On the Response to Aging of OPEFB/Acrylic Composites: A Fungal Degradation Perspective
Abstract
1. Introduction
2. Materials and Methods
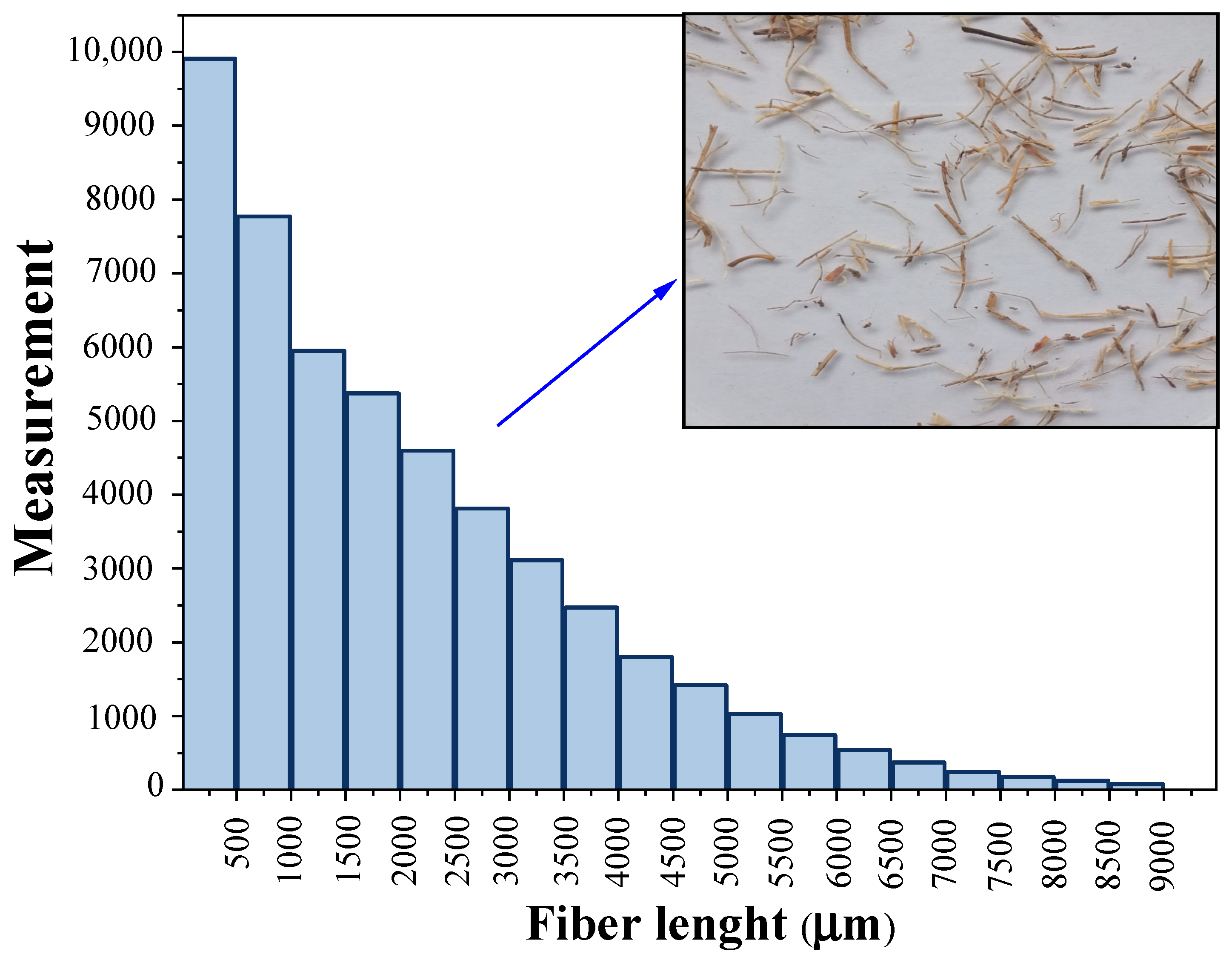
2.1. Materials
2.2. Composite Elaboration and Accelerated Aging
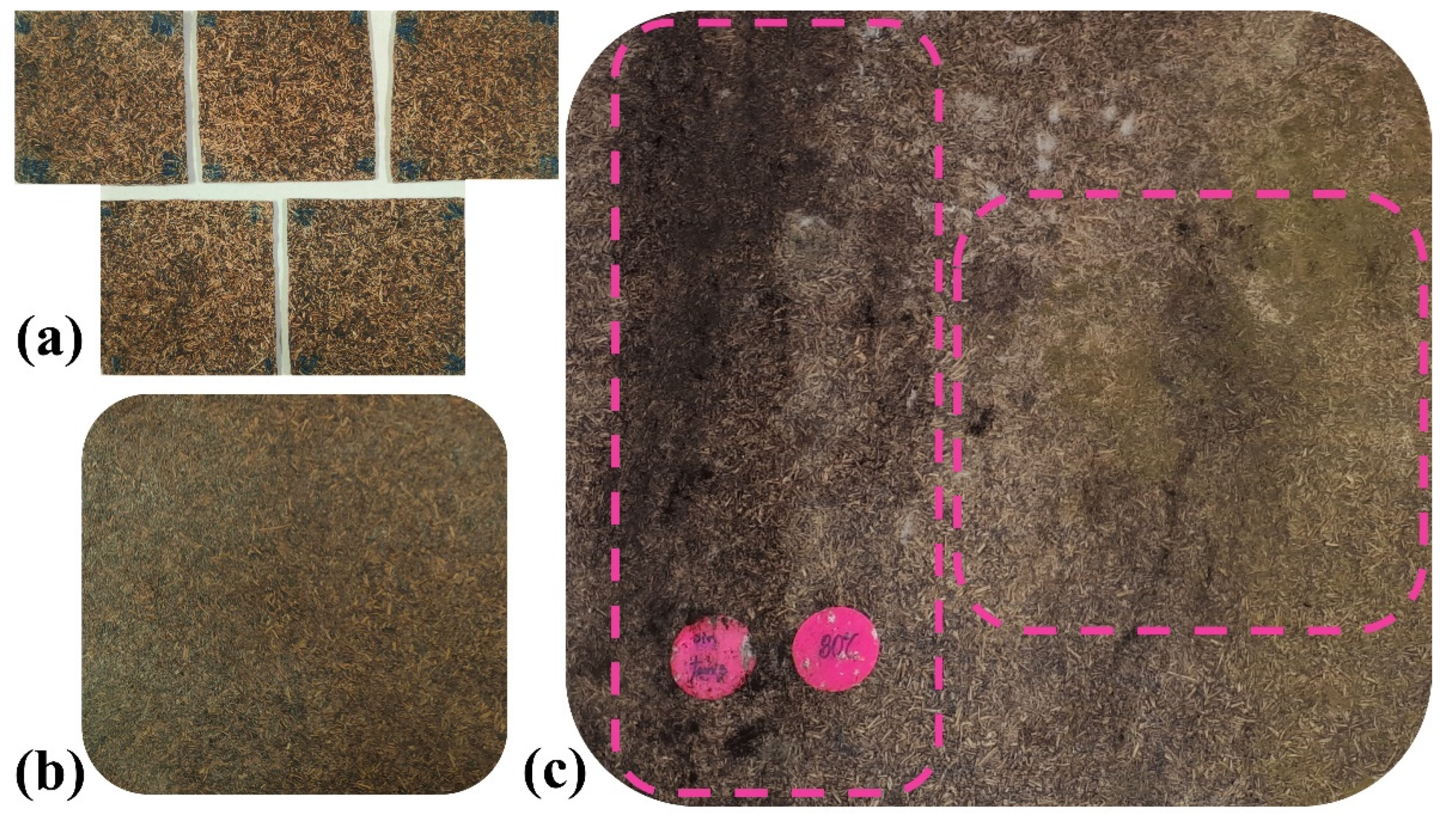
2.3. Fungal Degradation Assessment
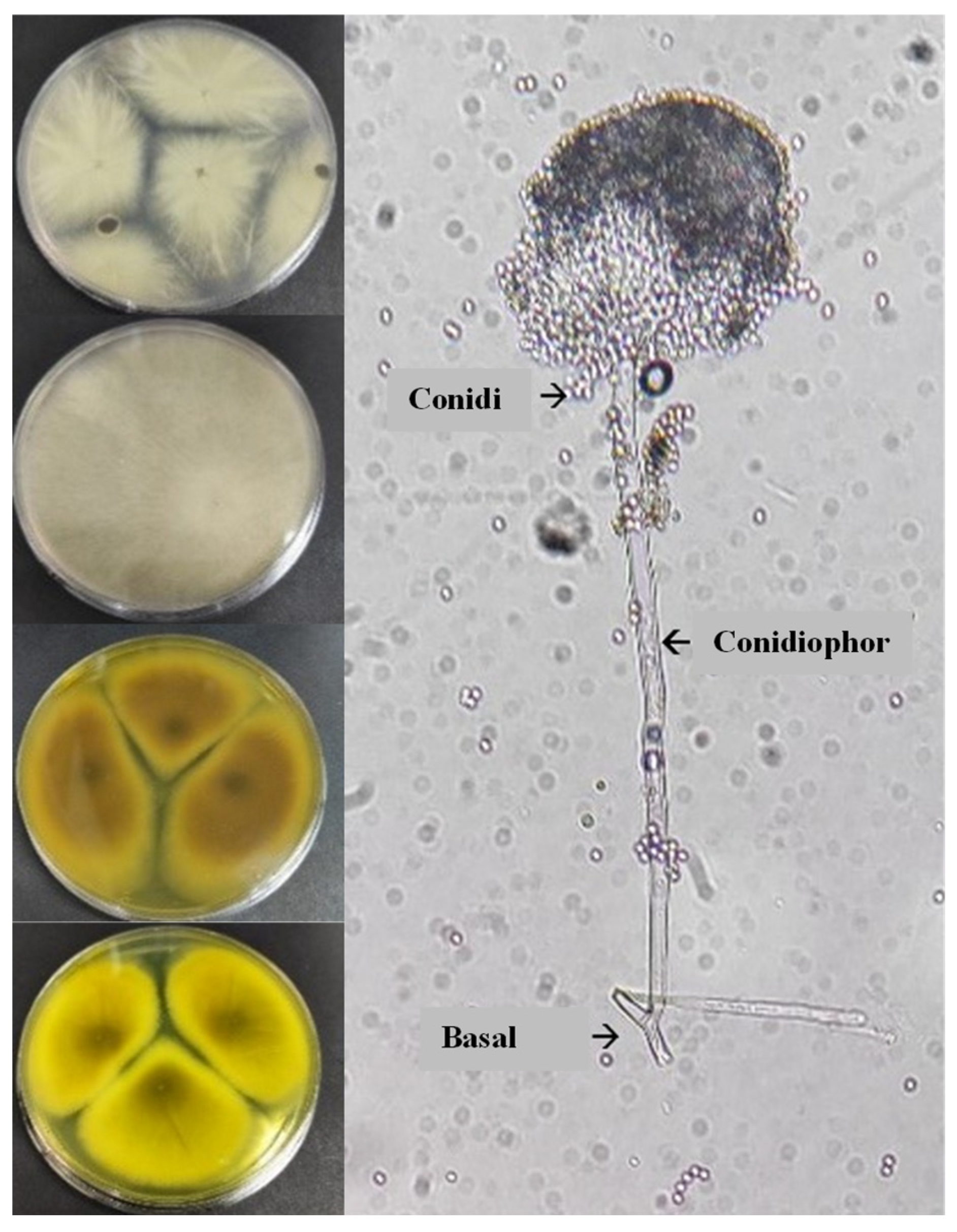
2.3.1. Fungal Identification
2.3.2. Testing and Characterization
3. Results and Discussion
3.1. Fungal Identification
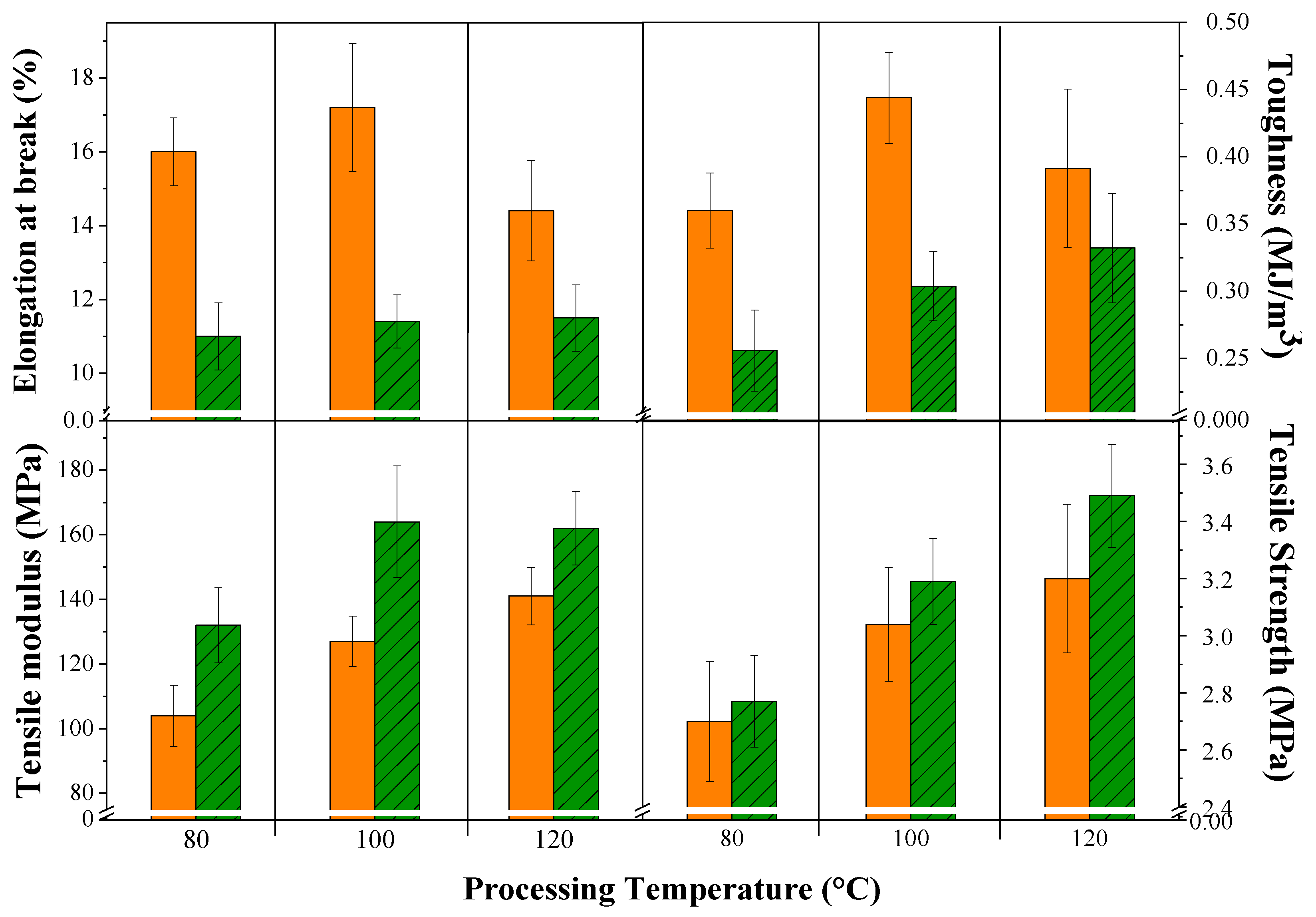
3.2. Weight Loss and Mechanical Evaluation
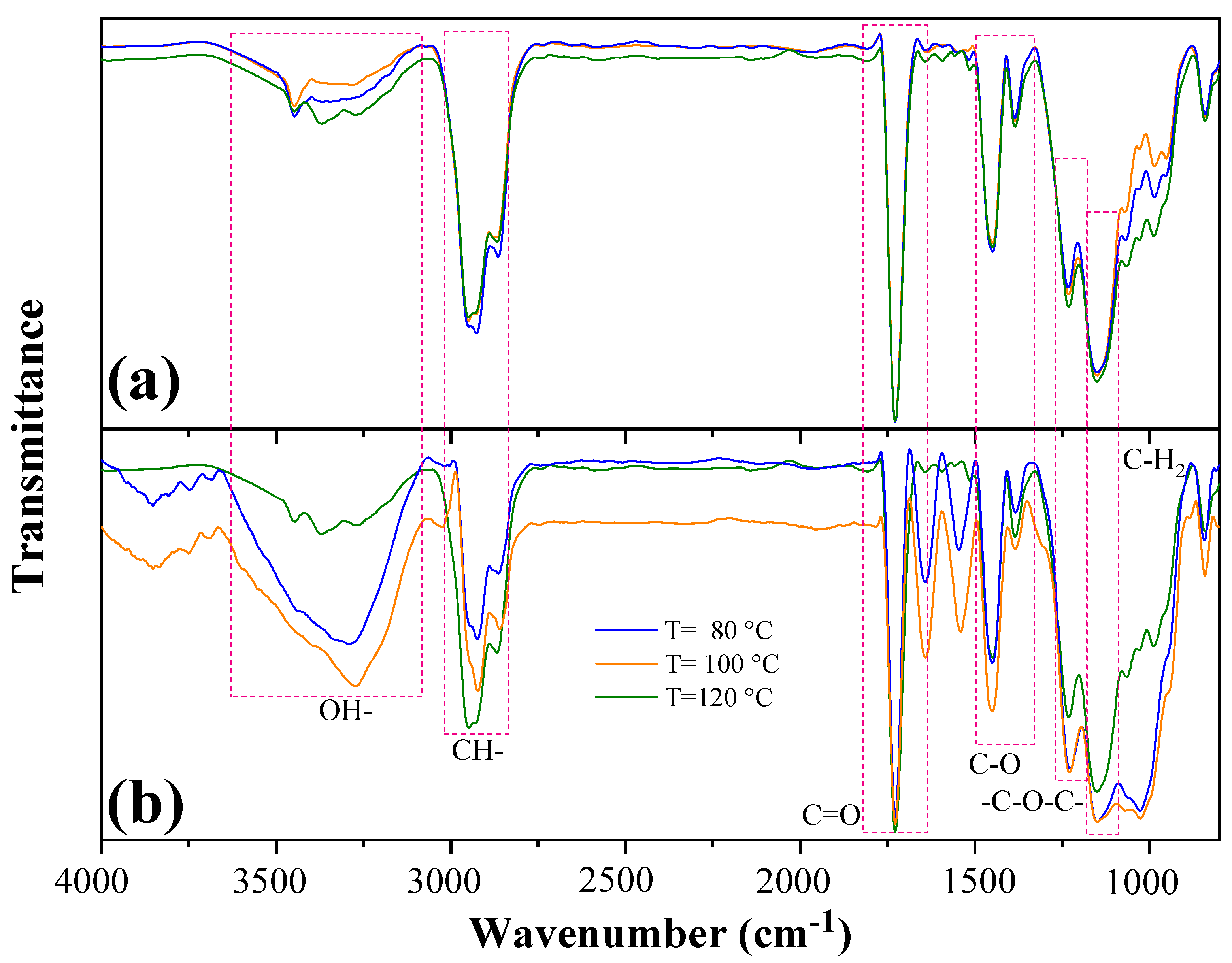
3.3. FTIR Analysis
3.4. TGA
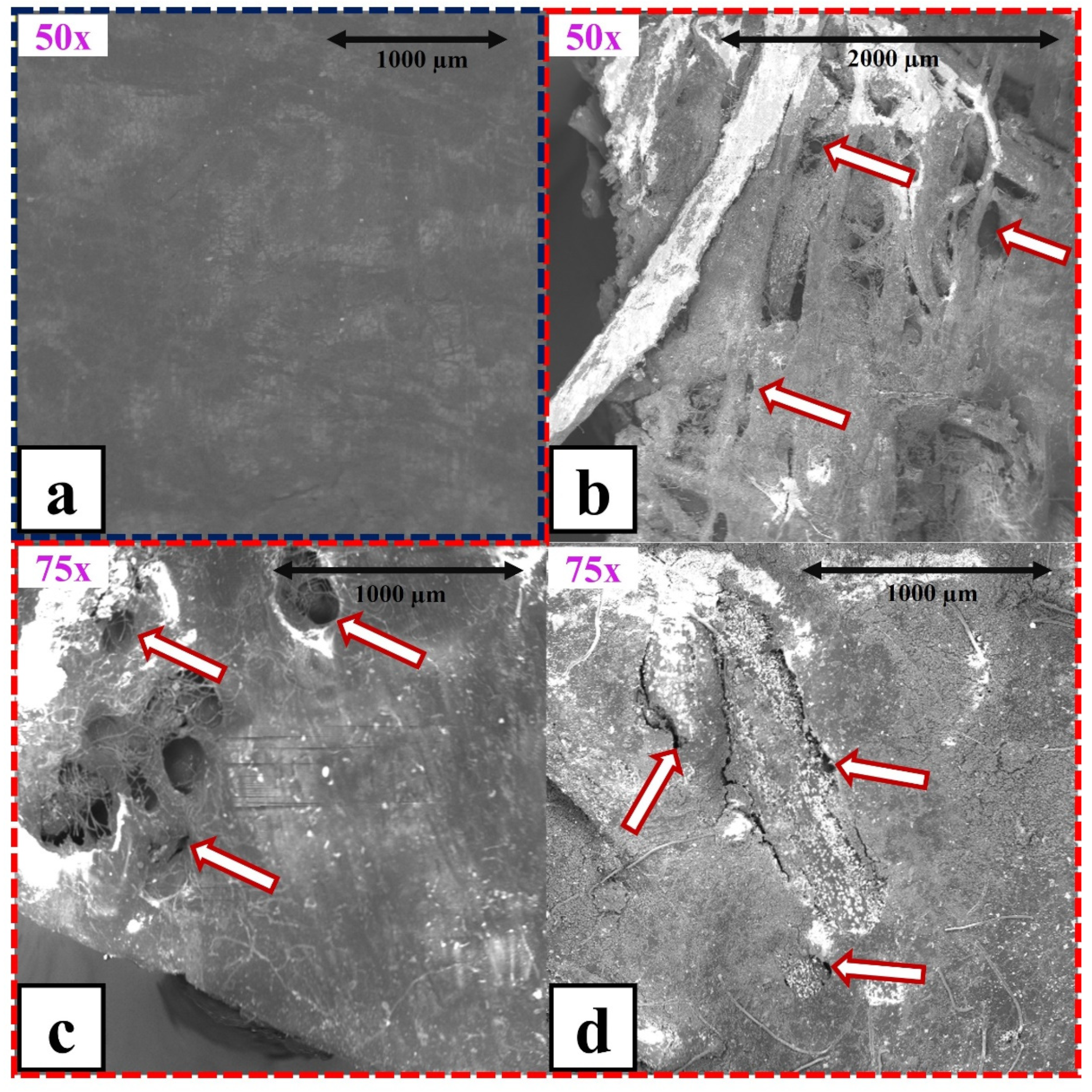
3.5. Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boniek, D.; de Abreu, C.; Batista, A.; Resende, M. Evaluation of Microbiological Air Parameters and the Fungal Community Involved in the Potential Risks of Biodeterioration in a Cultural Heritage of Humanity, Ouro Preto, Brazil. Folia Microbiol. 2021, 66, 797–807. [Google Scholar] [CrossRef]
- Kazemian, N.; Pakpour, S.; Milani, A.; Klironomos, J. Environmental Factors Influencing Fungal Growth on Gypsum Boards and Their Structural Biodeterioration: A University Campus Case Study. PLoS ONE 2019, 14, e0220556. [Google Scholar] [CrossRef]
- Joseph, E. Microorganisms in the Deterioration and Preservation of Cultural Heritage, 1st ed.; Springer Nature Switzerland: Cham, Switzerland, 2021. [Google Scholar]
- Madigan, M.; Bender, K.; Buckley, D.; Sattley, M.; Stahl, D. Brock Biology of Microorganisms, 16th ed.; Pearson Education Limited: Harlow, UK, 2022; ISBN 9780134874401. [Google Scholar]
- Stanaszek-Tomal, E. Environmental Factors Causing the Development of Microorganisms on the Surfaces of National Cultural Monuments Made of Mineral Building Materials—Review. Coatings 2020, 10, 1203. [Google Scholar] [CrossRef]
- Hernández, C.; Orjuela, D.; Solano, J. Degradation Methods of Recovered Expanded Polypropylene and Polystyrene Mixtures and Recovered Polypropylene, High Density Polyethylene, Rice Husk and Silane Coupling Agent. In Proceedings of the Congreso de Desarrollo Sostenible; ; 28-29 September 2020, Bogota, Colombia, 28–29 September 2020. [Google Scholar]
