Degradation of Bio-Based and Biodegradable Plastic and Its Contribution to Soil Organic Carbon Stock
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Design of Experiment
2.3. Soil Respiration
2.4. Source-Partitioning of CO2 Emitted from Soil and from PBSA
2.5. Statistical Analysis
3. Results
3.1. CO2-C Respired
3.2. 13C Signature of Respired CO2-C
3.3. Source Partitioning of the Respired CO2-C
3.4. Priming Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yaeko, S.; Fumikazu, A.; Rumiko, N.; Takashi, K. A novel method to discriminate between plant- and petroleum-derived plastics by stable carbon isotope analysis. Chem. Lett. 2010, 39, 998–999. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Narayan, R.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Dos and do nots when assessing the biodegradation of plastics. Environ. Sci. Technol. 2019, 53, 9967–9969. [Google Scholar] [CrossRef] [PubMed]
- Liwarska-Bizukojc, E. Effect of (bio)plastics on soil environment: A review. Sci. Total Environ. 2021, 795, 148889. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O.; Kartushinskaya, M.V. Degradation of bioplastics in natural environment. Dokl. Biol. Sci. 2004, 397, 330–332. [Google Scholar] [CrossRef]
- Van den Oever, M.; Molenveld, K.; Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics—Facts and Figures. Focus on Food Packaging in the Netherlands; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Tanunchai, B.; Kalkhof, S.; Guliyev, V.; Wahdan, S.F.M.; Krstic, D.; Schädler, M.; Geissler, A.; Glaser, B.; Buscot, F.; Blagodatskaya, E. Nitrogen fixing bacteria facilitate microbial biodegradation of a bio-based and biodegradable plastic in soils under ambient and future climatic conditions. Environ. Sci. Process. Impacts 2022, 24, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Morita, T.; Cao, X.-h.; Yoshida, S.; Koitabashi, M.; Watanabe, T.; Suzuki, K.; Sameshima-Yamashita, Y.; Nakajima-Kambe, T.; Fujii, T. Biodegradable plastic-degrading enzyme from Pseudozyma antarctica: Cloning, sequencing, and characterization. Appl. Microbiol. Biotechnol. 2013, 97, 2951–2959. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend in freshwater with sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Purahong, W.; Wahdan, S.F.M.; Heinz, D.; Jariyavidyanont, K.; Sungkapreecha, C.; Tanunchai, B.; Sansupa, C.; Sadubsarn, D.; Alaneed, R.; Heintz-Buschart, A.; et al. Back to the future: Decomposability of a biobased and biodegradable plastic in field soil environments and its microbiome under ambient and future climates. Environ. Sci. Technol. 