Recent Advances in Poly(Ionic Liquid)-Based Membranes for CO2 Separation
Abstract
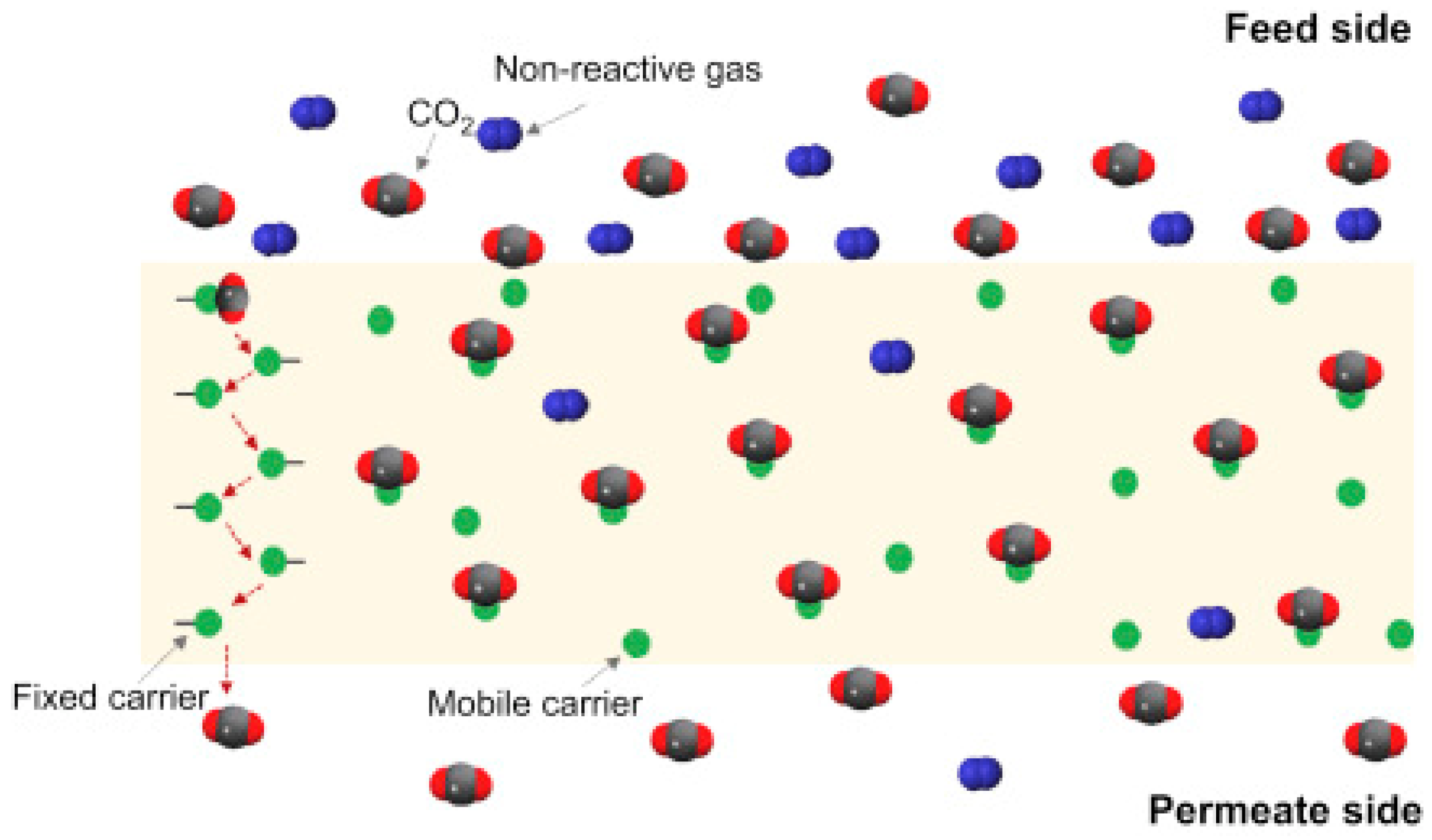
1. Introduction
2. Recent Studies
2.1. Neat PIL Membranes
2.2. PIL-IL Composite Membranes
2.3. PIL-Polymer Blend Membranes
2.4. PIL-Based Block Copolymer Membranes
2.5. PIL-Based Mixed Matrix Membranes (MMM)
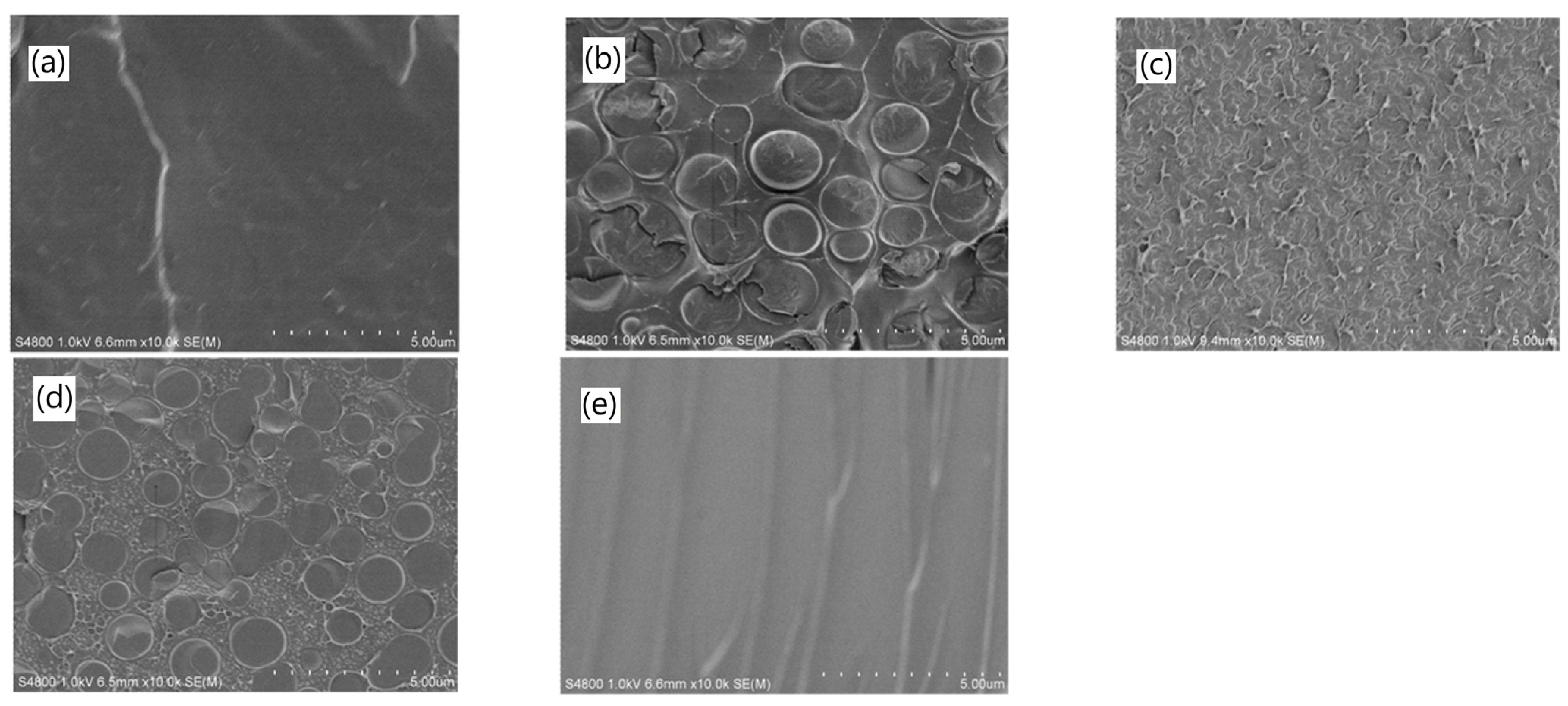
3. Advanced Structural Characterization of PIL-Based Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Popovski, V. The Implementation of the Paris Agreement on Climate Change (Law, Ethics and Governance); Routledge: Abingdon, UK, 2018. [Google Scholar]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Hou, M.; Qi, W.; Li, L.; Xu, R.; Xue, J.; Zhang, Y.; Song, C.; Wang, T. Carbon molecular sieve membrane with tunable microstructure for CO2 separation: Effect of multiscale structures of polyimide precursors. J. Membr. Sci. 2021, 635, 119541. [Google Scholar] [CrossRef]
- Araújo, T.; Andrade, M.; Bernardo, G.; Mendes, A. Stable cellulose-based carbon molecular sieve membranes with very high selectivities. J. Membr. Sci. 2022, 641, 119852. [Google Scholar] [CrossRef]
- Haider, S.; Lindbråthen, A.; Lie, J.A.; Andersen, I.C.T.; Hägg, M.-B. CO2 separation with carbon membranes in high pressure and elevated temperature applications. Sep. Purif. Technol. 2017, 190, 177–189. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; Lopes, T.D.S.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Lei, L.; Bai, L.; Lindbråthen, A.; Pan, F.; Zhang, X.; He, X. Carbon membranes for CO2 removal: Status and perspectives from materials to processes. Chem. Eng. J. 2020, 401, 126084. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Gao, H.; Bai, L.; Han, J.; Yang, B.; Zhang, S.; Zhang, X. Functionalized ionic liquid membranes for CO2 separation. Chem. Commun. 2018, 54, 12671–12685. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Zhang, M.; Semiat, R.; He, X. Recent advances in Poly(ionic liquids) membranes for CO2 separation. Sep. Purif. Technol. 2022, 299, 121784. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and Performance of Polymerizable Room-Temperature Ionic Liquids as Gas Separation Membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Tang, J.; Sun, W.; Tang, H.; Radosz, M.; Shen, Y. Enhanced CO2 Absorption of Poly(ionic liquid)s. Macromolecules 2005, 38, 2037–2039. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Radosz, M.; Shen, Y. Poly(ionic liquid)s as new materials for CO2 absorption. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5477–5489. [Google Scholar] [CrossRef]
