Carrier Variety Used in Immobilization of His6-OPH Extends Its Application Areas
Abstract
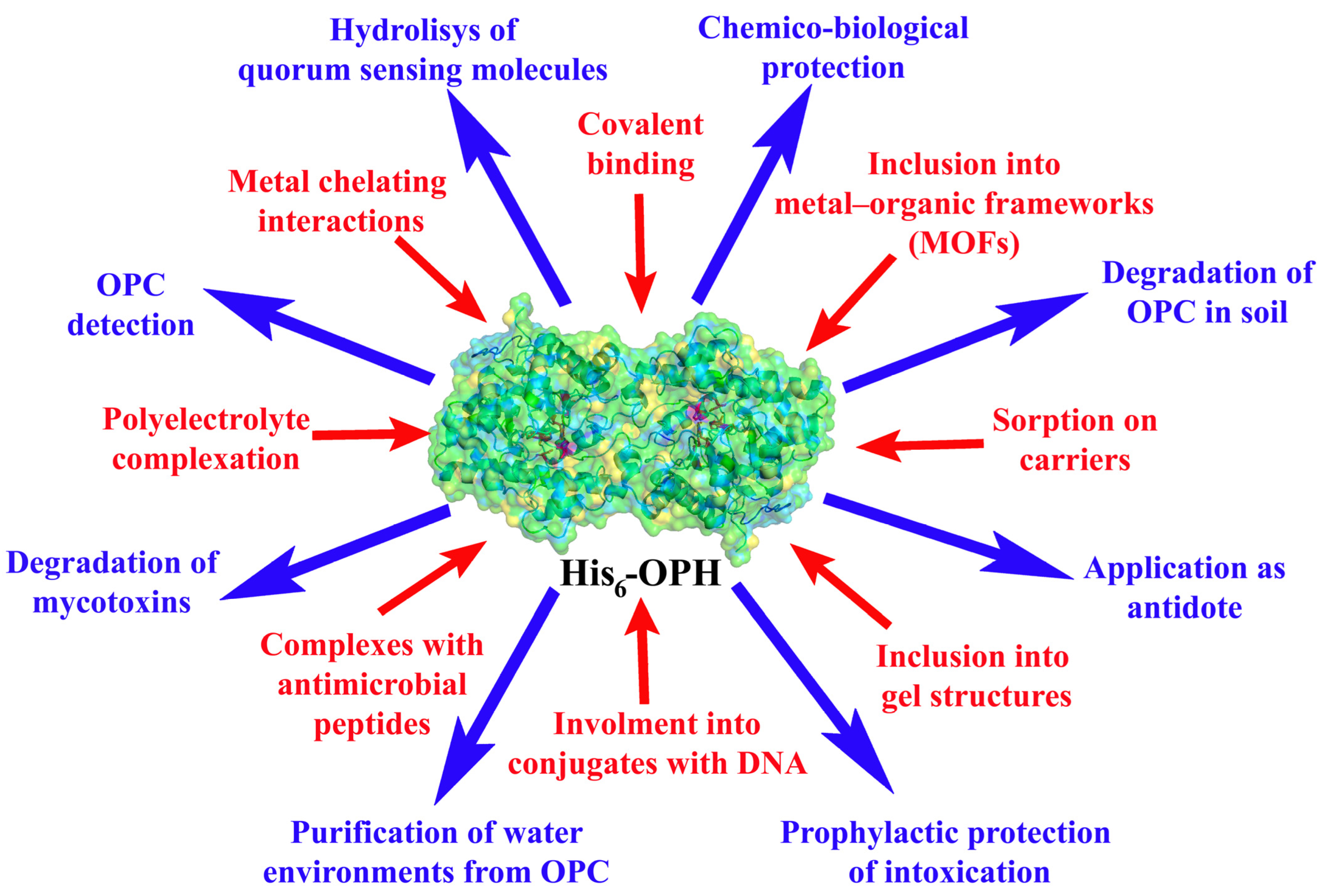
1. Introduction
2. Stabilization of Hexa-Histidine-Containing OPH for OPC Hydrolysis
3. His-Tagged OPH Involved in the Immobilized Antimicrobial Combinations
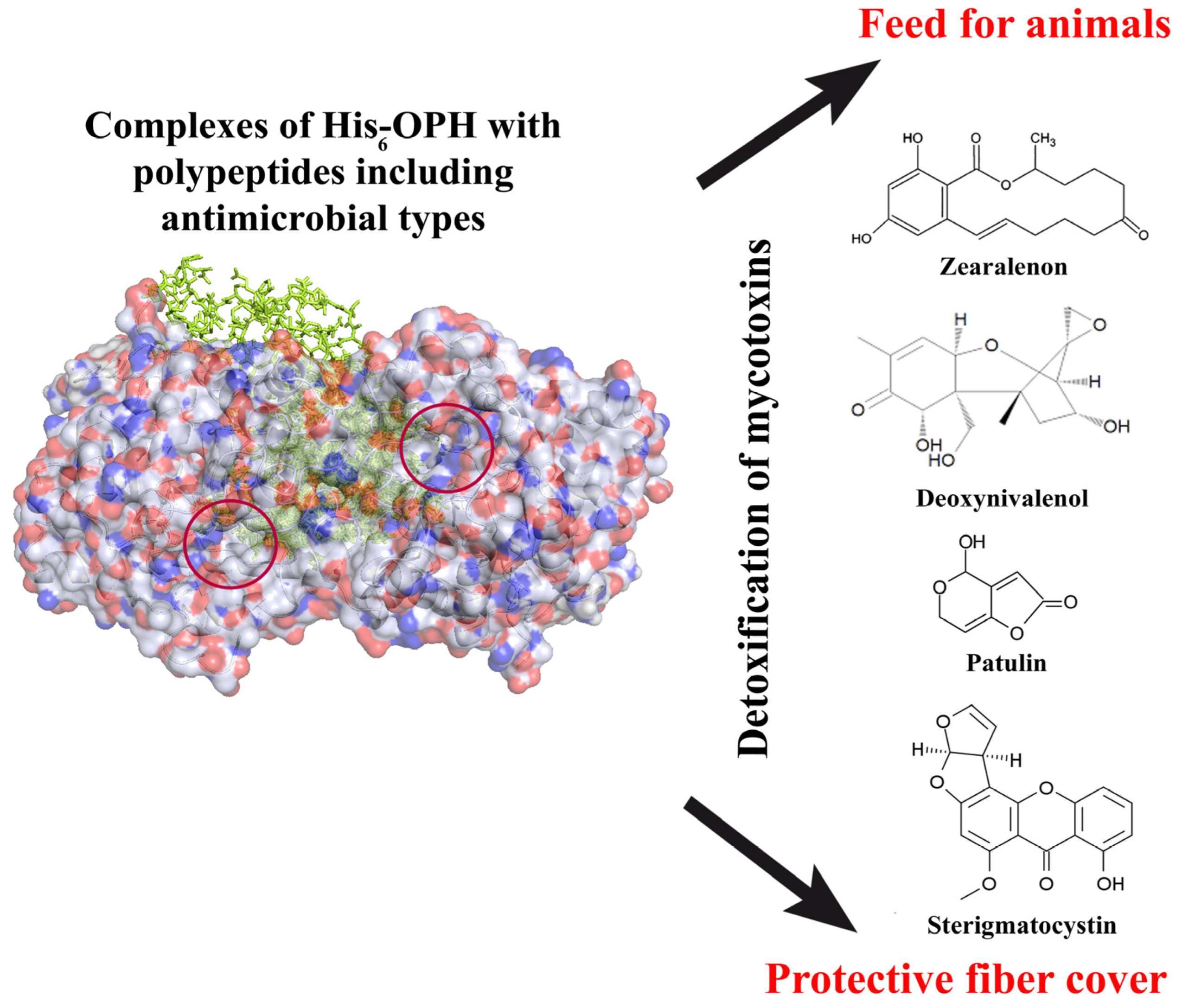
4. Stabilized and Immobilized His-Tagged OPH in the Hydrolysis of Mycotoxins
5. Comparative Analysis of Biocatalysts Based on Immobilized/Stabilized His6-OPH
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alejo-González, K.; Hanson-Viana, E.; Vazquez-Duhalt, R. Enzymatic detoxification of organophosphorus pesticides and related toxicants. J. Pestic. Sci. 2018, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, S.D.; Efremenko, E.N. Organophosphorus Neurotoxins; RIOR: Moscow, Russia, 2020; p. 380. ISBN 978-5-369-02026-5. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Overview of a bioremediation tool: Organophosphorus hydrolase and its significant application in the food, environmental, and therapy fields. Appl. Microbiol. Biotechnol. 2021, 105, 8241–8253. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes, reacting with organophosphorus compounds as detoxifiers: Diversity and functions. Int. J. Mol. Sci. 2021, 22, 1761. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Andrianova, M.S.; Efremenko, E.N. Extensive hydrolysis of phosphonates as unexpected behaviour of the known His6-organophosphorus hydrolase. Appl. Microbiol. Biotechnol. 2016, 100, 5829–5838. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Efremenko, E. Theoretical evaluation of suspected enzymatic hydrolysis of Novichok agents. Catal. Commun. 2019, 120, 91–94. [Google Scholar] [CrossRef]
- Latip, W.; Knight, V.F.; Abdul Halim, N.; Ong, K.K.; Mohd Kassim, N.A.; Wan Yunus, W.M.Z.; Mohd Noor, S.A.; Mohamad Ali, M.S. Microbial phosphotriesterase: Structure, function, and biotechnological applications. Catalysts 2019, 9, 671. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Klyachko, N.L.; Bronich, T.; Zavyalova, N.V.; Jiang, Y.; Kabanov, A.V. A simple and highly effective catalytic nanozyme scavenger for organophosphorus neurotoxins. J. Control. Release 2017, 247, 175–181. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, E.J.; Tsao, C.; Kasten, S.A.; Boeri, M.V.; Dao, T.L.; DeBus, S.J.; Cadieux, C.L.; Baker, C.A.; Otto, T.C.; et al. Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents. Sci. Transl. Med. 2019, 11, eaau7091. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, P.; Joshi, A.; Kodgire, P. Advances in detection of hazardous organophosphorus compounds using organophosphorus hydrolase based biosensors. Crit. Rev. Toxicol. 2019, 49, 387–410. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, S.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Recent advances and future prospective of organophosphorus-degrading enzymes: Identification, modification, and application. Crit. Rev. Biotechnol. 2021, 41, 1096–1113. [Google Scholar] [CrossRef]
- Chi, M.C.; Liao, T.Y.; Lin, M.G.; Lin, L.L.; Wang, T.F. Expression and physiochemical characterization of an N-terminal polyhistidine-tagged phosphotriesterase from the soil bacterium Brevundimonas diminuta. Biocatal. Agric. Biotechnol. 2020, 29, 101811. [Google Scholar] [CrossRef]
- Mali, H.; Shah, C.; Patel, D.; Trivedi, U.; Subramanian, R. Bio-catalytic system of metallohydrolases for remediation of neurotoxin organophosphates and applications with a future vision. J. Inorg. Biochem. 2022, 231, 111771. [Google Scholar] [CrossRef]
- Manco, G.; Porzio, E.; Suzumoto, Y. Enzymatic detoxification: A sustainable means of degrading toxic organophosphate pesticides and chemical warfare nerve agents. J. Chem. Technol. Biot. 2018, 93, 2064–2082. [Google Scholar] [CrossRef]
- Chi, M.-C.; Liao, T.-Y.; Lin, M.-G.; Lin, L.-L.; Wang, T.-F. Catalytic performance of a recombinant organophosphate-hydrolyzing phosphotriesterase from Brevundimonas diminuta in the presence of surfactants. Catalysts 2021, 11, 597. [Google Scholar] [CrossRef]
- Efremenko, E.; Lyagin, I.; Votchitseva, Y.; Sirotkina, M.; Varfolomeyev, S. Polyhistidine-containing organophosphorus hydrolase with outstanding properties. Biocatal. Biotransfor. 2007, 25, 103–108. [Google Scholar] [CrossRef]
- Bigley, A.N.; Raushel, F.M. The evolution of phosphotriesterase for decontamination and detoxification of organophosphorus chemical warfare agents. Chem. Biol. Interact. 2019, 308, 80–88. [Google Scholar] [CrossRef]
- Katyal, P.; Chu, S.; Montclare, J. Enhancing organophosphate hydrolase efficacy via protein engineering and immobilization strategies. Ann. N. Y. Acad. Sci. 2020, 1480, 54–72. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to quorum quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879. [Google Scholar] [CrossRef]
- Lyagin, I.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Efremenko, E. Not a mistake but a feature: Promiscuous activity of enzymes meeting mycotoxins. Catalysts 2022, 12, 1095. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Pourhassan-Moghaddam, M.; Wood, D.W.; Majdi, H.; Zarghami, N. Current affinity approaches for purification of recombinant proteins. Cogent Biol. 2019, 5, 1665406. [Google Scholar] [CrossRef]
