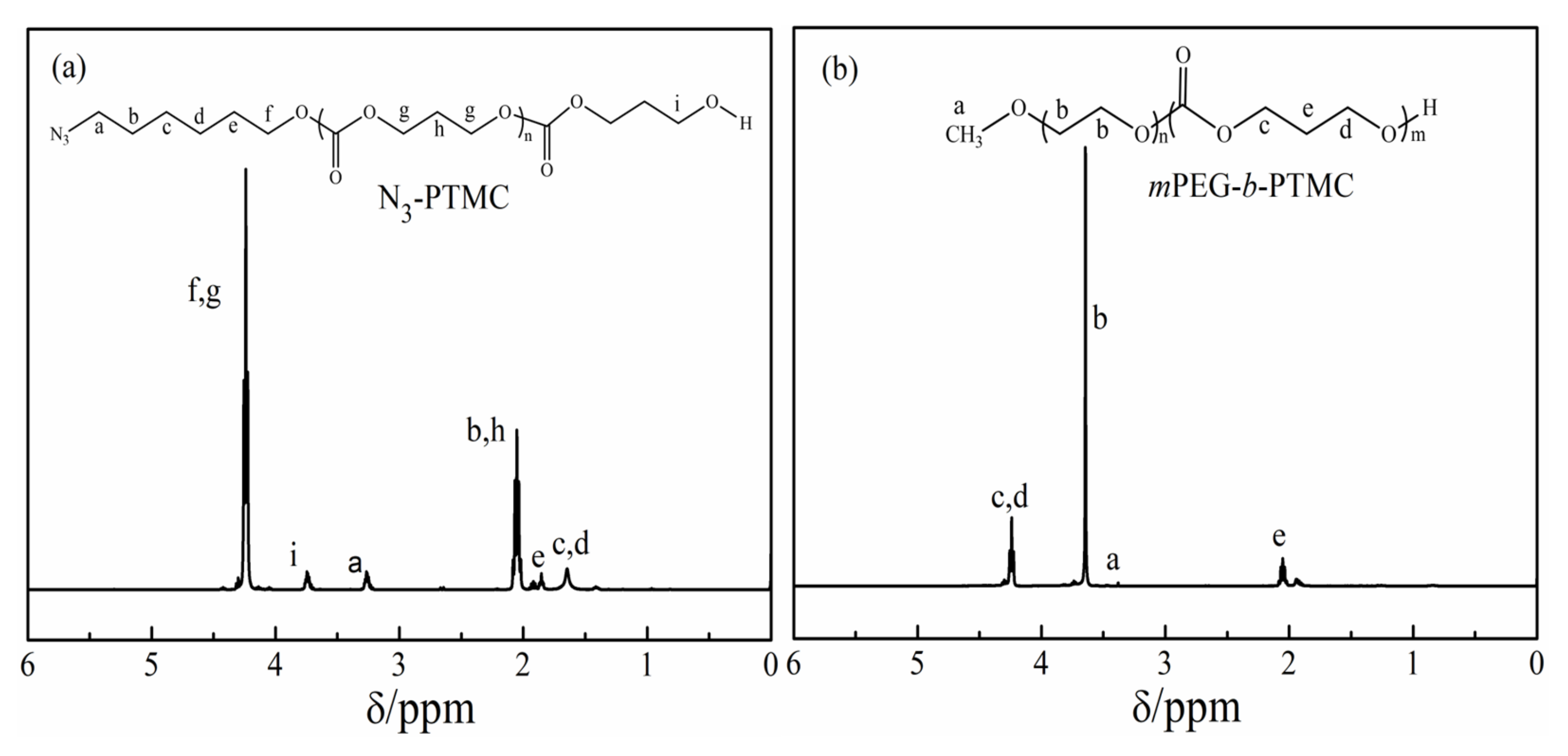
3.4. Preparation of Functional PTMC
3.5. Thermal Properties of the Obtained PTMC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carboue, Q.; Fadlallah, S.; Lopez, M.; Allais, F. Progress in Degradation Behavior of Most Common Types of Functionalized Polymers: A Review. Macromol. Rapid Commun. 2022, 43, e2200254. [Google Scholar] [CrossRef] [PubMed]
- Dirauf, M.; Muljajew, I.; Weber, C.; Schubert, U.S. Recent Advances in Degradable Synthetic Polymers for Biomedical Applications-Beyond Polyesters. Prog. Polym. Sci. 2022, 129, 101547. [Google Scholar] [CrossRef]
- Pagar, R.R.; Musale, S.R.; Pawar, G.; Kulkarni, D.; Giram, P.S. Comprehensive Review on the Degradation Chemistry and Toxicity Studies of Functional Materials. ACS Biomater. Sci. Eng. 2022, 8, 2161–2195. [Google Scholar] [CrossRef] [PubMed]
- Saghebasl, S.; Akbarzadeh, A.; Gorabi, A.M.; Nikzamir, N.; SeyedSadjadi, M.; Mostafavi, E. Biodegradable Functional Macromolecules as Promising Scaffolds for Cardiac Tissue Engineering. Polym. Adv. Technol. 2022, 33, 2044–2068. [Google Scholar] [CrossRef]
- Fukushima, K.; Fukushima, K. Biodegradable Functional Biomaterials Exploiting Substituted Trimethylene Carbonates and Organocatalytic Transesterification. Polym. J. 2016, 48, 1103–1114. [Google Scholar] [CrossRef]
- Yu, W.; Maynard, E.; Chiaradia, V.; Arno, M.C.; Dove, A.P. Aliphatic Polycarbonates from Cyclic Carbonate Monomers and Their Application as Biomaterials. Chem. Rev. 2021, 121, 10865–10907. [Google Scholar] [CrossRef]
- Feng, J.; Zhuo, R.-X.; Zhang, X.-Z. Construction of Functional Aliphatic Polycarbonates for Biomedical Applications. Prog. Polym. Sci. 2012, 37, 211–236. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X. Recent Development of Functional Aliphatic Polycarbonates for the Construction of Amphiphilic Polymers. Polym. Chem. 2017, 8, 7429–7437. [Google Scholar] [CrossRef]