- Bara, J.E.; Hatakeyama, E.S.; Gin, D.L.; Noble, R.D. Improving CO2 permeability in polymerized room-temperature ionic liquid gas separation membranes through the formation of a solid composite with a room-temperature ionic liquid. Polym. Adv. Technol. 2008, 19, 1415–1420. [Google Scholar] [CrossRef]
- Mulder, M. (Ed.) Transport in Membranes. In Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Araújo, T.; Bernardo, G.; Mendes, A. Cellulose-Based Carbon Molecular Sieve Membranes for Gas Separation: A Review. Molecules 2020, 25, 3532. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.C.; Sousa, J.; Mendes, A. Chapter 13—Facilitated Transport Membranes for CO2/H2 Separation. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Favvas, E.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–384. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M.; Jaschik, M.; Warmuzinski, K.; Jansen, J.C.; Vankelecom, I.F.J. Poly(vinylbenzyl chloride)-based poly(ionic liquids) as membranes for CO2 capture from flue gas. J. Mater. Chem. A 2017, 5, 19808–19818. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Loïs, S.; Sheridan, E.; et al. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, C.; Yu, Y.; Hao, T.; Wang, H.; Ding, X.; Meng, J. Tuning the microstructure of crosslinked Poly(ionic liquid) membranes and gels via a multicomponent reaction for improved CO2 capture performance. J. Membr. Sci. 2020, 593, 117405. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Ab Ranii, M.A.; Lickiss, P.D.; Welton, T.; Marrucho, I.M. Study on Gas Permeation and CO2 Separation through Ionic Liquid-Based Membranes with Siloxane-Functionalized Cations. Ind. Eng. Chem. Res. 2017, 56, 2229–2239. [Google Scholar] [CrossRef]
- Teodoro, R.M.; Tomé, L.C.; Mantione, D.; Mecerreyes, D.; Marrucho, I.M. Mixing poly(ionic liquid)s and ionic liquids with different cyano anions: Membrane forming ability and CO2/N2 separation properties. J. Membr. Sci. 2018, 552, 341–348. [Google Scholar] [CrossRef]
- Tomé, L.C.; Guerreiro, D.C.; Teodoro, R.M.; Alves, V.D.; Marrucho, I.M. Effect of polymer molecular weight on the physical properties and CO2/N2 separation of pyrrolidinium-based poly(ionic liquid) membranes. J. Membr. Sci. 2018, 549, 267–274. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Ventaja, L.; Tomé, L.C.; Marrucho, I.M. Towards Biohydrogen Separation Using Poly(Ionic Liquid)/Ionic Liquid Composite Membranes. Membranes 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.S.; Yáñez, M.; Alves, V.D.; Palomar, J.; Moya, C.; Gorri, D.; Tomé, L.C.; Marrucho, I.M. CO2/H2 separation through poly(ionic liquid)–ionic liquid membranes: The effect of multicomponent gas mixtures, temperature and gas feed pressure. Sep. Purif. Technol. 2021, 259, 118113. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Malcaitè, E.; Lozinskaya, E.I.; Shaplov, A.S.; Tomé, L.C.; Marrucho, I.M. Poly(ionic liquid)–Ionic Liquid Membranes with Fluorosulfonyl-Derived Anions: Characterization and Biohydrogen Separation. ACS Sustain. Chem. Eng. 2020, 8, 7087–7096. [Google Scholar] [CrossRef]
- Kammakakam, I.; Bara, J.E.; Jackson, E.M.; Lertxundi, J.; Mecerreyes, D.; Tomé, L.C. Tailored CO2-Philic Anionic Poly(ionic liquid) Composite Membranes: Synthesis, Characterization, and Gas Transport Properties. ACS Sustain. Chem. Eng. 2020, 8, 5954–5965. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Kim, J.H.; Nam, S.Y. Piperidinium functionalized poly(2,6 dimethyl 1,4 phenylene oxide) based polyionic liquid/ionic liquid (PIL/IL) composites for CO2 separation. J. Ind. Eng. Chem. 2021, 99, 81–89. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Gao, H.; Bai, Y.; Sun, Y.; Chen, Y. Synthesis and gas transport properties of poly(ionic liquid) based semi-interpenetrating polymer network membranes for CO2/N2 separation. J. Membr. Sci. 2017, 528, 72–81. [Google Scholar] [CrossRef]
- Nellepalli, P.; Tomé, L.C.; Vijayakrishna, K.; Marrucho, I.M. Imidazolium-Based Copoly(Ionic Liquid) Membranes for CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 2017–2026. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Chen, J.; Arnould, M.; Popovs, I.; Mahurin, S.M.; Chen, H.; Wang, T.; Dai, S. Synthesis of Poly(ionic Liquid)s-block-poly(methyl Methacrylate) Copolymer-Grafted Silica Particle Brushes with Enhanced CO2 Permeability and Mechanical Performance. Langmuir 2021, 37, 10875–10881. [Google Scholar] [CrossRef]
- Ravula, S.; O’Harra, K.E.; Watson, K.A.; Bara, J.E. Poly(ionic liquid)s with Dicationic Pendants as Gas Separation Membranes. Membranes 2022, 12, 264. [Google Scholar] [CrossRef]
- Dunn, C.A.; Shi, Z.; Zhou, R.; Gin, D.L.; Noble, R.D. (Cross-Linked Poly(Ionic Liquid)–Ionic Liquid–Zeolite) Mixed-Matrix Membranes for CO2/CH4 Gas Separations Based on Curable Ionic Liquid Prepolymers. Ind. Eng. Chem. Res. 2019, 58, 4704–4708. [Google Scholar] [CrossRef]