- Stanaszek-Tomal, E. Influence of Pore Structure on Humidity Parameters of Cement-Polymer Mortars Contaminated with Filamentous Fungi. PLoS ONE 2020, 15, e0231347. [Google Scholar] [CrossRef]
- Giri, B.; Varma, A. Microorganisms in Saline Environments: Strategies and Functions, 1st ed.; Springer Nature Switzerland: Cham, Switzerland, 2019; Volume 56. [Google Scholar]
- Muscat, Y.; Farrugia, C.; Camilleri, L.; Arias-Moliz, M.; Valdramidis, V.; Camilleri, J. Investigation of Acrylic Resin Disinfection Using Chemicals and Ultrasound. J. Prosthodont. 2018, 27, 461–468. [Google Scholar] [CrossRef]
- Barlow, D.; Biffinger, J.; Estrella, L.; Lu, Q.; Hung, C.; Nadeau, L.; Crouch, A.; Russell, J.; Crookes-Goodson, W. Edge-Localized Biodeterioration and Secondary Microplastic Formation by Papiliotrema Laurentii Unsaturated Biofilm Cells on Polyurethane Films. Langmuir 2020, 36, 1596–1607. [Google Scholar] [CrossRef]
- Lors, C.; Feugeas, F.; Tribollet, B. Interactions Materials—Microorganisms: Concretes and Metals More Resistant to Biodeterioration, 1st ed.; EDP Sciences: Les Ulis, France, 2018; ISBN 9782759822003. [Google Scholar]
- Özdemir, A.; Erguven, G.; Adar, E.; Nuhoglu, Y. Investigation on Microbial Biodeterioration of the Stone Monuments in Yildiz Technical University—Yildiz Campus—Istanbul—Turkey. Curr. Microbiol. 2020, 77, 3288–3299. [Google Scholar] [CrossRef]
- Janczak, K.; Dąbrowska, G.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the Plastics PLA and PET in Cultivated Soil with the Participation of Microorganisms and Plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sanmartín, P. Interactions of Microorganisms and Synthetic Polymers in Cultural Heritage Conservation. Int. Biodeterior. Biodegrad. 2021, 163, 105282. [Google Scholar] [CrossRef]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current Status on the Biodegradability of Acrylic Polymers: Microorganisms, Enzymes and Metabolic Pathways Involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef]
- Zhao, L.; Song, T.; Han, D.; Bao, M.; Lu, J. Hydrolyzed Polyacrylamide Biotransformation in an Up-Flow Anaerobic Sludge Blanket Reactor System: Key Enzymes, Functional Microorganisms, and Biodegradation Mechanisms. Bioprocess Biosyst. Eng. 2019, 42, 941–951. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Ahlgren, J. Microbial Degradation of Polyacrylamide and the Deamination Product Polyacrylate. Int. Biodeterior. Biodegrad. 