- Van Bochove, B.; Grijpma, D.W. Mechanical Properties of Porous Photo-Crosslinked Poly(trimethylene carbonate) Network Films. Eur. Polym. J. 2021, 143, 101456. [Google Scholar] [CrossRef]
- Zhai, H.; Chen, K.; Meng, Y.; Wu, Z.; Deng, R.; Bai, Y.; Zhou, J.; Quan, D. Synthesis and Self-Assembly of Amphiphilic Diblock Polycarbonates with Various Pendant Hydrophilic Groups. Polymer 2022, 244, 10234. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Kaźmierski, S.; Lewinski, P.; Cypryk, M.; Penczek, S. Cationic Polymerization of Cyclic Trimethylene Carbonate Induced with Initiator and Catalyst in One Molecule: Polymer Structure, Kinetics and DFT. J. Catal. 2022, 415, 200–217. [Google Scholar] [CrossRef]
- Han, J.; Branford-White, C.J.; Zhu, L.-M. Preparation of Poly(ε-caprolactone)/Poly(trimethylene carbonate) Blend Nanofibers by Electrospinning. Carbohydr. Polym. 2010, 79, 214–218. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhuo, R.-X.; Liu, L.-J.; He, F.; Liu, G. Synthesis and Characterization of Novel Aliphatic Polycarbonates. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 70–75. [Google Scholar] [CrossRef]
- Ang, P.; Mothe, S.R.; Chennamaneni, L.R.; Aidil, F.; Khoo, H.H.; Thoniyot, P. Laboratory-Scale Life-Cycle Assessment: A Comparison of Existing and Emerging Methods of Poly(ε-caprolactone) Synthesis. ACS Sustain. Chem. Eng. 2020, 9, 669–683. [Google Scholar] [CrossRef]
