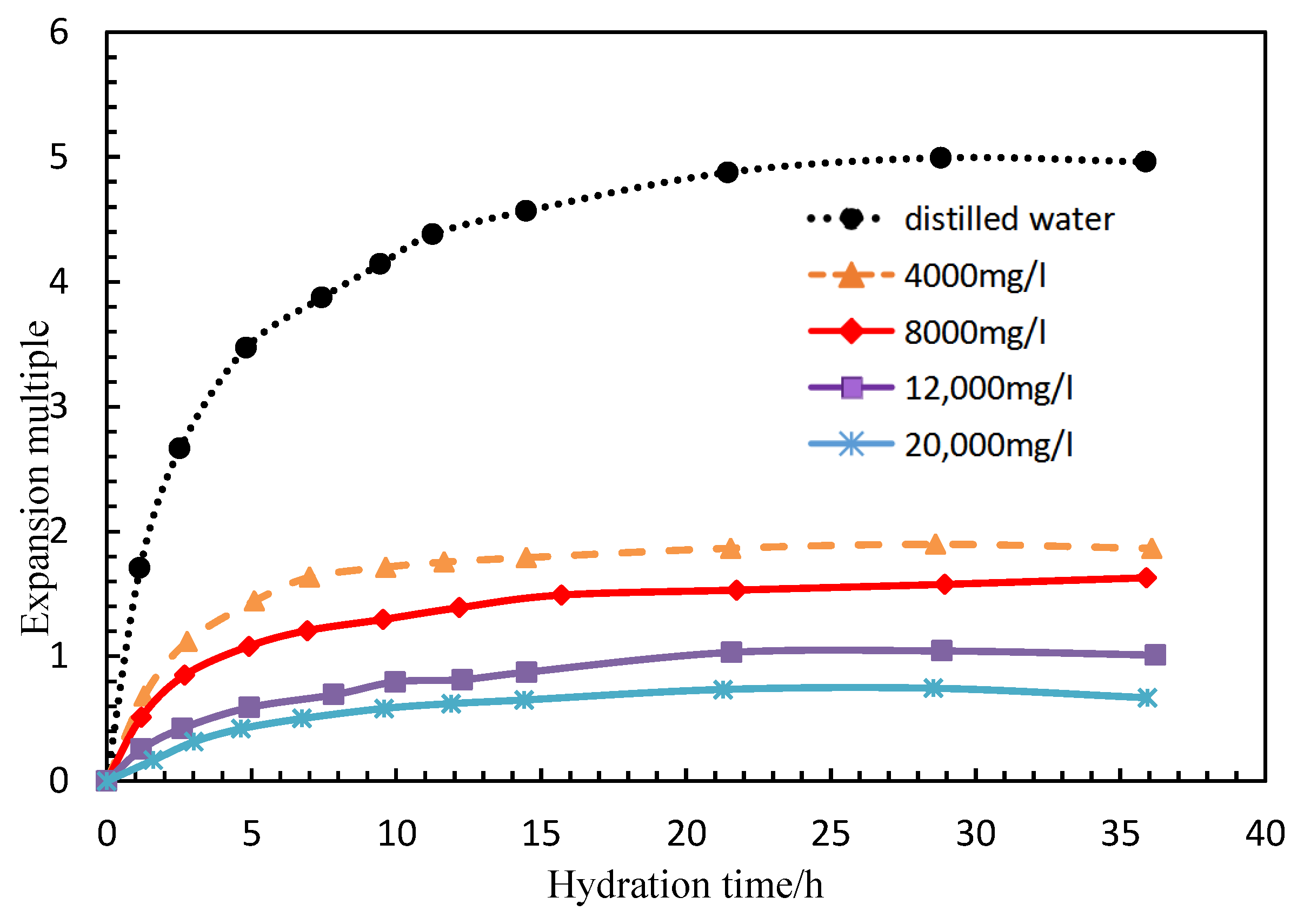
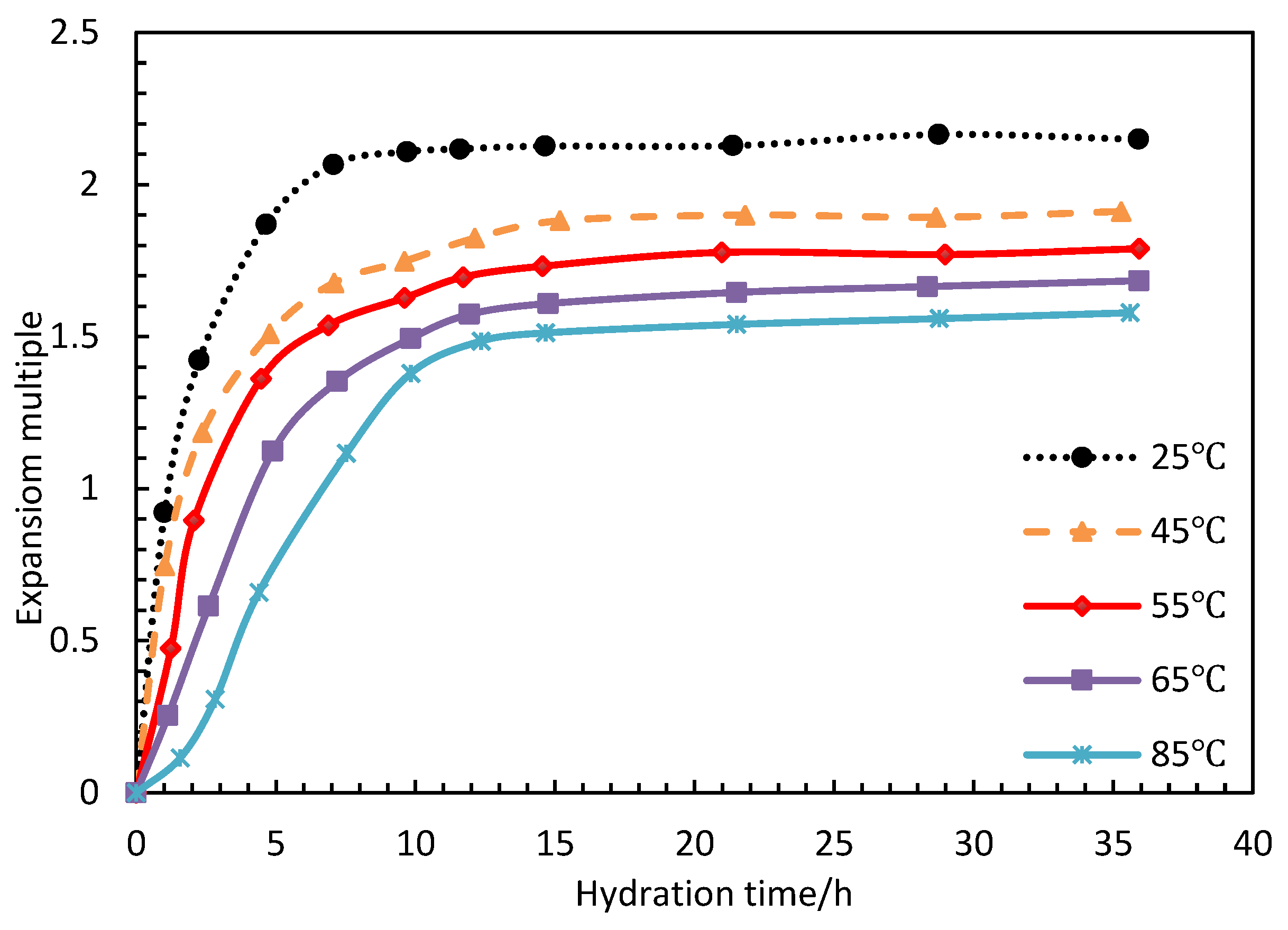
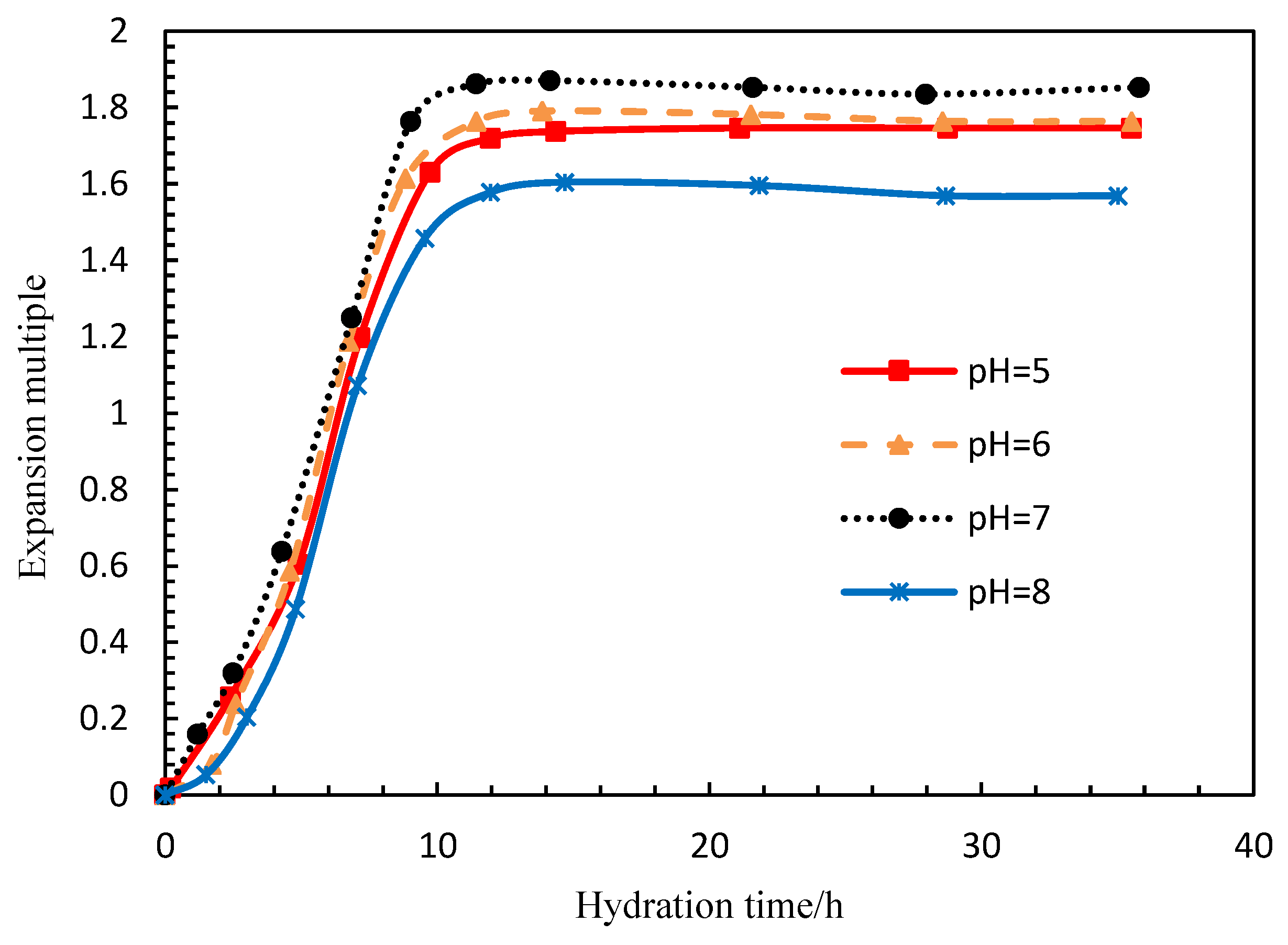
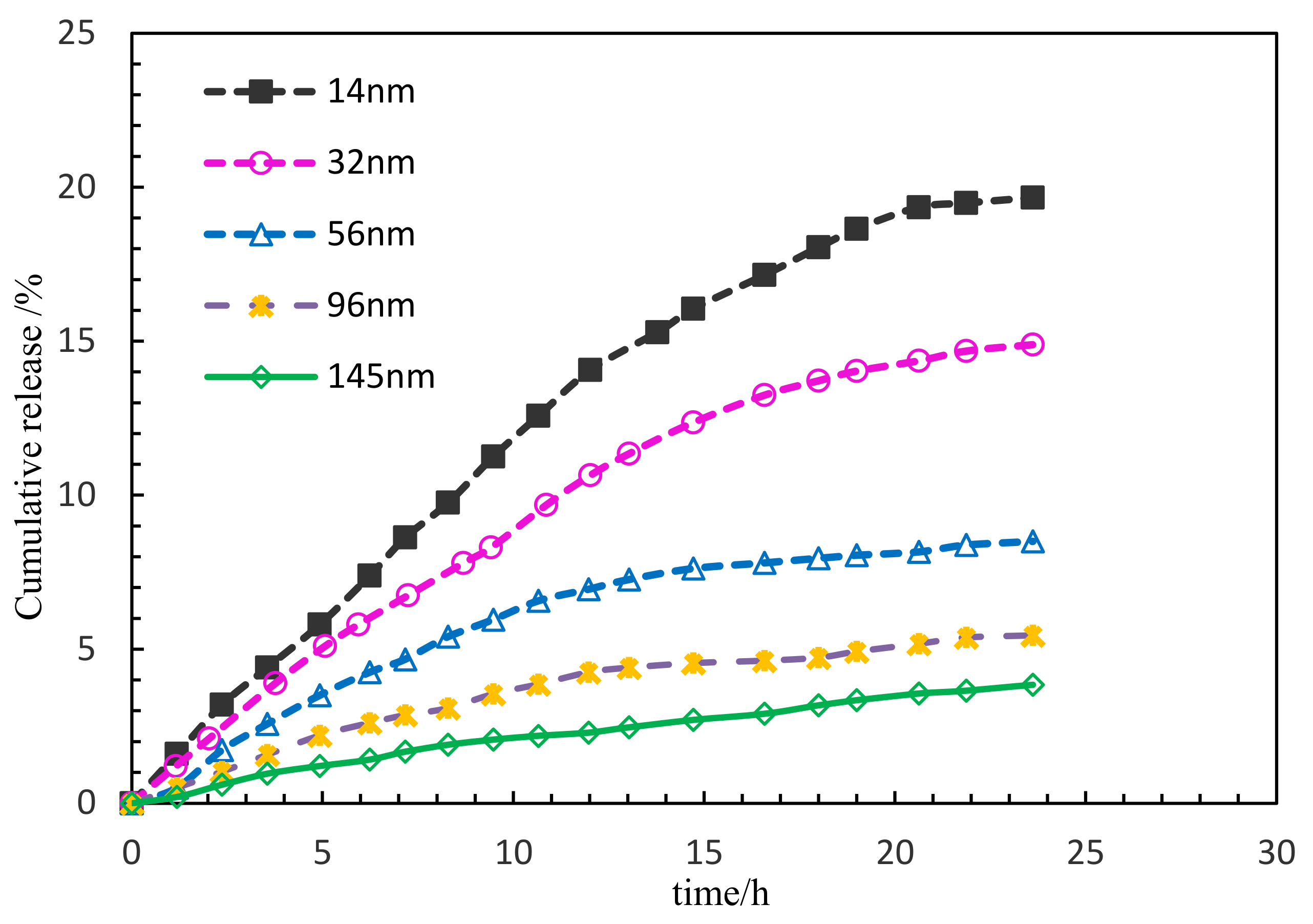
During the development of low permeability reservoirs, which is affected by dominant seepage channels, heterogeneity and fractures, water levels rise rapidly, leading to the restricted development of the oil production rate and a low recovery degree [
1]. There is still a large amount of oil remaining in the late stage of water flooding. Based on improving the plane and microheterogeneity of water flooding and realizing the technical requirements of fluid flow diversion in oil layers, it is necessary to carry out effective profile control and displacement treatment of reservoirs [
2,
3,
4,
5]. At present, polymer microspheres and polymer capsules are mainly used for plugging to induce a diversion of the fluid flow of the displaced medium, and then chemical displacement agents are added for oil displacement [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. The application of polymer capsules can effectively block high permeability layers—even high permeability bands or large pores—and increase the utilization of medium and low permeability layers. However, field tests have also shown that during the injection of polymer capsules into underground reservoirs, factors such as the high viscosity and large molecular size of the polymer capsule will cause wellbore plugging and the pollution of the low and medium permeability layers. In actual field tests, the effect of polymer capsules is also affected by the reservoir’s physical properties, the concentration of polymer and crosslinking agent, the types of chemical agent and other factors. According to actual needs, some polymer capsules also need to be unblocked [
10,
11]. The current method of comprehensive profile control and use of displacement agents, whether focusing on profile control or oil displacement technology, has certain limitations. Only a combination of the two can achieve the dual effects of profile control and oil displacement.