2019, 139, 24–33. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Hamidian, A.; Yang, M. Biodegradation of Low Molecular Weight Polyacrylamide under Aerobic and Anaerobic Conditions: Effect of the Molecular Weight. Water Sci. Technol. 2020, 81, 301–308. [Google Scholar] [CrossRef]
- Ayu, R.; Khalina, A.; Harmaen, A.; Zaman, K.; Isma, T.; Liu, Q.; Ilyas, R.; Lee, C. Characterization Study of Empty Fruit Bunch (EFB) Fibers Reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers 2020, 12, 1571. [Google Scholar] [CrossRef]
- Pekhtasheva, E.; Neverov, A.; Zaikov, G. Biodamage and Biodegradation of Polymeric Materials: New Frontiers, 1st ed.; Smithers Rapra Technology Ltd: Shawbury, UK, 2012; ISBN 9781847357519. [Google Scholar]
- Naranjo-Ortiz, M.; Gabaldón, T. Fungal Evolution: Major Ecological Adaptations and Evolutionary Transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef]
- Han, Y.; Huang, X.; Wang, Y.; Du, J.; Ma, K.; Chen, Y.; Li, N.; Zhang, Z.; Pan, J. Fungal Community and Biodeterioration Analysis of Hull Wood and Its Storage Environment of the Nanhai No. 1 Shipwreck. Front. Microbiol. 2021, 11, 609475. [Google Scholar] [CrossRef]
- Sarkar, M.; Maiti, M.; Malik, M.; Xu, S. Development of Anti-Bio Deteriorate Sustainable Geopolymer by SiO2 NPs Decorated ZnO NRs. Adv. Mater. Lett. 2019, 10, 128–131. [Google Scholar] [CrossRef]
- Yeh, S.; Hu, C.; Rizkiana, M.; Kuo, C. Effect of Fiber Size, Cyclic Moisture Absorption and Fungal Decay on the Durability of Natural Fiber Composites. Constr. Build. Mater. 2021, 286, 122819. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Dominic, M.; Parameswaranpillai, J. Natural Fillers as Potential Modifying Agents for Epoxy Composition: A Review. Polymers 2022, 14, 265. [Google Scholar] [CrossRef]
- Kalimuthu, M.; Nagarajan, R.; Ayrilmis, N.; Indira-Devi, M.; Siengchin, S.; Mohammad, F.; Al-Lohedan, H. An Overview of Endurance and Ageing Performance under Various Environmental Conditions of Hybrid Polymer Composites. J. Mater. Res. Technol. 2020, 9, 15962–15988. [Google Scholar] [CrossRef]
- Kudva, A.; Gt, M.; Pai, D. Physical, Thermal, Mechanical, Sound Absorption and Vibration Damping Characteristics of Natural Fiber Reinforced Composites and Hybrid Fiber Reinforced Composites: A Review. Cogent. Eng. 2022, 9, 2107770. [Google Scholar] [CrossRef]