- Kumar, A. (Ed.) Supermacroporous Cryogels: Biomedical and Biotechnological Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 496. ISBN 9780367869472. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Gao, J.; Liu, H.; Xue, S.; Ma, L.; Cao, G.; Huang, Z.; Jiang, Y. Facile oriented immobilization and purification of His-tagged organophosphohydrolase on virus-like mesoporous silica nanoparticles for organophosphate bioremediation. ACS Sustain. Chem. Eng. 2018, 6, 13588–13598. [Google Scholar] [CrossRef]
- Xue, S.; Li, J.; Zhou, L.; Gao, J.; Liu, G.; Ma, L.; He, Y.; Jiang, Y. Simple purification and immobilization of his-tagged organophosphohydrolase from cell culture supernatant by metal organic frameworks for degradation of organophosphorus pesticides. J. Agric. Food Chem. 2019, 67, 13518–13525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Shi, Q.; Dong, X.; Sun, Y. Facile purification and immobilization of organophosphorus hydrolase on protein-inorganic hybrid phosphate nanosheets. Chin. J. Chem. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Mehta, J.; Dhaka, S.; Paul, A.K.; Dayananda, S.; Deep, A. Organophosphate hydrolase conjugated UiO-66-NH2 MOF based highly sensitive optical detection of methyl parathion. Environ. Res. 2019, 174, 46–53. [Google Scholar] [CrossRef]
- Mehta, J.; Dhaka, S.; Bhardwaj, N.; Paul, A.K.; Dayananda, S.; Lee, S.-E.; Kim, K.-H.; Deep, A. Application of an enzyme encapsulated metal-organic framework composite for convenient sensing and degradation of methyl parathion. Sens. Actuators B Chem. 2019, 290, 267–274. [Google Scholar] [CrossRef]
- Li, Y.X.; Luan, P.Q.; Zhou, L.Y.; Xue, S.G.; Liu, Y.H.; Liu, Y.T.; Jiang, Y.J.; Gao, J. Purification and immobilization of His-tagged organophosphohydrolase on yolk–shell Co/C@SiO2@Ni/C nanoparticles for cascade degradation and detection of organophosphates. Biochem. Eng. J. 2021, 167, 107895. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Zhang, H.; Xin, Y.; Shi, Y.; Gu, Z.; Zhang, L.; Zhong, J.; Guo, X.; Li, Y.; et al. Development of a multimetal-based phosphotriesterase hybrid nanoflowers for decontamination of environmental organophosphorus compounds pollution. Chem. Eng. J. 2022, 446, 136933. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Yang, Y.; Guan, S.; Wang, X.; Li, H.; Zheng, X.; Zhou, L.; Jiang, Y.; Gao, J. Visible light assisted enzyme-photocatalytic cascade degradation of organophosphorus pesticides. Green Chem. Eng. 2022, in press. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y. A hybrid nanobiocatalyst with in situ encapsulated enzyme and exsolved Co nanoclusters for complete chemoenzymatic conversion of methyl parathion to 4-aminophenol. J. Hazard. Mater. 2022, 424, 127755. [Google Scholar] [CrossRef]
- Latip, W.; Knight, V.F.; Khim, O.K.; Mohd Kasim, N.A.; Wan Yunus, W.M.Z.; Mohamad Ali, M.S.; Mohd Noor, S.A. Immobilization of mutant phosphotriesterase on fuller’s earth enhanced the stability of the enzyme. Catalysts 2021, 11, 983. [Google Scholar] [CrossRef]
- Day, G.J.; Zhang, W.H.; Carter, B.M.; Xiao, W.; Sambrook, M.R.; Perriman, A.W. A rationally designed supercharged protein-enzyme chimera self-assembles in situ to yield bifunctional composite textiles. ACS Appl. Mater. Interfaces 2021, 13, 60433–60445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Carter, B.M.; Day, G.J.; Govan, N.; Jackson, C.; Perriman, A.W. Sequential electrostatic assembly of a polymer surfactant corona increases activity of the phosphotriesterase arPTE. Bioconjug. Chem. 2019, 30, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Day, G.J.; Zampetakis, I.; Carrabba, M.; Zhang, Z.; Carter, B.M.; Govan, N.; Jackson, C.; Chen, M.; Perriman, A.W. Three-dimensional printable enzymatically active plastics. CS Appl. Polym. Mater. 2021, 3, 6070–6077. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhao, Y.; Luo, X.-J.; Xu, D.-S.; Cao, X.; Xu, J.-H.; Zhang, X.-Y.; Ge, J.; Bai, Y. Cross-linked enzyme–polymer conjugates with excellent stability and detergent-enhanced activity for efficient organophosphate degradation. Bioresour. Bioprocess. 2018, 5, 49. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Yu, L.; Shi, Q.; Dong, X.; Sun, Y. Zwitterionic polymer-mediated immobilization of organophosphorus hydrolase enhances hydrolysis of methyl parathion by substrate enrichment. Biochem. Eng. J. 2022, 184, 108491. [Google Scholar] [CrossRef]
- Moon, Y.; Jafry, A.T.; Kang, S.B.; Seo, J.Y.; Baek, K.Y.; Kim, E.J.; Pan, J.G.; Choi, J.Y.; Kim, H.J.; Lee, K.H.; et al. Organophosphorus hydrolase-poly-β-cyclodextrin as a stable self-decontaminating bio-catalytic material for sorption and degradation of organophosphate pesticide. J. Hazard. Mater. 2019, 365, 261–269. [Google Scholar] [CrossRef]