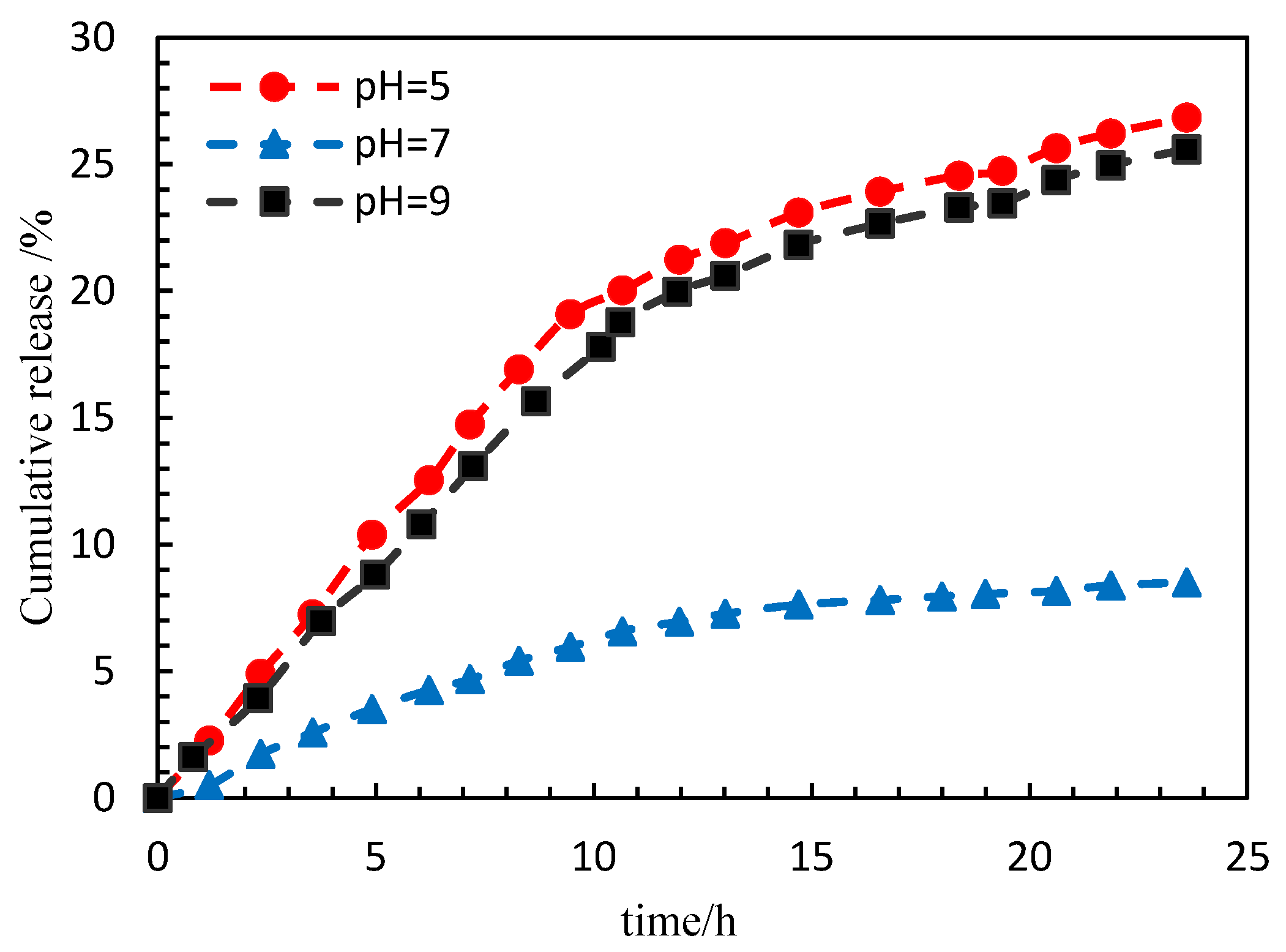
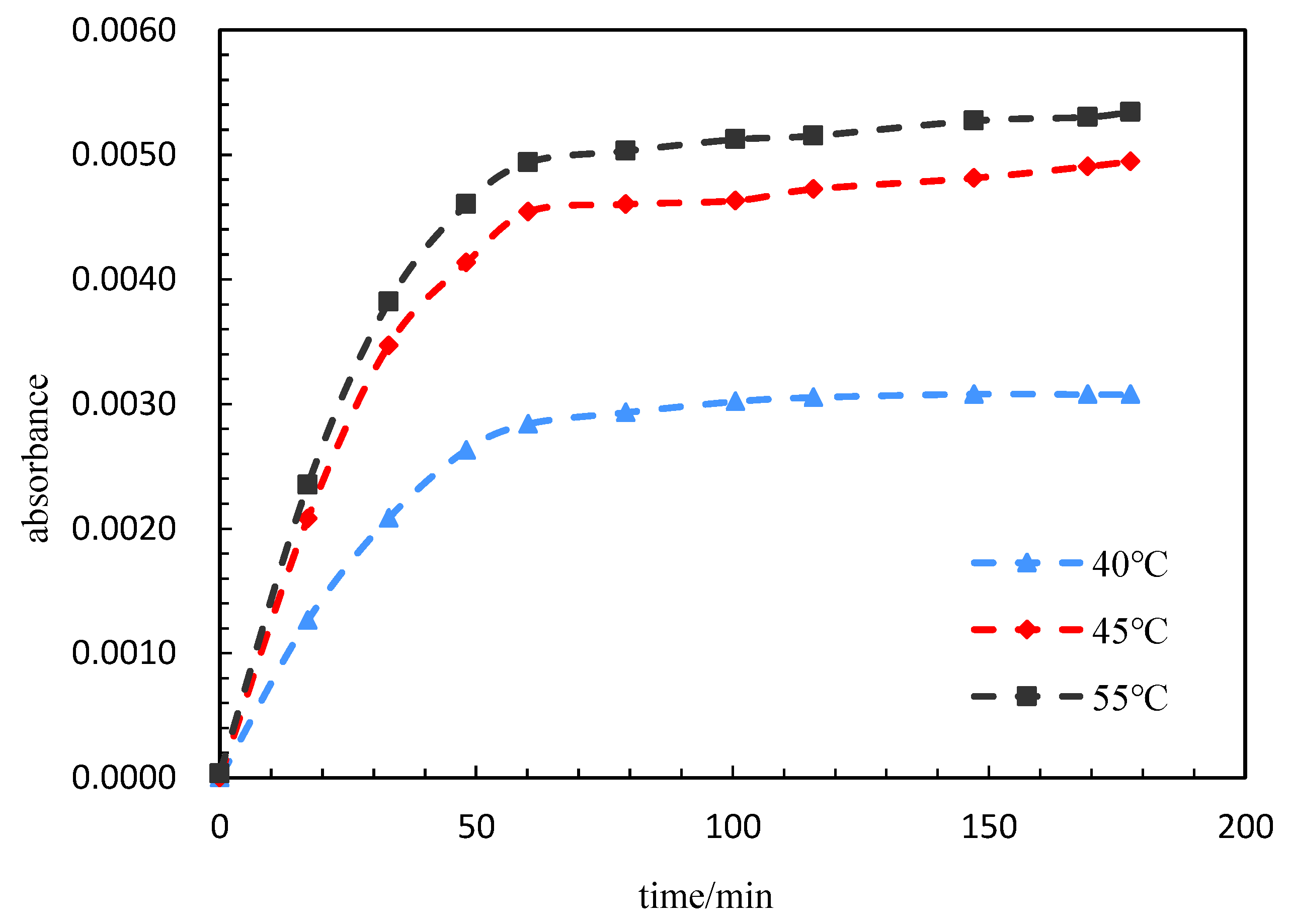
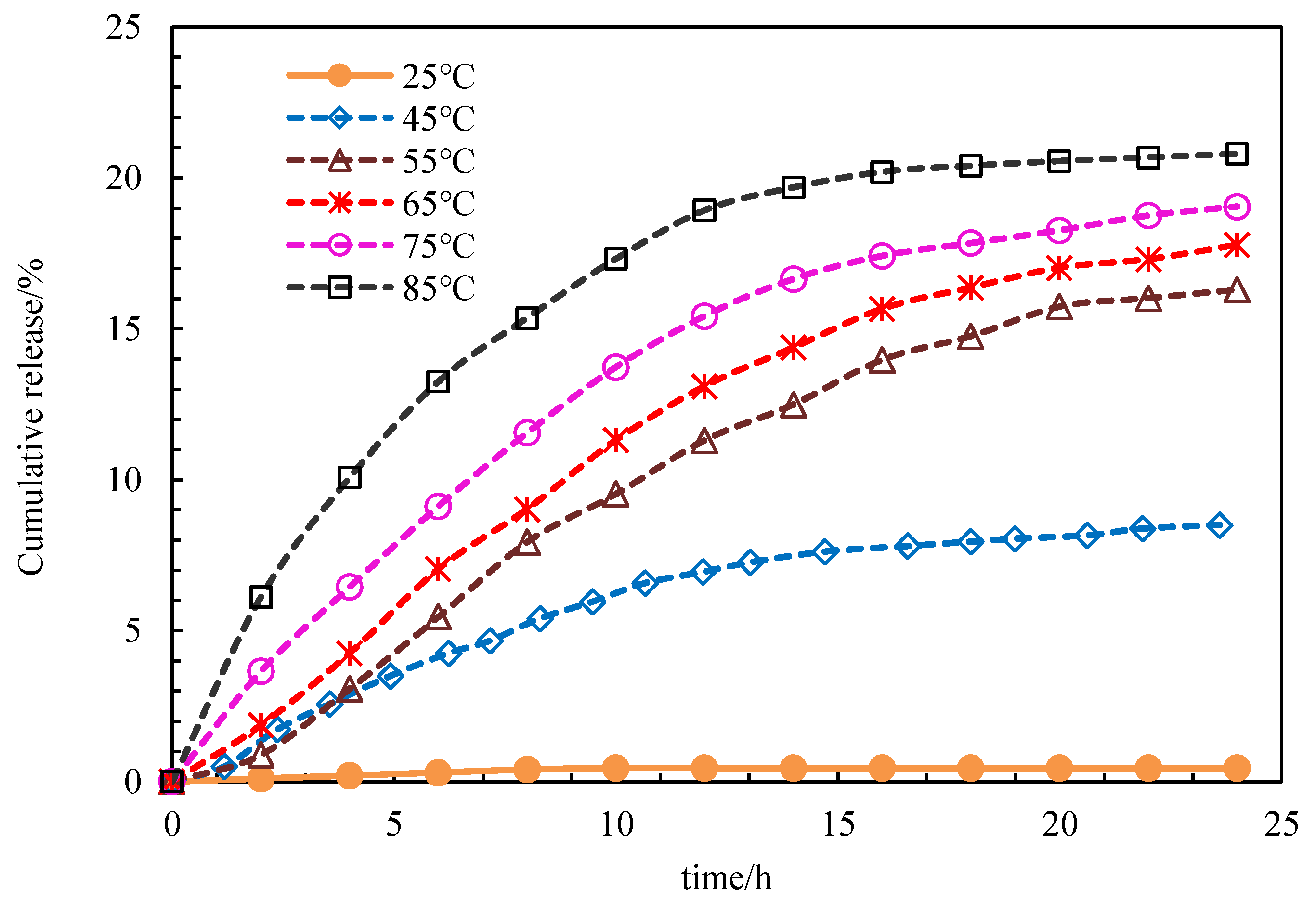
In order to improve the plugging effect in the dominant leaking area, controllable release micro/nanocapsules and displacement agents with low initial viscosity and environmental response have been screened and evaluated in the laboratory. The results show that intelligent nanocapsule systems can effectively block the water channel, can change the flow direction of the injected fluid, can improve the reservoir heterogeneity, can improve the sweep and oil displacement efficiency and have the potential to improve the recovery of low-permeability reservoirs [
12].
Environmental response-controlled nanocapsules are a matrix with fully dispersed and uniform effective components. The principle of controlled release mainly includes five forms: diffusion (1), dissolution (2), dissolution (3), osmotic pressure (4) and ion exchange (5) [