2021, 55, 12337–12351. [Google Scholar] [CrossRef] [PubMed]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Díaz, F.J.; Belmonte-Ureña, L.J.; Camacho-Ferre, F.; Tello-Marquina, J.C. The management of agriculture plastic waste in the framework of circular economy. Case of the Almeria Greenhouse (Spain). Int J Environ. Res Public Health 2021, 18, 12042. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Gayathri, R.; Vijayalakshmi, S. Microbial degradation of plastics: Sustainable approach to tackling environmental threats facing big cities of the future. J. King Saud Univ.—Sci. 2021, 33, 101362. [Google Scholar] [CrossRef]
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int. Biodeterior. Biodegrad. 2011, 65, 451–459. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of microplastic fibers and drought on plant communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Pathan, S.I.; Arfaioli, P.; Bardelli, T.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G. Soil Pollution from micro- and nanoplastic debris: A hidden and unknown biohazard. Sustainability 2020, 12, 7255. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Sawada, H.; Yokota, M.; Tsuji, M.; Fukuda, K.; Kimura, M. Influence of weather conditions and soil properties on degradation of biodegradable plastics in soil. Soil Sci. Plant Nutr. 2001, 47, 35–43. [Google Scholar] [CrossRef]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.-H.; Linne, U.; Erb, T.; Maier, U.G. Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell Factories 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Bhuyar, P.; Sundararaju, S.; Feng, H.X.; Rahim, M.H.A.; Muniyasamy, S.; Maniam, G.P.; Govindan, N. Evaluation of microalgae’s plastic biodeterioration property by a consortium of Chlorella sp. and Cyanobacteria sp. Environ. Res. Eng. Manag. 2021, 77, 86–98. [Google Scholar] [CrossRef]
- Thielen, M. Bioplastics: Plants and Crops Raw Materials Products; Fachagentur für Nachwachsende Rohstoffe eV: Gülzow-Prüzen, Germany, 2019. [Google Scholar]
- Abdul-Latif, N.-I.S.; Ong, M.Y.; Nomanbhay, S.; Salman, B.; Show, P.L. Estimation of carbon dioxide (CO2) reduction by utilization of algal biomass bioplastic in Malaysia using carbon emission pinch analysis (CEPA). Bioengineered 2020, 11, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.; Karageorgiou, A.; Calabrò, P.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Blagodatsky, S.A.; Anderson, T.H.; Kuzyakov, Y. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl. Soil Ecol. 2007, 37, 95–105. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Bei, S.; Li, X.; Kuyper, T.W.; Chadwick, D.R.; Zhang, J. Nitrogen availability mediates the priming effect of soil organic matter by preferentially altering the straw carbon-assimilating microbial community. Sci. Total Environ. 2022, 815, 152882. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic effects on carbon cycling processes in soils. PLOS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Hao, X. Fertilization accelerates the decomposition of microplastics in mollisols. Sci. Total Environ. 2020, 722, 137950. [Google Scholar] [CrossRef]
- Berto, D.; Rampazzo, F.; Gion, C.; Noventa, S.; Ronchi, F.; Traldi, U.; Giorgi, G.; Cicero, A.M.; Giovanardi, O. Preliminary study to characterize plastic polymers using elemental analyser/isotope ratio mass spectrometry (EA/IRMS). Chemosphere 2017, 176, 47–56. [Google Scholar] [CrossRef]
- Amelung, W.; Brodowski, S.; Sandhage-Hofmann, A.; Bol, R. Chapter 6: Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2008; Volume 100, pp. 155–250. [Google Scholar]
- Blagodatskaya, E.; Yuyukina, T.; Blagodatsky, S.; Kuzyakov, Y. Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol. Biochem. 2011, 43, 778–786. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Yuyukina, T.; Blagodatsky, S.; Kuzyakov, Y. Turnover of soil organic matter and of microbial biomass under C3–C4 vegetation change: Consideration of 13C fractionation and preferential substrate utilization. Soil Biol. Biochem. 2011, 43, 159–166. [Google Scholar] [CrossRef]
- Glaser, B.; Gross, S. Compound-specific δ13C analysis of individual amino sugars—A tool to quantify timing and amount of soil microbial residue stabilization. Rapid Commun. Mass Spectrom. 2005, 19, 1409–1416. [Google Scholar] [CrossRef]
- Glaser, B. Compound-specific stable-isotope (δ13C) analysis in soil science. J. Plant Nutr. Soil Sci. 2005, 168, 633–648. [Google Scholar] [CrossRef]
- Boschker, H.; Middelburg, J. Boschker HTS, Middelburg JJ.. Stable isotopes and biomarkers in microbial ecology. FEMS Microbiol. Ecol. 2002, 40, 85–95. [Google Scholar] [CrossRef]
- Hobbie, A.E.; Werner, R.A. Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: A review and synthesis. New Phytol. 2004, 161, 371–385. [Google Scholar] [CrossRef] [PubMed]
- ŠantRůČková, H.; Bird, M.I.; Lloyd, J. Microbial processes and carbon-isotope fractionation in tropical and temperate grassland soils. Funct. Ecol. 2000, 14, 108–114. [Google Scholar] [CrossRef]