- Hege, C.S.; Siegel-Axel, D.; Kohler, K.; Delorme, N.; Le Houérou, V.; Schiller, S.M.; Dolderer, J.H. Biopolymer Systems in Soft Tissue Engineering: Cell Compatibility and Effect Studies Including Material, Catalyst, and Surface Properties. ACS Appl. Polym. Mater. 2020, 2, 3251–3258. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Le Grognec, E.; Chretien, J.M.; Zammattio, F.; Quintard, J.P. Methodologies Limiting or Avoiding Contamination by Organotin Residues in Organic Synthesis. Chem. Rev. 2015, 115, 10207–10260. [Google Scholar] [CrossRef]
- Nikulin, M.; Svedas, V. Prospects of Using Biocatalysis for the Synthesis and Modification of Polymers. Molecules 2021, 26, 2750. [Google Scholar] [CrossRef]
- Matsumura, S.; Tsukada, K.; Toshima, K. Enzyme-Catalyzed Ring-Opening Polymerization of 1,3-Dioxan-2-one to Poly(trimethylene carbonate). Macromolecules 1997, 30, 3122–3124. [Google Scholar] [CrossRef]
- Al-Azemi, T.F.; Bisht, K.S. Novel Functional Polycarbonate by Lipase-Catalyzed Ring-Opening Polymerization of 5-Methyl-5-benzyloxycarbonyl-1,3-dioxan-2-one. Macromolecules 1999, 32, 6536–6540. [Google Scholar] [CrossRef]
- Li, F.; Shi, Y.; Li, P.; Jiang, T. Research Progress of Ring-opening Polymerization of ε-Caprolactone Initiated by Degradable Biopolymers. Curr. Org. Chem. 2020, 24, 1507–1516. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding. Polymers 2019, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Cammas-Marion, S.; Coulembier, O. Organocatalysis Applied to the Ring-Opening Polymerization of β-Lactones: A Brief Overview. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 657–672. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, J. Macromolecular Architectures Based on Organocatalytic Ring-Opening (co)Polymerization of Epoxides. Polymer 2018, 143, 343–361. [Google Scholar] [CrossRef]