- Nabais, A.R.; Martins, A.P.S.; Alves, V.D.; Crespo, J.G.; Marrucho, I.M.; Tomé, L.C.; Neves, L.A. Poly(ionic liquid)-based engineered mixed matrix membranes for CO2/H2 separation. Sep. Purif. Technol. 2019, 222, 168–176. [Google Scholar] [CrossRef]
- Sampaio, A.M.; Nabais, A.R.; Tomé, L.C.; Neves, L.A. Impact of MOF-5 on Pyrrolidinium-Based Poly(ionic liquid)/Ionic Liquid Membranes for Biogas Upgrading. Ind. Eng. Chem. Res. 2019, 59, 308–317. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Loïs, S.; Dahl, P.I.; Sandru, M.; Jaschik, J.; Tanczyk, M.; Fuoco, A.; Jansen, J.C.; Vankelecom, I.F.J. Water Vapour Promotes CO2 Transport in Poly(ionic liquid)/Ionic Liquid-Based Thin-Film Composite Membranes Containing Zinc Salt for Flue Gas Treatment. Appl. Sci. 2020, 10, 3859. [Google Scholar] [CrossRef]
- Liu, H.; Paddison, S.J. Direct Comparison of Atomistic Molecular Dynamics Simulations and X-ray Scattering of Polymerized Ionic Liquids. ACS Macro Lett. 2016, 5, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Paddison, S.J. Alkyl Chain Length Dependence of Backbone-to-Backbone Distance in Polymerized Ionic Liquids: An Atomistic Simulation Perspective on Scattering. Macromolecules 2017, 50, 2889–2895. [Google Scholar] [CrossRef]
- Iacob, C.; Matsumoto, A.; Brennan, M.; Liu, H.; Paddison, S.J.; Urakawa, O.; Inoue, T.; Sangoro, J.; Runt, J. Polymerized Ionic Liquids: Correlation of Ionic Conductivity with Nanoscale Morphology and Counterion Volume. ACS Macro Lett. 2017, 6, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Doughty, B.; Genix, A.-C.; Popov, I.; Li, B.; Zhao, S.; Saito, T.; Lutterman, D.A.; Sacci, R.L.; Sumpter, B.G.; Wojnarowska, Z.; et al. Structural correlations tailor conductive properties in polymerized ionic liquids. Phys. Chem. Chem. Phys. 2019, 21, 14775–14785. [Google Scholar] [CrossRef]
- Corvo, T.O.; Jourdain, A.; O’Brien, S.; Restagno, F.; Drockenmuller, E.; Chennevière, A. Multiscale Structure of Poly(ionic liquid)s in Bulk and Solutions by Small-Angle Neutron Scattering. Macromolecules 2022, 55, 4111–4118. [Google Scholar] [CrossRef]
- Cabry, C.P.; D’Andrea, L.; Shimizu, K.; Grillo, I.; Li, P.; Rogers, S.; Bruce, D.W.; Canongia Lopes, J.N.; Slattery, J.M. Exploring the bulk-phase structure of ionic liquid mixtures using small-angle neutron scattering. Faraday Discuss. 2018, 206, 265–289. [Google Scholar] [CrossRef]