- Fiore, V.; Sanfilippo, C.; Calabrese, L. Dynamic Mechanical Behavior Analysis of Flax/Jute Fiber-Reinforced Composites under Salt-Fog Spray Environment. Polymers 2020, 12, 716. [Google Scholar] [CrossRef]
- Aguilar, A.; Valle, V.; Almeida-Naranjo, C.; Naranjo, Á.; Cadena, F.; Kreiker, J.; Raggiotti, B. Characterization Dataset of Oil Palm Empty Fruit Bunch (OPEFB) Fibers—Natural Reinforcement/Filler for Materials Development. Data Brief 2022, 45, 108618. [Google Scholar] [CrossRef]
- Valle, V.; Aguilar, A.; Kreiker, J.; Raggiotti, B.; Cadena, F. Oil Palm Empty Fruit Bunch (OPEFB) Fiber-Reinforced Acrylic Thermoplastic Composites: Effect of Salt Fog Aging on Tensile, Spectrophotometric, and Thermogravimetric Properties. Int. J. Polym. Sci. 2022, 2022, 6372264. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.; Valle, V.; Aguilar, A.; Cadena, F.; Kreiker, J.; Raggiotti, B. Water Absorption Behavior of Oil Palm Empty Fruit Bunch (OPEFB) and Oil Palm Kernel Shell (OPKS) as Fillers in Acrylic Thermoplastic Composites. Materials 2022, 15, 5015. [Google Scholar] [CrossRef]
- Zhai, X.; Ren, Y.; Wang, N.; Guan, F.; Agievich, M.; Duan, J.; Hou, B. Microbial Corrosion Resistance and Antibacterial Property of Electrodeposited Zn–Ni–Chitosan Coatings. Molecules 2019, 24, 1974. [Google Scholar] [CrossRef]
- Lani, N.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, Characterization, and Application of Nanocellulose from Oil Palm Empty Fruit Bunch Fiber as Nanocomposites. J. Nanomater. 2014, 2014, 702538. [Google Scholar] [CrossRef]
- Tsang, C.; Tang, J.; Lau, S.; Woo, P. Taxonomy and Evolution of Aspergillus, Penicillium and Talaromyces in the Omics Era—Past, Present and Future. Comput. Struct. Biotechnol. J. 2018, 16, 197–210. [Google Scholar] [CrossRef]
- Nyongesa, B.; Okoth, S.; Ayugi, V. Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi County, Kenya. Adv. Microbiol. 2015, 5, 205–229. [Google Scholar] [CrossRef]
- Ortega-Rosas, C.; Calderón-Ezquerro, M.; Gutiérrez-Ruacho, O. Fungal Spores and Pollen Are Correlated with Meteorological Variables: Effects in Human Health at Hermosillo, Sonora, Mexico. Int. J. Environ. Health Res. 2019, 30, 677–695. [Google Scholar] [CrossRef]