13,
18,
19,
20]. Of these, (1), the diffusion principle, is the process in which the effective components are first dissolved and then diffused out of the nanocapsule. (2) is the dissolution principle, which requires that the solubility of the effective components is significantly reduced in advance so that a concentration difference is formed in the release process, so as to increase the particle size of the nanocapsule, reduce the dissolution rate and realize controlled release. (3), the principle of dissolution, is the dual function of diffusion and dissolution. (4), the principle of osmotic pressure, is that a polymer is used as the coating on the outside and the core material is water-soluble. The coating forms a semi permeable membrane, and water can penetrate the membrane to maintain the saturation state of the internal components, so as to achieve the effect of controlled release. (5), the principle of ion exchange, requires the exchange of components in the inner and outer space and the release of effective components after this exchange. Generally speaking, the release can be summarized as the way in which the permeability is changed or the permeable membrane is dissolved or degraded by water immersion or heating [
21,
22]. Considering that the pore throat size matching problem exists in the migration of nanocapsules in porous media, the sensitive response of nanocapsules is not only the temperature response of the target layer of the reservoir, but also the timed dissolution response formed by coordinating and regulating the thickness of the capsule wall. After a nanocapsule is injected from the wellhead, and before it has responded to the temperature, it stays at the position with relatively small pore throat size [
23,
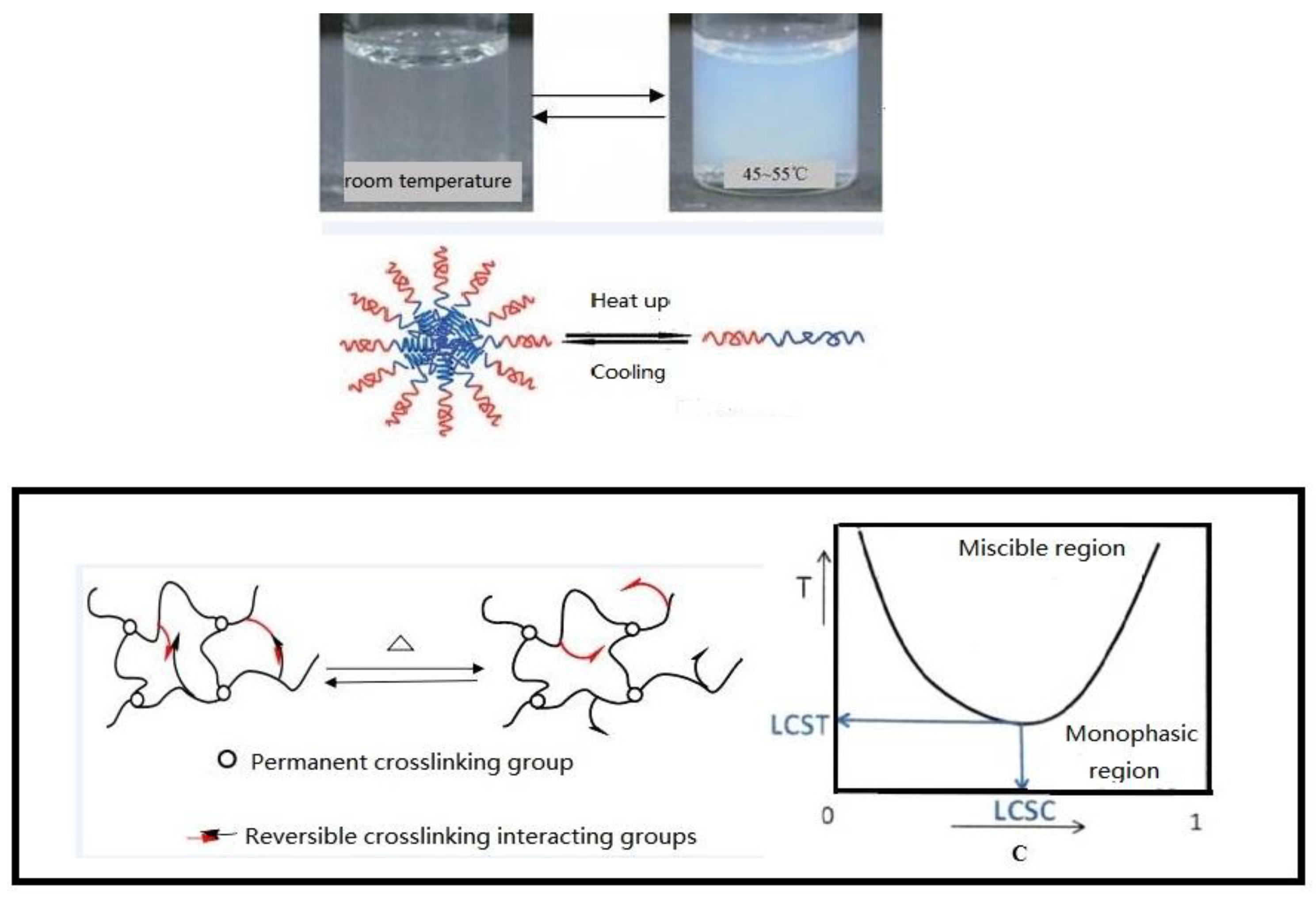