- Cabry, C.P.; D’Andrea, L.; Elstone, N.S.; Kirchhecker, S.; Riccobono, A.; Khazal, I.; Li, P.; Rogers, S.E.; Bruce, D.W.; Slattery, J.M. Small-angle neutron scattering from mixtures of long- and short-chain 3-alkyl-1-methyl imidazolium bistriflimides. Phys. Chem. Chem. Phys. 2022, 24, 15811–15823. [Google Scholar] [CrossRef] [PubMed]
- Russina, O.; Lo Celso, F.; Plechkova, N.V.; Triolo, A. Emerging Evidences of Mesoscopic-Scale Complexity in Neat Ionic Liquids and Their Mixtures. J. Phys. Chem. Lett. 2017, 8, 1197–1204. [Google Scholar] [CrossRef]
- Gaspar, H.; Teixeira, P.; Santos, R.; Fernandes, L.; Hilliou, L.; Weir, M.P.; Parnell, A.J.; Abrams, K.J.; Hill, C.J.; Bouwman, W.G.; et al. A Journey along the Extruder with Polystyrene:C60 Nanocomposites: Convergence of Feeding Formulations into a Similar Nanomorphology. Macromolecules 2017, 50, 3301–3312. [Google Scholar] [CrossRef]
- Gaspar, H.; Santos, R.; Teixeira, P.; Hilliou, L.; Weir, M.P.; Duif, C.P.; Bouwman, W.G.; Parnell, S.R.; King, S.M.; Covas, J.A.; et al. Evolution of dispersion in the melt compounding of a model polymer nanocomposite system: A multi-scale study. Polym. Test. 2019, 76, 109–118. [Google Scholar] [CrossRef]
- Parnell, S.R.; Washington, A.L.; Parnell, A.J.; Walsh, A.; Dalgliesh, R.M.; Li, F.; Hamilton, W.A.; Prevost, S.; Fairclough, J.P.A.; Pynn, R. Porosity of silica Stöber particles determined by spin-echo small angle neutron scattering. Soft Matter 2016, 12, 4709–4714. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, Z.; van der Goot, A.J.; Bouwman, W.G. Air bubbles in fibrous caseinate gels investigated by neutron refraction, X-ray tomography and refractive microscope. Food Hydrocoll. 2018, 83, 287–295. [Google Scholar] [CrossRef]
- Bouwman, W.G. Spin-echo small-angle neutron scattering for multiscale structure analysis of food materials. Food Struct. 2021, 30, 100235. [Google Scholar] [CrossRef]
- Bakker, J.H.; Washington, A.L.; Parnell, S.R.; van Well, A.A.; Pappas, C.; Bouwman, W.G. Analysis of SESANS data by numerical Hankel transform implementation in SasView. J. Neutron Res. 2020, 22, 57–70. [Google Scholar] [CrossRef]
- Bernardo, G.; Melle-Franco, M.; Washington, A.L.; Dalgliesh, R.M.; Li, F.; Mendes, A.; Parnell, S.R. Different agglomeration properties of PC61BM and PC71BM in photovoltaic inks—A spin-echo SANS study. RSC Adv. 2020, 10, 4512–4520. [Google Scholar] [CrossRef]



| Membrane Type | Supported or Unsupported | Membrane Name | Temp (°C) | P CO2 (Barrer) | P N2 | P H2 | P CH4 | α CO2/N2 | α CO2/H2 | α CO2/CH4 | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neat PIL | S | P[VBTMA][Tf2N] | 26 | 502 | 19 | ----- | ----- | 27.0 (a) | ----- | ----- | 2017 | [28] |