- Zulkifli, N.; Zakaria, L. Morphological and Molecular Diversity of Aspergillus From Corn Grain Used as Livestock Feed. Hayati 2017, 24, 26–34. [Google Scholar] [CrossRef]
- de Vries, R.; Visser, J. Aspergillus Enzymes Involved in Degradation of Plant Cell Wall Polysaccharides. Microbiol. Mol. Biol. Rev. 2001, 65, 497–522. [Google Scholar] [CrossRef] [PubMed]
- Culleton, H.; Mckie, V.; de Vries, R. Physiological and Molecular Aspects of Degradation of Plant Polysaccharides by Fungi: What Have We Learned from Aspergillus? Biotechnol. J. 2013, 8, 884–894. [Google Scholar] [CrossRef]
- Ottenheim, C.; Verdejo, C.; Zimmermann, W.; Wu, J. Hemicellulase Production by Aspergillus Niger DSM 26641 in Hydrothermal Palm Oil Empty Fruit Bunch Hydrolysate and Transcriptome Analysis. J. Biosci. Bioeng. 2014, 118, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.; Pakpour, S.; Kazemian, N.; Klironomos, J.; Stoeffler, K.; Rho, D.; Denault, J.; Milani, A. Effect of Fungal Deterioration on Physical and Mechanical Properties of Hemp and Flax Natural Fiber Composites. Materials 2017, 10, 1252. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Ungtrakul, T.; Muthukumar, C.; Thiagamani, S.; Nagarajan, R.; Siengchin, S.; Pulikkalparambil, H.; Parameswaranpillai, J.; Ayrilmis, N. Performance of Sisal/Hemp Bio-Based Epoxy Composites Under Accelerated Weathering. J. Polym. Environ. 2021, 29, 624–636. [Google Scholar] [CrossRef]
- Moshiul-Alam, A.; Beg, M.; Mina, M.; Mamun, A.; Bledzki, A. Degradation and Stability of Green Composites Fabricated from Oil Palm Empty Fruit Bunch Fiber and Polylactic Acid: Effect of Fiber Length. J. Compos. Mater. 2014, 49, 3103–3114. [Google Scholar] [CrossRef]
- Célino, A.; Gonçalves, O.; Jacquemin, F.; Fréour, S. Qualitative and Quantitative Assessment of Water Sorption in Natural Fibres Using ATR-FTIR Spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, L.; Yang, W.; Shi, W.; Tong, Y.; Chai, L.; Gao, S.; Liao, Q. Simultaneous Immobilization of Cadmium and Lead in Contaminated Soils by Hybrid Bio-Nanocomposites of Fungal Hyphae and Nano-Hydroxyapatites. Environ. Sci. Pollut. Res. 2018, 25, 11970–11980. [Google Scholar] [CrossRef]