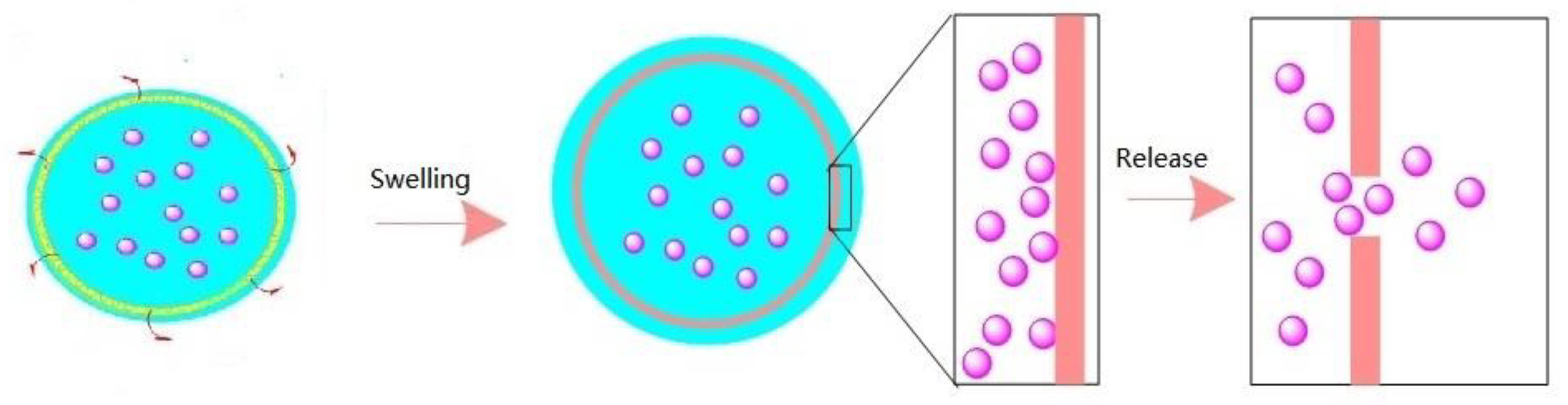
24]. After a certain amount of time, the capsule wall dissolves, so that a blocking phenomenon is observed. Temporary delays to this process can also play a role in expanding the swept volume and play a positive role in improving the subsequent displacement efficiency. Using supramolecular interactions or dynamic covalent bonds as molecular switches can temporarily alter the shape of a nanocapsule. As shown in
Figure 1, when the ambient temperature reaches the lower critical solution temperature (LOST), the chemical stability of the outer surface of the core–shell structure changes. When the temperature is higher than the LOST, hydrophobic association occurs in the side chain of the polymer, which is hydrophobic. At this time, the polymer as a whole is amphiphilic [
25]. At this time, due to physical crosslinking, the polymer has shear thickening and good salt resistance.