- Mezzasalma, L.; Dove, A.P.; Coulembier, O. Organocatalytic Ring-Opening Polymerization of l-Lactide in Bulk: A Long Standing Challenge. Eur. Polym. J. 2017, 95, 628–634. [Google Scholar] [CrossRef]
- Coady, D.J.; Horn, H.W.; Jones, G.O.; Sardon, H.; Engler, A.C.; Waymouth, R.M.; Rice, J.E.; Yang, Y.Y.; Hedrick, J.L. Polymerizing Base Sensitive Cyclic Carbonates Using Acid Catalysis. ACS Macro Lett. 2013, 2, 306–312. [Google Scholar] [CrossRef]
- Zhang, L.; Nederberg, F.; Pratt, R.C.; Waymouth, R.M.; Hedrick, J.L.; Wade, C.G. Phosphazene Bases: A New Category of Organocatalysts for the Living Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2007, 40, 4154–4158. [Google Scholar] [CrossRef]
- Boileau, S.; Illy, N. Activation in Anionic Polymerization: Why Phosphazene Bases Are Very Exciting Promoters. Prog. Polym. Sci. 2011, 36, 1132–1151. [Google Scholar] [CrossRef]
- Liu, S.; Ren, C.; Zhao, N.; Shen, Y.; Li, Z. Phosphazene Bases as Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromol. Rapid Commun. 2018, 39, e1800485. [Google Scholar] [CrossRef]
- Helou, M.; Miserque, O.; Brusson, J.M.; Carpentier, J.F.; Guillaume, S.M. Organocatalysts for the Controlled “Immortal” Ring-Opening Polymerization of Six-Membered-Ring Cyclic Carbonates: A Metal-Free, Green Process. Chemistry 2010, 16, 13805–13813. [Google Scholar] [CrossRef]
- Brignou, P.; Carpentier, J.-F.; Guillaume, S.M. Metal- and Organo-Catalyzed Ring-Opening Polymerization of α-Methyl-Trimethylene Carbonate: Insights into the Microstructure of the Polycarbonate. Macromolecules 2011, 44, 5127–5135. [Google Scholar] [CrossRef]
- Kiesewetter, M.K.; Shin, E.J.; Hedrick, J.L.; Waymouth, R.M. Organocatalysis Opportunities and Challenges for Polymer Synthesis. Macromolecules 2010, 43, 2093–2107. [Google Scholar] [CrossRef]