- Pană, A.; Ordodi, V.; Rusu, G.; Gherman, V.; Bandur, G.; Rusnac, L.; Dumitrel, G. Biodegradation Pattern of Glycopolymer Based on D-Mannose Oligomer and Hydroxypropyl Acrylate. Polymers 2020, 12, 704. [Google Scholar] [CrossRef]
- Geng, W.; Venditti, R.; Pawlak, J.; Chang, H.; Pal, L.; Ford, E. Carboxymethylation of Hemicellulose Isolated from Poplar (Populus Grandidentata) and Its Potential in Water-Soluble Oxygen Barrier Films. Cellulose 2020, 27, 3359–3377. [Google Scholar] [CrossRef]
- Morales, A.; Labidi, J.; Gullón, P. Effect of the Formulation Parameters on the Absorption Capacity of Smart Lignin-Hydrogels. Eur. Polym. J. 2020, 129, 109631. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, T.; Li, C.; Jiang, X.; Wu, J.; Wu, B. Electrical Strength and Physicochemical Performances of HTV Silicone Rubber under Salt-Fog Environment with DC Energized. Polymers 2020, 12, 324. [Google Scholar] [CrossRef]
- Nguyen, D.; Makke, A.; Montay, G. A Pull-Out Test to Characterize the Fiber/Matrix Interfaces Aging of Hemp Fiber Reinforced Polypropylene Composites. In Proceedings of the Proceedings of the International Conference on Advances in Computational Mechanics, Phu Quoc Island, Vietnam, 2–4 August 2017; Nguyen-Xuan, H., Phung-Van, P., Rabczuk, T., Eds.; Springer Nature: Singapore, 2018; Volume 5, pp. 477–484. [Google Scholar]
- Bayerl, T.; Geith, M.; Somashekar, A.; Bhattacharyya, D. Influence of Fibre Architecture on the Biodegradability of FLAX/PLA Composites. Int. Biodeterior. Biodegrad. 2014, 96, 18–25. [Google Scholar] [CrossRef]
- Odalanowska, M.; Cofta, G.; Woźniak, M.; Ratajczak, I.; Rydzkowski, T.; Borysiak, S. Bioactive Propolis-Silane System as Antifungal Agent in Lignocellulosic-Polymer Composites. Materials 2022, 15, 3435. [Google Scholar] [CrossRef]
- Wiejak, A.; Francke, B. Testing and Assessing Method for the Resistance of Wood-Plastic Composites to the Action of Destroying Fungi. Materials 2021, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Naumann, A.; Seefeldt, H.; Stephan, I.; Braun, U.; Noll, M. Material Resistance of Flame Retarded Wood-Plastic Composites against Fire and Fungal Decay. Polym. Degrad. Stab. 2012, 97, 1189–1196. [Google Scholar] [CrossRef]