| P[VBHEDMA][Tf2N] | 349 | 8.3 | ----- | ----- | 41.6 (a) | ----- | ----- | |||||
| P[VBMP][Tf2N] | 4802 | 280 | ----- | ----- | 17.2 (a) | ----- | ----- | |||||
| S | P[CA][Tf2N] | 25 | 8.9 | 0.3 | 8.7 | 0.4 | 26.8 | 1.0 | 22.3 | 2018 | [29] | |
| P[DADMA][Tf2N] (reference) | 6.8 | 0.4 | 6.6 | 0.3 | 18.4 | 1.0 | 22.7 | |||||
| U | LP(1:2) | 35 | 170 | 4.7 | ----- | ----- | 36 | ----- | ----- | 2020 | [30] | |
| HP(1:2) | 167 | 9.3 | ----- | ----- | 18 | ----- | ----- | |||||
| PIL-IL composite membranes | U | PIL NTf2—40 IL Si NTf2 | 20 | 181 | ----- | ----- | ----- | 16.8 | ----- | 9.8 | 2017 | [31] |
| PIL NTf2—60 IL Si NTf2 | 426 | ----- | ----- | ----- | 24.7 | ----- | 11.5 | |||||
| PIL C(CN)3—40 IL Si C(CN)3 | 57 | ----- | ----- | ----- | 29.3 | ----- | 10.7 | |||||
| PIL C(CN)3—60 IL Si C(CN)3 | 238 | ----- | ----- | ----- | 35.2 | ----- | 11.5 | |||||
| U | PIL N(CN)2—60 IL C(CN)3 | 20 | 249.0 | 4.1 | ----- | ----- | 61.3 | ----- | ----- | 2018 | [32] | |
| PIL C(CN)3—60 IL B(CN)4 | 472.7 | 8.7 | ----- | ----- | 54.4 | ----- | ----- | |||||
| PIL C(CN)3—40 IL N(CN)2 | 198.8 | 3.0 | ----- | ----- | 67.0 | ----- | ----- | |||||
| PIL B(CN)4—60 IL C(CN)3 | 502.1 | 11.6 | ----- | ----- | 43.1 | ----- | ----- | |||||
| U | Medium Mw PIL—20 IL | 20 | 14.6 | ----- | ----- | ----- | 35.9 | ----- | ----- | 2018 | [33] | |
| Medium Mw PIL—40 IL | 193 | ----- | ----- | ----- | 52.3 | ----- | ----- | |||||
| Medium Mw PIL—60 IL | 542 | ----- | ----- | ----- | 54.0 | ----- | ----- | |||||
| U | PIL C(CN)3—40 [C2mim][C(CN)3] | 20 | 139 | ----- | 14.5 | ----- | ----- | 9.6 | ----- | 2018 | [34] | |
| 35 | 209 | ----- | 25.7 | ----- | ----- | 8.1 | ----- | |||||
| PIL C(CN)3—60 [C2mim][C(CN)3] | 20 | 438 | ----- | 29.1 | ----- | ----- | 15.1 | ----- | ||||
| 35 | 505 | ----- | 40.3 | ----- | ----- | 12.5 | ----- | |||||
| PIL NTf2—40 [C4mpyr][NTf2] | 20 | 119 | ----- | 21.9 | ----- | ----- | 5.4 | ----- | ||||
| 35 | 164 | ----- | 34.4 | ----- | ----- | 4.8 | ----- | |||||
| PIL NTf2—60 [C4mpyr][NTf2] | 20 | 232 | ----- | 29.8 | ----- | ----- | 7.8 | ----- | ||||
| 35 | 288 | ----- | 46.0 | ----- | ----- | 6.3 | ----- | |||||
| PIL NTf2—40 [C2mim][NTf2] | 20 | 214 | ----- | 26.2 | ----- | ----- | 8.2 | ----- | ||||
| 35 | 287 | ----- | 43.8 | ----- | ----- | 6.5 | ----- | |||||
| U | PIL C(CN)3—40 [C2mim][C(CN)3] | 35 | 129.7 | ----- | 15.7 | ----- | ----- | 8.2 (a) | ----- | 2021 | [35] | |