- Brossier, T.; Volpi, G.; Lapinte, V.; Blanquer, S. Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity. Polymers 2021, 13, 280. [Google Scholar] [CrossRef]
- Ren, C.; Zhu, X.; Zhao, N.; Zhen, Y.; Chen, L.; Liu, S.; Li, Z. Polystyrene Beads Supported Phosphazene Superbase as Recyclable Organocatalyst for Ring-Opening Polymerization of δ-Valerolactone. Eur. Polym. J. 2019, 119, 130–135. [Google Scholar] [CrossRef]
- Ren, C.; Zhu, X.; Zhao, N.; Fang, S.; Li, Z. Using Recyclable Polystyrene Supported Cyclic Trimeric Phosphazene Base as Catalyst to Directly Prepare Hypotoxic Polyesters via Ring-Opening Polymerizations. Polymer 2020, 204, 122797. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, W.; Zhang, J.; Bu, M.; Zhang, X.; Chen, A.; Xu, J.; Lei, C. Development of Recoverable and Recyclable Fe3O4-Supported Organocatalysts for Ring-Opening Polymerization. J. Polym. Sci. 2020, 58, 3411–3418. [Google Scholar] [CrossRef]
- Mezzasalma, L.; Harrisson, S.; Saba, S.; Loyer, P.; Coulembier, O.; Taton, D. Bulk Organocatalytic Synthetic Access to Statistical Copolyesters from l-Lactide and epsilon-Caprolactone Using Benzoic Acid. Biomacromolecules 2019, 20, 1965–1974. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Lewinski, P.; Kaźmierski, S.; Penczek, S. Catalysis in Polymerization of Cyclic Esters. Catalyst and Initiator in One molecule. Polymerization of ε-Caprolactone. J. Catal. 2020, 392, 97–107. [Google Scholar] [CrossRef]
- Lewinski, P.; Kaluzynski, K.; Pretula, J.; Mielniczak, G.; Penczek, S. Catalysis in polymerization of Cyclic Esters. Catalyst and Initiator in One Molecule. Polymerization of Lactide. J. Catal. 2022, 405, 249–264. [Google Scholar] [CrossRef]