- Catto, A.; Montagna, L.; Almeida, S.; Silveira, R.; Santana, R. Wood Plastic Composites Weathering: Effects of Compatibilization on Biodegradation in Soil and Fungal Decay. Int. Biodeterior. Biodegrad. 2016, 109, 11–22. [Google Scholar] [CrossRef]
- Sudár, A.; López, M.; Keledi, G.; Vargas-García, M.; Suárez-Estrella, F.; Moreno, J.; Burgstaller, C.; Pukánszky, B. Ecotoxicity and Fungal Deterioration of Recycled Polypropylene/Wood Composites: Effect of Wood Content and Coupling. Chemosphere 2013, 93, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Darabi, P.; Gril, J.; Thevenon, M.; Karimi, A.; Azadfalah, M. Evaluation of High Density Polyethylene Composite Filled with Bagasse after Accelerated Weathering Followed by Biodegradation. Bioresources 2012, 7, 5258–5267. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.; Freitag, C.; Morrell, J. Effects of Wood Fiber Esterification on Properties, Weatherability and Biodurability of Wood Plastic Composites. Polym. Degrad. Stab. 2013, 98, 1348–1361. [Google Scholar] [CrossRef]
- Schirp, A.; Wolcott, M. Influence of Fungal Decay and Moisture Absorption on Mechanical Properties of Extruded Wood-Plastic Composites. Wood Fiber Sci. 2005, 37, 643–652. [Google Scholar]
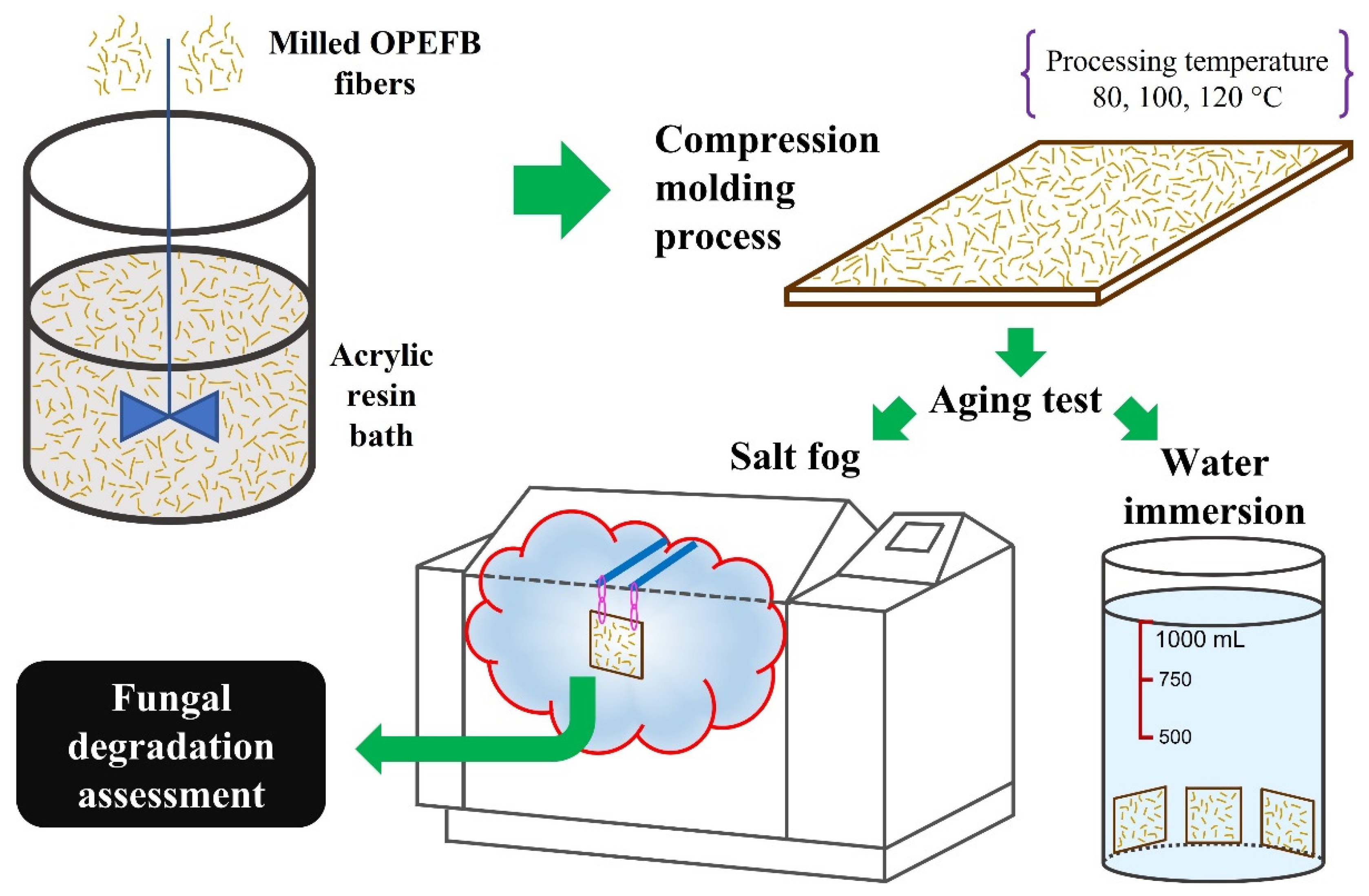


| Aging Test | Reference Standards | Conditions * | Sample Dimensions (Length × Width × Thickness) | Exposure Time |
|---|---|---|---|---|
| Water immersion | ASTM D5529 and UNE-EN-2378 | Bath temperature: 23 °C | 50 mm × 50 mm × 2 mm | 800 h |
| Salt fog | ASTM G85—Annex 5 Prohesion cycle | E.S.: 0.05 wt.% NaCl + 0.35 wt.% (NH4)2SO4 E.C.: fog (25 °C, 1 h) + dry-off (35 °C, 1 h) | 250 mm × 250 mm × 2 mm | 400 h |
| ASTM B117 ** | E.S.: 8.00 wt.% NaCl E.C.: continuous fog (35 °C) |
| Composite | Aging Testing | Fungal Identification | Deterioration Effects | Reference |
|---|---|---|---|---|
| pine wood flour/PP | Modified European standard EN 113 | Coriolus versicolor | W.L. = 2.5% Increase E.B. = 43.7% Decrease T.S. = 3.1% Increase T.M. = 2.7% | [52] |
| Coniophora puteana | W.L. = 2.8% Increase E.B. = 8.2% Increase T.S. = 2.4% Increase T.M. = 0.6% | |||
| Chaetomium globosumdurante | W.L. = 0.6% Decrease E.B. = 29.1% Increase T.S. = 0.5% Increase T.M. = 4.6% | |||
| wood flour/HDPE | European standard ENV 12038 | Coniophora puteana | W.L. = 2.4% Decrease F.R. = 7.6% | [53] |
| wood flour/PP | W.L. = 2.7% Decrease F.R. = 9.6% | |||