- Formánek, P.; Ambus, P. Assessing the use of δ13C natural abundance in separation of root and microbial respiration in a Danish beech (Fagus sylvatica L.) forest. Rapid Commun. Mass Spectrom. RCM 2004, 18, 897–902. [Google Scholar] [CrossRef]
- Boström, B.; Comstedt, D.; Ekblad, A. Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 2007, 153, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Breecker, D.; Bergel, S.; Nadel, M.; Tremblay, M.; Osuna Orozco, R.; Larson, T.; Sharp, Z. Minor stable carbon isotope fractionation between respired carbon dioxide and bulk soil organic matter during laboratory incubation of topsoil. Biogeochemistry 2014, 123, 83–98. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. 13C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol. Biochem. 2010, 42, 1372–1384. [Google Scholar] [CrossRef]
- Volk, M.; Bassin, S.; Lehmann, M.F.; Johnson, M.G.; Andersen, C.P. 13C isotopic signature and C concentration of soil density fractions illustrate reduced C allocation to subalpine grassland soil under high atmospheric N deposition. Soil Biol. Biochem. 2018, 125, 178–184. [Google Scholar] [CrossRef]
- Altermann, M.; Rinklebe, J.; Merbach, I.; Körschens, M.; Langer, U.; Hofmann, B. Chernozem—Soil of the Year 2005. J. Plant Nutr. Soil Sci. 2005, 168, 725–740. [Google Scholar] [CrossRef]
- Schädler, M.; Buscot, F.; Klotz, S.; Reitz, T.; Durka, W.; Bumberger, J.; Merbach, I.; Michalski, S.G.; Kirsch, K.; Remmler, P.; et al. Investigating the consequences of climate change under different land-use regimes: A novel experimental infrastructure. Ecosphere 2019, 10, e02635. [Google Scholar] [CrossRef]
- Tanunchai, B.; Juncheed, K.; Wahdan, S.F.M.; Guliyev, V.; Udovenko, M.; Lehnert, A.-S.; Alves, E.G.; Glaser, B.; Noll, M.; Buscot, F.; et al. Analysis of microbial populations in plastic–soil systems after exposure to high poly(butylene succinate-co-adipate) load using high-resolution molecular technique. Environ. Sci. Eur. 2021, 33, 105. [Google Scholar] [CrossRef]
- Guliyev, V.; Tanunchai, B.; Noll, M.; Buscot, F.; Purahong, W.; Blagodatskaya, E. Links among microbial communities, soil properties and functions: Are fungi the sole players in decomposition of bio-based and biodegradable plastic? Polymers 2022, 14, 2801. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, A. Apparatus for the continuous, long-term monitoring of soil respiration rate in large numbers of samples. Soil Biol. Biochem. 1988, 20, 955–957. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Balesdent, J.; Mariotti, A. Measurement of Soil Organic Matter Turnover Using 13C Natural Abundance; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 83–111. [Google Scholar]