- Se, K.; Miyawaki, K.; Hirahara, K.; Takano, A.; Fujimoto, T. Model Block-Graft Copolymer via Anionic Living Polymerization: Preparation and Characterization of Polystyrene-block-[Poly(p-hydroxystyrene)-graft-Poly(ethylene oxide)]-block-Polystyrene. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 3021–3034.41. [Google Scholar] [CrossRef]
- Mountrichas, G.; Mantzaridis, C.; Pispas, S. Well-Defined Flexible Polyelectrolytes with Two Cationic Sites per Monomeric Unit. Macromol. Rapid Comm. 2006, 27, 289–294. [Google Scholar] [CrossRef]
- Mundil, R.; Zhigunov, A.; Uchman, M. Metal-Free Synthesis and Self-Assembly of Poly(ethylene glycol) Methyl Ether-block-Poly(ε-decalactone)-block-Poly(methyl methacrylate) Triblock Terpolymers. Eur. Polym. J. 2022, 166, 110966. [Google Scholar] [CrossRef]
- Engler, A.C.; Ke, X.; Gao, S.; Chan, J.M.W.; Coady, D.J.; Ono, R.J.; Lubbers, R.; Nelson, A.; Yang, Y.Y.; Hedrick, J.L. Hydrophilic Polycarbonates: Promising Degradable Alternatives to Poly(ethylene glycol)-Based Stealth Materials. Macromolecules 2015, 48, 1673–1678. [Google Scholar] [CrossRef]
- De los Santos Pereira, A.; Sheikh, S.; Blaszykowski, C.; Pop-Georgievski, O.; Fedorov, K.; Thompson, M.; Rodriguez-Emmenegger, C. Antifouling Polymer Brushes Displaying Antithrombogenic Surface Properties. Biomacromolecules 2016, 17, 1179–1185. [Google Scholar] [CrossRef]
- Díaz-Celorio, E.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Synthesis of Glycolide/Trimethylene Carbonate Copolymers: Influence of Microstructure on Properties. Eur. Polym. J. 2012, 48, 60–73. [Google Scholar] [CrossRef]
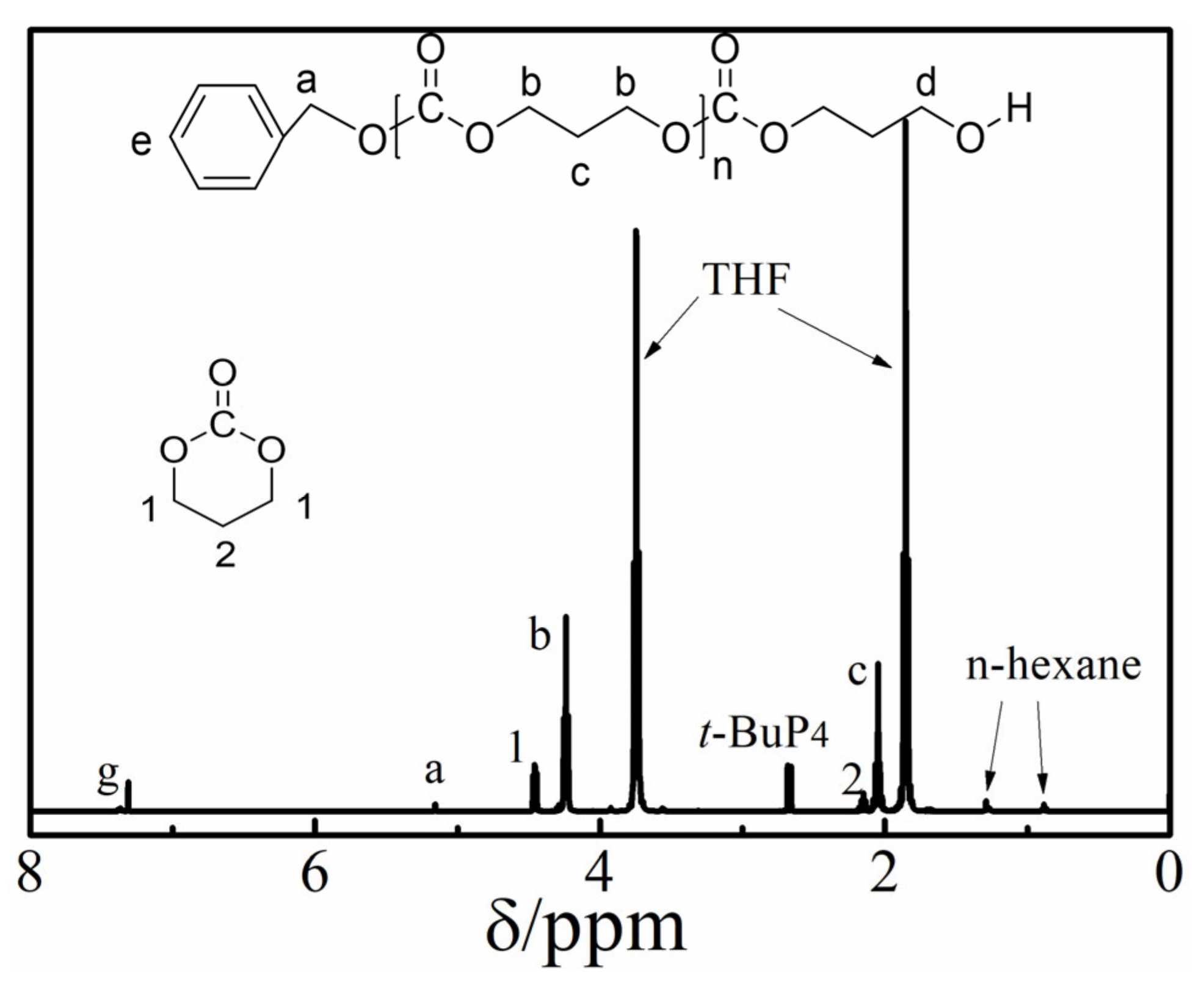
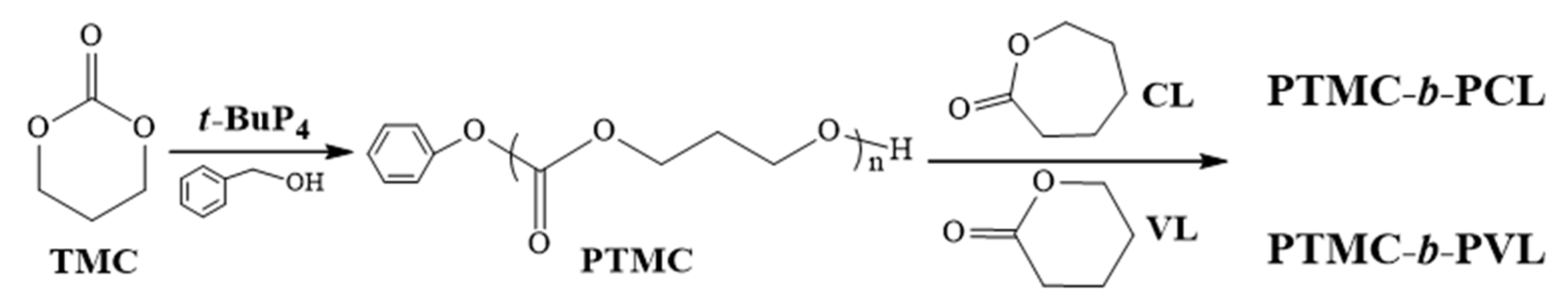
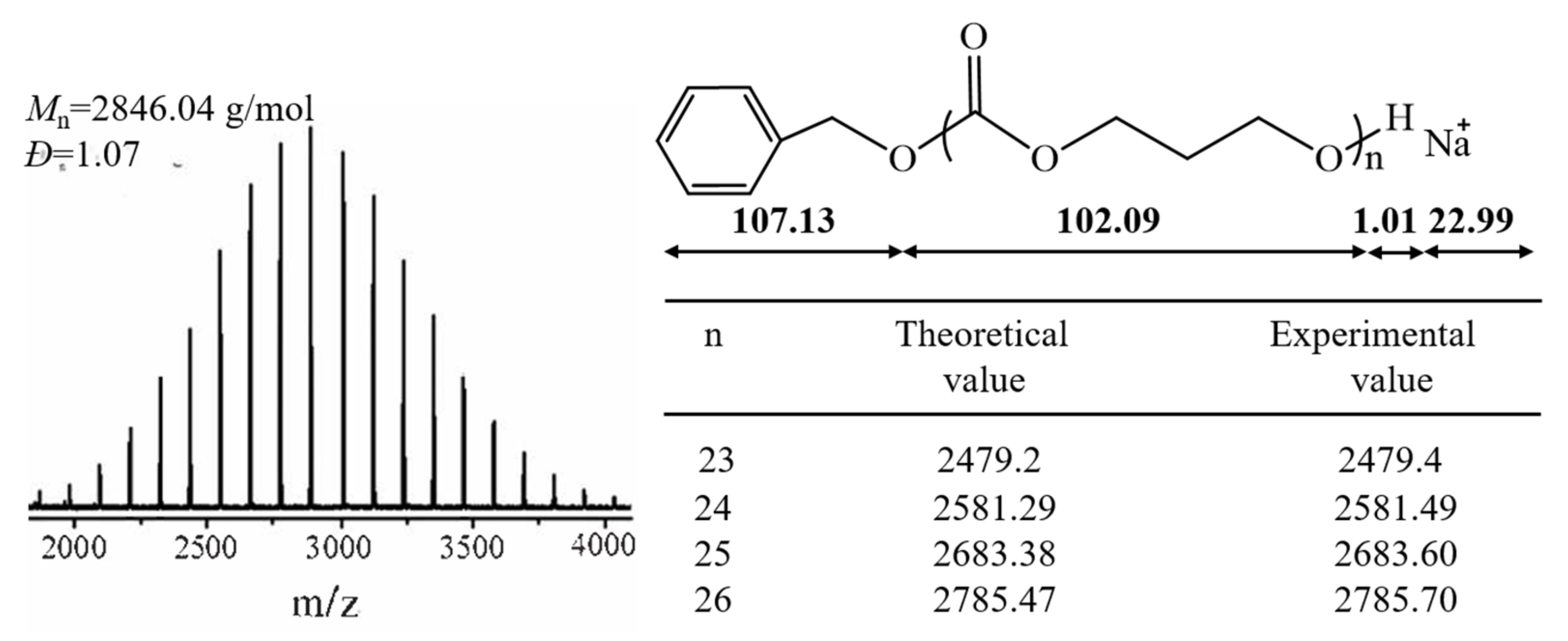




| Samples | Monomer | [M]0/[I]0 b | Time (h) | Conv. (%) c | Mn,NMR × 10−4 (g/mol) c | Mn,theo × 10−4 (g/mol) d | Mn,SEC × 10−4 (g/mol) e | Ðe |