| wood flour/PP | European standard EN 113 | Laetiporus sulfureus + Lenzites betulina | W.L. = 0.1–1.0% | [24] |
| hemp/PP | Non-specified | Chaetomium globosum | W.L. = 9.8% Decrease T.M. = 38.0% | [41] |
| flax/PP | W.L. = 10.9% Decrease T.M. = 53.9% | |||
| eucalyptus wood flour/PP-EVA | Method of [54] | Fuscoporia ferrea | W.L. = 14.0% | [55] |
| Trametes villosa | W.L. = 4.2% | |||
| Trametes versicolor | W.L. = 5.4% | |||
| Pycnoporus sanguineus | W.L. = 1.8% | |||
| wood/PP-EPDE | ISO 846 | Aspergillus niger + Penicillium funiculosum + Paecilomyces variotii + Gliocladium virens + Chaetomium globosum | W.L. < 3.0% | [56] |
| sugar cane bagasse/HDPE-PP | Agar-plate test method | Coniophora puteana | W.L. = 0.2–.6% | [57] |
| poplar wood fibers/HDPE | AWPA standard E10-06 | Trametes versicolor | W.L. = −2.0–0.5% | [58] |
| Gloeophyllum trabeum | W.L. = −0.7–0.1% | |||
| wood/PE | ASTM D2017 | Trametes versicolor | W.L. = 1.0–6.0% Decrease F.S. = 83.0% | [59] |
| OPEFB/acrylic | Modified ASTM B117 | Aspergillus spp. | W.L. = 0.4–2.7% Decrease E.B. = 20.0–34.0% Decrease T.S. = 15.0–32.0% Increase T.M. = 15.0–29.0% | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, V.; Aguilar, A.D.; Yánez, P.; Almeida-Naranjo, C.E.; Cadena, F.; Kreiker, J.; Raggiotti, B. On the Response to Aging of OPEFB/Acrylic Composites: A Fungal Degradation Perspective. Polymers 2023, 15, 704. https://doi.org/10.3390/polym15030704
Valle V, Aguilar AD, Yánez P, Almeida-Naranjo CE, Cadena F, Kreiker J, Raggiotti B. On the Response to Aging of OPEFB/Acrylic Composites: A Fungal Degradation Perspective. Polymers. 2023; 15(3):704. https://doi.org/10.3390/polym15030704
Chicago/Turabian StyleValle, Vladimir, Alex Darío Aguilar, Paola Yánez, Cristina E. Almeida-Naranjo, Francisco Cadena, Jerónimo Kreiker, and Belén Raggiotti. 2023. "On the Response to Aging of OPEFB/Acrylic Composites: A Fungal Degradation Perspective" Polymers 15, no. 3: 704. https://doi.org/10.3390/polym15030704
APA StyleValle, V., Aguilar, A. D., Yánez, P., Almeida-Naranjo, C. E., Cadena, F., Kreiker, J., & Raggiotti, B. (2023). On the Response to Aging of OPEFB/Acrylic Composites: A Fungal Degradation Perspective. Polymers, 15(3), 704. https://doi.org/10.3390/polym15030704