- Potthoff, M.; Loftfield, N.; Buegger, F.; Wick, B.; John, B.; Joergensen, R.G.; Flessa, H. The determination of δ13C in soil microbial biomass using fumigation-extraction. Soil Biol. Biochem. 2003, 35, 947–954. [Google Scholar] [CrossRef]
- Tu, K.; Dawson, T. Partitioning ecosystem respiration using stable carbon isotope analyses of CO2. In Stable Isotopes and Biosphere Atmosphere Interactions; Flanagan, L.B., Ehleringer, J.R., Pataki, D.E., Eds.; Academic Press: San Diego, CA, USA, 2005; pp. 125–153. [Google Scholar]
- Pelz, O.; Abraham, W.-R.; Saurer, M.; Siegwolf, R.; Zeyer, J. Microbial assimilation of plant-derived carbon in soil traced by isotope analysis. Biol. Fertil. Soils 2005, 41, 153–162. [Google Scholar] [CrossRef]
- Ghashghaie, J.; Duranceau, M.; Badeck, F.-W.; Cornic, G.; Adeline, M.-T.; Deleens, E. δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: Comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ. 2001, 24, 505–515. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. Three-source partitioning of CO2 efflux from maize field soil by 13C natural abundance. J. Plant Nutr. Soil Sci. 2009, 172, 487–499. [Google Scholar] [CrossRef]
- Ågren, G.I.; Bosatta, E.; Balesdent, J. Isotope discrimination during decomposition of organic matter: A theoretical analysis. Soil Sci. Soc. Am. J. 1996, 60, 1121–1126. [Google Scholar] [CrossRef]
- Crow, S.E.; Sulzman, E.W.; Rugh, W.D.; Bowden, R.D.; Lajtha, K. Isotopic analysis of respired CO2 during decomposition of separated soil organic matter pools. Soil Biol. Biochem. 2006, 38, 3279–3291. [Google Scholar] [CrossRef]
- Salomé, C.; Nunan, N.; Pouteau, V.; Lerch, T.Z.; Chenu, C. Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob. Chang. Biol. 2010, 16, 416–426. [Google Scholar] [CrossRef]
- Qiao, N.; Xu, X.; Hu, Y.; Blagodatskaya, E.; Liu, Y.; Schaefer, D.; Kuzyakov, Y. Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Sci. Rep. 2016, 6, 19865. [Google Scholar] [CrossRef] [PubMed]
- Moran, K.K.; Six, J.; Horwath, W.R.; van Kessel, C. Role of mineral-nitrogen in residue decomposition and stable soil organic matter formation. Soil Sci. Soc. Am. J. 2005, 69, 1730–1736. [Google Scholar] [CrossRef]
- Gómez, E.F.; Michel, F.C. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591. [Google Scholar] [CrossRef]