| PIL C(CN)3—60 [C2mim][C(CN)3] | 324.7 | ----- | 28.3 | ----- | ----- | 11.4 (a) | ----- | |||||
| PIL NTf2—40 [C4mpyr][NTf2] | 118.9 | ----- | 23.6 | ----- | ----- | 5.0 (a) | ----- | |||||
| PIL NTf2—60 [C4mpyr][NTf2] | 254.2 | ----- | 38.3 | ----- | ----- | 6.6 (a) | ----- | |||||
| PIL NTf2—40 [C2mim][NTf2] | 201.6 | ----- | 29.0 | ----- | ----- | 6.9 (a) | ----- | |||||
| U | PIL TFSAM—20 IL TFSAM | 35 | 40 | 1.1 | 12.4 | ----- | ----- | 3.2 | ----- | 2020 | [36] | |
| PIL TFSAM—40 IL TFSAM | 177 | 5.0 | 24.6 | ----- | ----- | 7.2 | ----- | |||||
| PIL FSI—20 IL FSI | 38 | 0.9 | 10.5 | ----- | ----- | 3.6 | ----- | |||||
| PIL FSI—40 IL FSI | 201 | 4.5 | 22.7 | ----- | ----- | 8.9 | ----- | |||||
| PIL TSAC—20 IL TSAC | 72 | 2.8 | 20.2 | ----- | ----- | 3.5 | ----- | |||||
| U | MIL—CF3/PEGDA(20%)/IL(0.5 equiv) | 20 | 7.69 | 0.165 | 2.83 | 0.136 | 46.6 | 2.71 | 56.54 | 2020 | [37] | |
| MIL—CF3/PEGDA(20%)/IL(1 equiv) | 5.94 | 0.115 | 1.87 | 0.096 | 51.65 | 3.18 | 61.88 | |||||
| MIL—C7H7/PEGDA(20%)/IL(0.5 equiv) | 4.67 | 0.071 | 1.65 | 0.066 | 65.77 | 2.83 | 70.75 | |||||
| MIL—C7H7/PEGDA(20%)/IL(1 equiv) | 20.4 | 0.235 | 4.97 | 0.172 | 86.81 | 4.1 | 118.6 | |||||
| U | HT—66 wt% IL | 2070 | 84.1 | 24.6 | 2020 | [30] | ||||||
| U | ILPPO | 25 | 69.58 | 11.20 | ----- | ----- | 6.21 | ----- | ----- | 2021 | [38] | |
| ILPPO/Br-6-MPRD-2 | 907.20 | 70.10 | ----- | ----- | 12.94 | ----- | ----- | |||||
| ILPPO/Br-6-MPRD-5 | 1.30 | 0.40 | ----- | ----- | 3.25 | ----- | ----- | |||||
| PIL-polymer blend membranes | U | PVAc/ c-PIL-50 semi-IPN | 20 | 18.43 | 0.26 | ----- | ----- | 70.61 | ----- | ----- | 2017 | [39] |
| 30 | 29.47 | 0.46 | ----- | ----- | 64.20 | ----- | ----- | |||||
| 40 | 38.86 | 0.74 | ----- | ----- | 52.30 | ----- | ----- | |||||
| 50 | 41.72 | 0.83 | ----- | ----- | 50.56 | ----- | ----- | |||||
| 60 | 42.72 | 0.89 | ----- | ----- | 48.00 | ----- | ----- | |||||
| 70 | 43.88 | 0.92 | ----- | ----- | 47.70 | ----- | ----- | |||||
| Block-copolymer | U | poly(ViPenIm)(Sty)NTf2—10% IL | 20 | 21.6 | 0.68 | ----- | ----- | 31.7 | ----- | ----- | 2019 | [40] |
| poly(ViBenIm)(Sty)NTf2—25% IL | 16.5 | 0.50 | ----- | ----- | 32.9 | ----- | ----- | |||||
| poly(ViNapIm)(Sty)NTf2—30% IL | 24.5 | 0.71 | ----- | ----- | 34.4 | ----- | ----- | |||||
| U | SiO2-g-PMMA-b-PIL | 54.1 | 2.9 | 18.5 | 2021 | [41] | ||||||
| SiO2-g-PIL | 53.4 | 2.8 | 19.1 | |||||||||
| U | HM | 20 | 6.34 | 0.29 | 3.98 | 0.31 | 20.76 | 1.59 | 20.92 | 2022 | [42] | |