|---|---|---|---|---|---|---|---|---|
| S1 | TMC | 10 | 1 | 99 | 0.10 | 0.11 | 0.13 | 1.07 |
| S2 | TMC | 30 | 4 | 97 | 0.30 | 0.33 | 0.29 | 1.07 |
| S3 | TMC | 50 | 8 | 95 | 0.48 | 0.54 | 0.55 | 1.18 |
| S4 | TMC | 100 | 10 | 94 | 1.10 | 1.04 | 1.22 | 1.19 |
| S5 | TMC | 200 | 17 | 95 | 1.90 | 2.07 | 2.16 | 1.26 |
| S6 | TMC | 500 | 24 | 85 | 3.52 | 4.85 | 4.32 | 1.30 |
| S7 | VL | 30 | 1 | 99 | 0.30 | 0.30 | 0.41 | 1.18 |
| S8 | VL | 50 | 2 | 98 | 0.42 | 0.51 | 0.52 | 1.20 |
| S9 | CL | 100 | 4 | 98 | 0.94 | 1.08 | 0.98 | 1.25 |
| S10 | CL | 50 | 4 | 98 | 0.45 | 0.49 | 0.52 | 1.55 |
| Run | Monomer | Initiator | Conv.(%) b | Mn,NMR (g/mol) b | Mn,theo (g/mol) c | Mn,SEC (g/mol) d | Ðd |
|---|---|---|---|---|---|---|---|
| 1 | TMC | AHA | 97 | 1960 | 2148 | 2500 | 1.07 |
| 2 | TMC | mPEG | 98 | 5960 | 6000 | 6900 | 1.17 |
| 3 | VL | PTMC e | 99 | -- | -- | 7500 | 1.24 |
| 4 | CL | PTMC e | 90 | -- | -- | 8000 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Luo, X.; Li, X. Ring-Opening Polymerization of Trimethylene Carbonate with Phosphazene Organocatalyst. Polymers 2023, 15, 720. https://doi.org/10.3390/polym15030720
Zhu J, Luo X, Li X. Ring-Opening Polymerization of Trimethylene Carbonate with Phosphazene Organocatalyst. Polymers. 2023; 15(3):720. https://doi.org/10.3390/polym15030720
Chicago/Turabian StyleZhu, Jianglin, Xiaoming Luo, and Xin Li. 2023. "Ring-Opening Polymerization of Trimethylene Carbonate with Phosphazene Organocatalyst" Polymers 15, no. 3: 720. https://doi.org/10.3390/polym15030720
APA StyleZhu, J., Luo, X., & Li, X. (2023). Ring-Opening Polymerization of Trimethylene Carbonate with Phosphazene Organocatalyst. Polymers, 15(3), 720. https://doi.org/10.3390/polym15030720