- Blagodatsky, S.; Larionova, A.; Yevdokimov, I. Effect of mineral nitrogen on the respiration rate and growth efficiency of soil microorganisms. Eurasian Soil Sci. 1993, 25, 85–95. [Google Scholar]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Griffiths, H.; Chamberlain, P.M.; Stott, A.W.; Tanner, E.V.J. Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl. Soil Ecol. 2009, 42, 183–190. [Google Scholar] [CrossRef]
- Liming, Y.; Zhang, T.; Dijkstra, F.; Huo, C.; Wang, P.; Cheng, W. Priming effect varies with root order: A case of Cunninghamia Lanceolata. Soil Biol. Biochem. 2021, 160, 108354. [Google Scholar] [CrossRef]
- Wang, Q.; He, T.; Liu, J. Litter input decreased the response of soil organic matter decomposition to warming in two subtropical forest soils. Sci. Rep. 2016, 6, 33814. [Google Scholar] [CrossRef]
- Chao, L.; Liu, Y.; Freschet, G.T.; Zhang, W.; Yu, X.; Zheng, W.; Guan, X.; Yang, Q.; Chen, L.; Dijkstra, F.A.; et al. Litter carbon and nutrient chemistry control the magnitude of soil priming effect. Funct. Ecol. 2019, 33, 876–888. [Google Scholar] [CrossRef]
- Brant, J.B.; Sulzman, E.W.; Myrold, D.D. Microbial community utilization of added carbon substrates in response to long-term carbon input manipulation. Soil Biol. Biochem. 2006, 38, 2219–2232. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Khomyakov, N.; Myachina, O.; Bogomolova, I.; Blagodatsky, S.; Kuzyakov, Y. Microbial interactions affect sources of priming induced by cellulose. Soil Biol. Biochem. 2014, 74, 39–49. [Google Scholar] [CrossRef]
- Fontaine, S.; Bardoux, G.; Abbadie, L.; Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 2004, 7, 314–320. [Google Scholar] [CrossRef]
- Dalenberg, J.W.; Jager, G. Priming effect of some organic additions to 14C-labelled soil. Soil Biol. Biochem. 1989, 21, 443–448. [Google Scholar] [CrossRef]
- Cheng, W.; Coleman, D.C. Effect of living roots on soil organic matter decomposition. Soil Biol. Biochem. 1990, 22, 781–787. [Google Scholar] [CrossRef]
- Qiu, Q.; Wu, L.; Ouyang, Z.; Li, B.; Xu, Y.; Wu, S.; Gregorich, E.G. Priming effect of maize residue and urea N on soil organic matter changes with time. Appl. Soil Ecol. 2016, 100, 65–74. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Wei, W.; He, Z.; Kuzyakov, Y.; Bol, R.; Hu, R. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
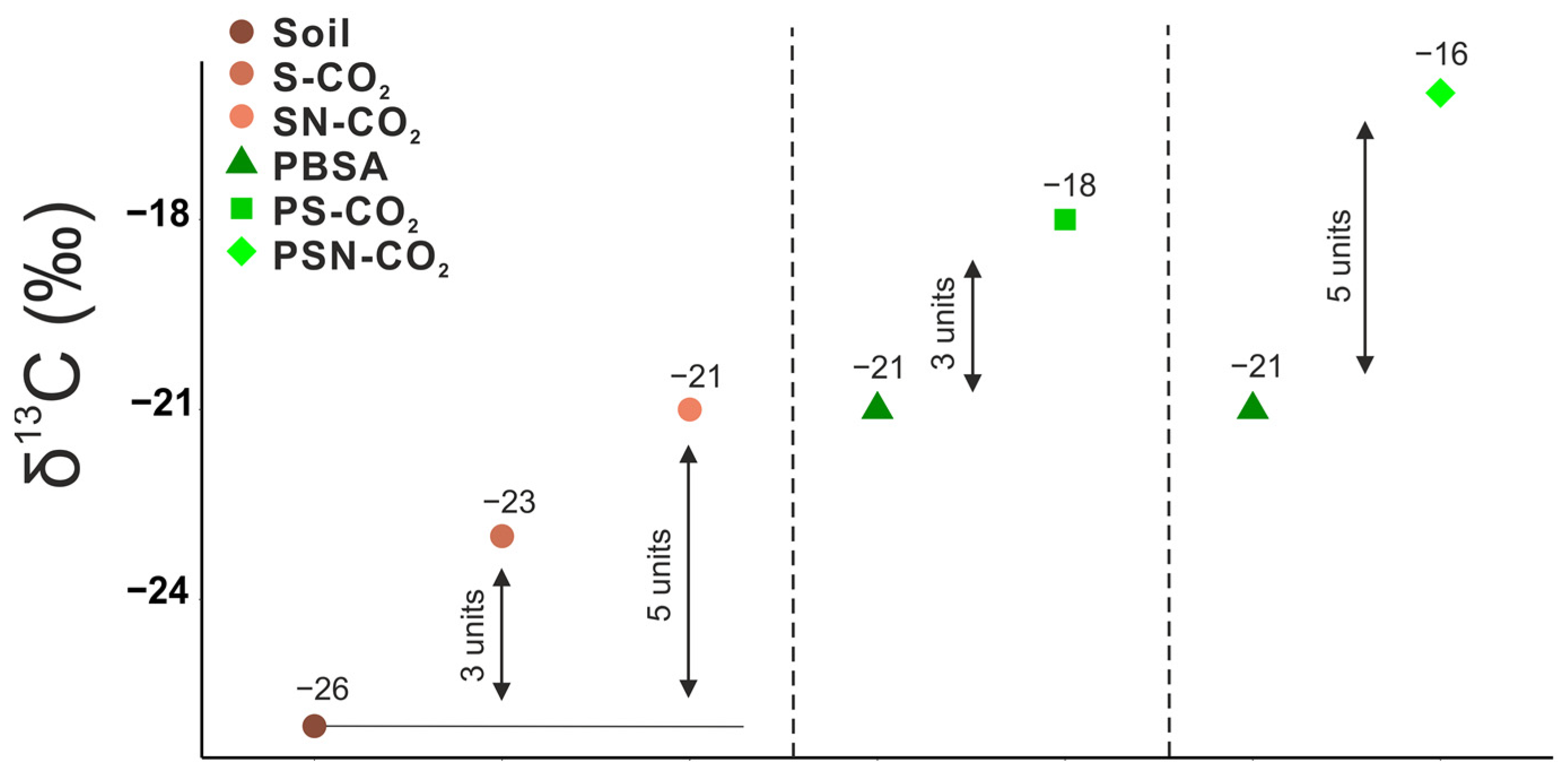
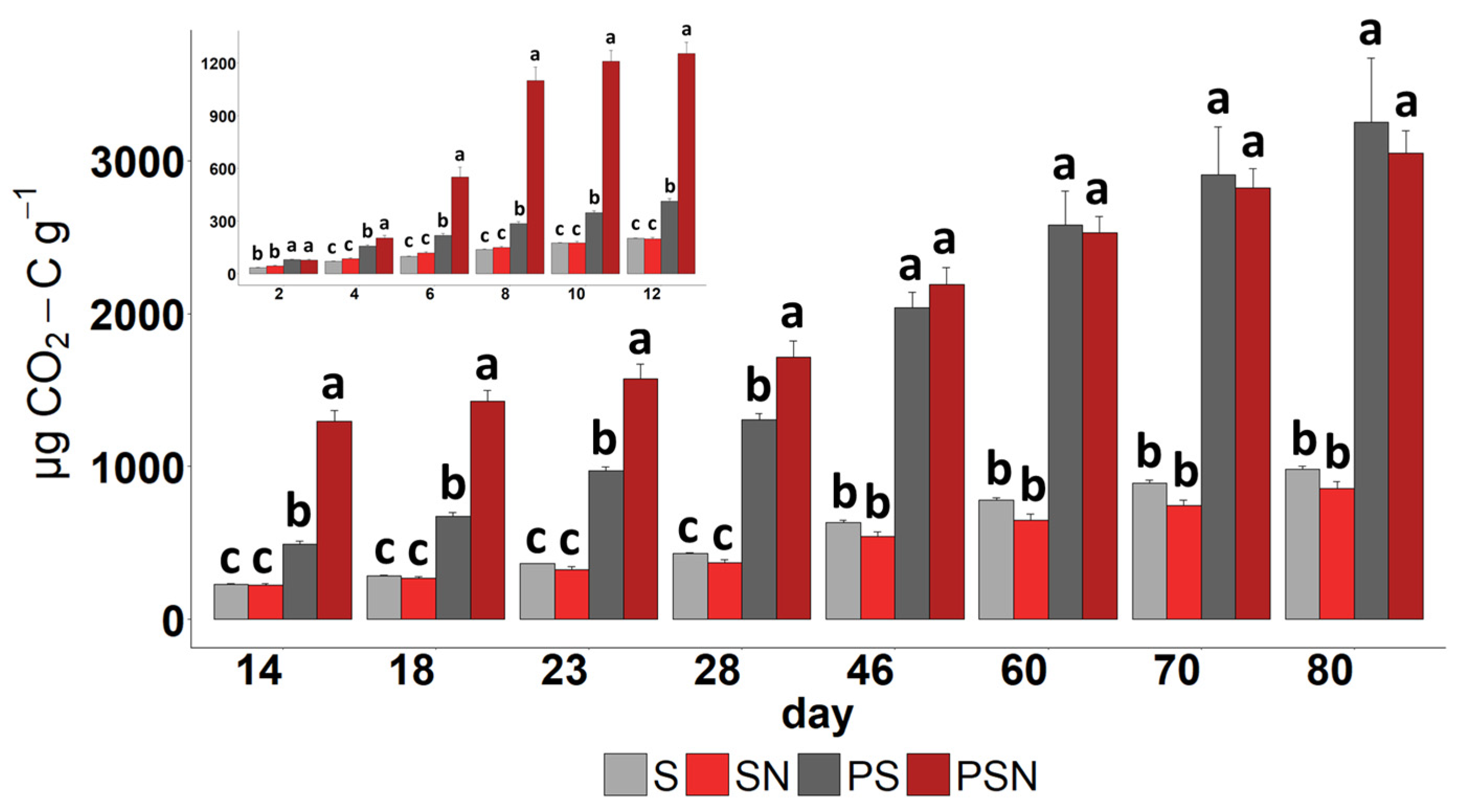



| Labeled Substrate | Soil Type/Texture | Fraction of Substrate in Soil (%) | Mineralization to CO2 (% of Added) | Duration (Day) | Primed C to CO2 (% of Control) | Reference |
|---|---|---|---|---|---|---|