| BCP1-C4 | 9.61 | 0.43 | 5.54 | 0.55 | 19.72 | 1.69 | 17.80 | |||||
| BCP2-C4 | 7.26 | 0.23 | 4.92 | 0.29 | 31.99 | 1.45 | 27.61 | |||||
| BCP1-C6 | 11.23 | 0.51 | 7.69 | 0.58 | 21.82 | 1.48 | 19.61 | |||||
| BCP2-C6 | 6.05 | 0.21 | 3.86 | 0.34 | 30.82 | 1.57 | 16.28 | |||||
| Mixed Matrix Membrane (MMM) | S | 1d/IL/zeolite (64/16/20) | 47 | 1.1 | 42 | 2019 | [43] | |||||
| U | PIL-IL | 30 | 47.1 | ------ | ------ | ------ | ------ | 2.2 | ------ | 2019 | [44] | |
| MMM/10% MIL-53 | 35.2 | ------ | ------ | ------ | ------ | 3.4 | ------ | |||||
| MMM/20% MIL-53 | 50.0 | ------ | ------ | ------ | ------ | 6.5 | ------ | |||||
| MMM/30% MIL-53 | 89.0 | ------ | ------ | ------ | ------ | 13.3 | ------ | |||||
| MMM/10% Cu3(BTC)2 | 40.0 | ------ | ------ | ------ | ------ | 2.6 | ------ | |||||
| MMM/20% Cu3(BTC)2 | 74.3 | ------ | ------ | ------ | ------ | 3.0 | ------ | |||||
| MMM/30% Cu3(BTC)2 | 77.1 | ------ | ------ | ------ | ------ | 6.4 | ------ | |||||
| MMM/10% ZIF-8 | 54.8 | ------ | ------ | ------ | ------ | 3.0 | ------ | |||||
| MMM/20% ZIF-8 | 83.8 | ------ | ------ | ------ | ------ | 3.6 | ------ | |||||
| MMM/30% ZIF-8 | 97.2 | ------ | ------ | ------ | ------ | 4.5 | ------ | |||||
| U | Tf2N/40 IL BETI | 146 | 10 | 14.5 | 2020 | [45] | ||||||
| Tf2N/40 IL BETI/10 MOF-5 | 261 | 33 | 7.9 | |||||||||
| Tf2N/40 IL BETI/20 MOF-5 | 282 | 19 | 14.8 | |||||||||
| Tf2N/40 IL BETI/30 MOF-5 | 340 | 189 | 1.8 | |||||||||
| S | P9IL0Zn0 | 25 | 7.6 | 0.4 | ------ | ------ | 17.6 | ------ | ------ | 2020 | [46] | |
| P9IL6Zn0 | 166 | 5.4 | ------ | ------ | 30.5 | ------ | ------ | |||||
| P9IL6Zn1 | 80.9 | 7.2 | ------ | ------ | 11.2 | ------ | ------ | |||||
| P9IL6Zn9 | 20.2 | 0.9 | ------ | ------ | 23.5 | ------ | ------ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, G.; Gaspar, H. Recent Advances in Poly(Ionic Liquid)-Based Membranes for CO2 Separation. Polymers 2023, 15, 667. https://doi.org/10.3390/polym15030667
Bernardo G, Gaspar H. Recent Advances in Poly(Ionic Liquid)-Based Membranes for CO2 Separation. Polymers. 2023; 15(3):667. https://doi.org/10.3390/polym15030667
Chicago/Turabian StyleBernardo, Gabriel, and Hugo Gaspar. 2023. "Recent Advances in Poly(Ionic Liquid)-Based Membranes for CO2 Separation" Polymers 15, no. 3: 667. https://doi.org/10.3390/polym15030667
APA StyleBernardo, G., & Gaspar, H. (2023). Recent Advances in Poly(Ionic Liquid)-Based Membranes for CO2 Separation. Polymers, 15(3), 667. https://doi.org/10.3390/polym15030667