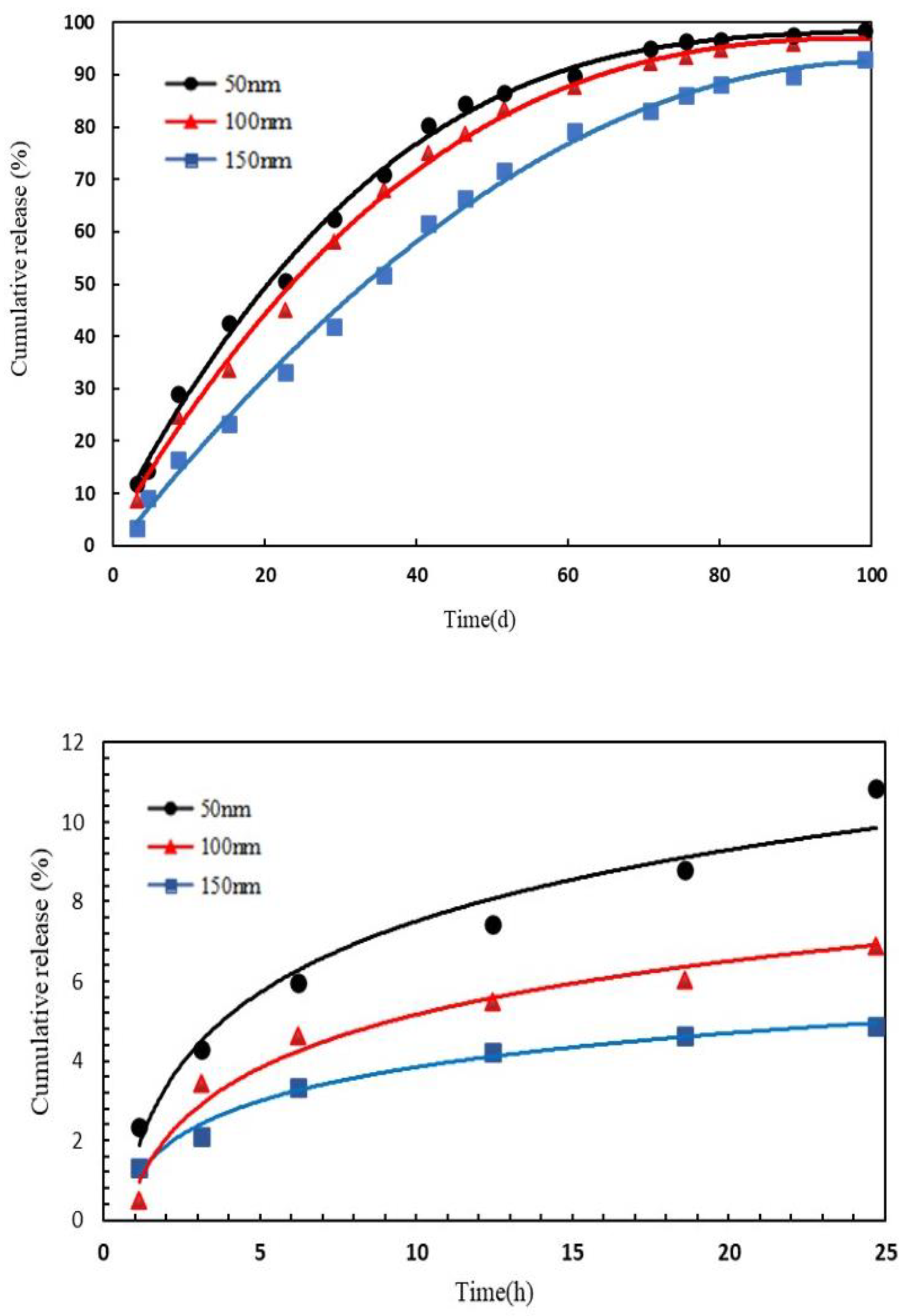
The release mechanism is shown in
Figure 1. Based on the original intention of preparing the target nanocapsule, the core–shell structure and shell are dissolved, the effective components are dissolved into solution, and then are diffused out of the nanocapsule into the porous medium. The release rate of the nanocapsule is affected by several main factors; (1) the core material solubility; (2) the core material diffusion coefficient; (3) the shell properties and thickness; and (4) the release environmental temperature. The nanocapsule shell developed in this paper is an amphiphilic copolymer, AP-g-PNIPAAM, which has a certain viscosity and can emulsify with crude oil. Janus functional particles are embedded in the amphiphilic AP-g-PNIPAAM, and AP-g-PNIPAAM gradually dissolves at the response temperature, releasing at a constant rate with time. Based on the above analysis, it is necessary to clarify the release characteristics of the nanocapsule in porous media.