| 13C Leaf (wheat) | Luvisol | 0.5–1 | 46–54 | 120 | 31–42 | [70] |
| 13C Leaf (maize) | Alluvial, sand silt | 0.6 | 13–19 | 32 | 44–67 | [71] |
| 13C Stem (wheat) | Luvisol | 0.5–1 | 38–49 | 120 | 66–68 | [70] |
| 13C Root (wheat) | Luvisol | 0.5–1 | 29 | 120 | 65–89 | [70] |
| 13C Root (tree) | Mollisol | 0.6 | 13–19 | 85 | 5–31 | [72] |
| 13C Leaf litter | Ultisols (broad-leaved forest) | 5 | 10–12 | 42 | (−7)–9 | [73] |
| 13C Leaf litter | Ultisols (coniferous forest) | 5 | 10–12 | 42 | 6–25 | [73] |
| 13C Leaf litter | Clay loam | 5 | 24 | 125 | (−7)–25 | [74] |
| 14C Glucose | Gleyic Cambisol | 0.01–0.1 | 48–65 | 54 | 110–125 | [39] |
| 13C Glucose | Typic Hapludands | 0.005 | 20–35 | 14 | 10–22 | [75] |
| 14C Glucose | Luvic Chernozem | 0.005–0.5 | 25–50 | 12 | (−87)–60 | [31] |
| 14C Cellulose | Gleyic Cambisol | 0.04 | 29 | 103 | 25 | [76] |
| 13C Cellulose | Sandy silt | 0.05 | 64–73 | 70 | 21–32 | [77] |
| 14C Cellulose | Sandy loam | 0.5−1.2 | 52–75 | 90 | 28–37 | [78] |
| 14C Straw (wheat) | Sandy loam | 0.5–1.2 | 24–33 | 32 | 7–9 | [78] |
| 14C Straw (rye) | Typic Kanadult | 0.04 | 21–23 | 49 | 11 | [79] |
| 13C Straw (maize) | Fluvisol | 0.2–0.3 | 16–18 | 250 | 9.1 | [80] |
| 13C Straw (rice) | Ferralic Cambisol | 0.5 | 34–45 | 70 | 43–122 | [81] |
| 13C PBSA (bioplastic) | Haplic Chernozem | 5 | 4.1–5.4 | 80 | 100–132 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guliyev, V.; Tanunchai, B.; Udovenko, M.; Menyailo, O.; Glaser, B.; Purahong, W.; Buscot, F.; Blagodatskaya, E. Degradation of Bio-Based and Biodegradable Plastic and Its Contribution to Soil Organic Carbon Stock. Polymers 2023, 15, 660. https://doi.org/10.3390/polym15030660
Guliyev V, Tanunchai B, Udovenko M, Menyailo O, Glaser B, Purahong W, Buscot F, Blagodatskaya E. Degradation of Bio-Based and Biodegradable Plastic and Its Contribution to Soil Organic Carbon Stock. Polymers. 2023; 15(3):660. https://doi.org/10.3390/polym15030660
Chicago/Turabian StyleGuliyev, Vusal, Benjawan Tanunchai, Maria Udovenko, Oleg Menyailo, Bruno Glaser, Witoon Purahong, François Buscot, and Evgenia Blagodatskaya. 2023. "Degradation of Bio-Based and Biodegradable Plastic and Its Contribution to Soil Organic Carbon Stock" Polymers 15, no. 3: 660. https://doi.org/10.3390/polym15030660
APA StyleGuliyev, V., Tanunchai, B., Udovenko, M., Menyailo, O., Glaser, B., Purahong, W., Buscot, F., & Blagodatskaya, E. (2023). Degradation of Bio-Based and Biodegradable Plastic and Its Contribution to Soil Organic Carbon Stock. Polymers, 15(3), 660. https://doi.org/10.3390/polym15030660