- Ogura, K.; Rehm, B.H.A. Alginate encapsulation of bioengineered protein-coated polyhydroxybutyrate particles: A new platform for multifunctional composite materials. Adv. Funct. Mater. 2019, 29, 1901893. [Google Scholar] [CrossRef]
- Wong, J.X.; Gonzalez-Miro, M.; Sutherland-Smith, A.J.; Rehm, B.H.A. Covalent functionalization of bioengineered polyhydroxyalkanoate spheres directed by specific protein-protein interactions. Front. Bioeng. Biotechnol. 2020, 8, 44. [Google Scholar] [CrossRef]
- Breger, J.C.; Buckhout-White, S.; Walper, S.A.; Oh, E.; Susumu, K.; Ancona, M.G.; Medintz, I.L. Assembling high activity phosphotriesterase composites using hybrid nanoparticle peptide-DNA scaffolded architectures. Nano Futures 2017, 1, 011002. [Google Scholar] [CrossRef]
- Samanta, A.; Breger, J.C.; Susumu, K.; Oh, E.; Walper, S.A.; Bassim, N.; Medintz, I.L. DNA–nanoparticle composites synergistically enhance organophosphate hydrolase enzymatic activity. ACS Appl. Nano Mater. 2018, 1, 3091–3097. [Google Scholar] [CrossRef]
- Hong, X.; Cholko, T.; Chang, C.A.; Wheeldon, I. Multiscale simulation-guided design of enzyme bioconjugates with enhanced catalysis. Chem Catal. 2022, 2, 2691–2703. [Google Scholar] [CrossRef]
- Chen, J.; Webb, J.; Shariati, K.; Guo, S.; Montclare, J.-K.; Scott McArt, S.; Ma, M. Pollen-inspired enzymatic microparticles to reduce organophosphate toxicity in managed pollinators. Nat. Food 2021, 2, 339–347. [Google Scholar] [CrossRef]
- Senko, O.; Maslova, O.; Efremenko, E. Optimization of the use of His6-OPH-based enzymatic biocatalysts for the destruction of chlorpyrifos in soil. Int. J. Environ. Res. Public Health 2017, 14, 1438. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121. [Google Scholar] [CrossRef]
- DelRe, C.; Huang, C.; Dennis, P.; Drockenmuller, E.; Xu, T. Reusable enzymatic fiber mats for neurotoxin remediation in water. ACS Appl. Mater. Interfaces 2018, 10, 44216–44220. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A critical review on metal-organic frameworks and their composites as advanced materials for adsorption and photocatalytic degradation of emerging organic pollutants from wastewater. Polymers 2020, 12, 2648. [Google Scholar] [CrossRef]
- Ye, N.; Kou, X.; Shen, J.; Huang, S.; Chen, G.; Ouyang, G. Metal-organic frameworks: A new platform for enzyme immobilization. ChemBioChem 2020, 21, 2585–2590. [Google Scholar] [CrossRef]
- An, H.; Song, J.; Wang, T.; Xiao, N.; Zhang, Z.; Cheng, P.; Ma, S.; Huang, H.; Chen, Y. Metal-organic framework disintegrants: Enzyme preparation platforms with boosted activity. Angew. Chem. Int. Ed. Engl. 2020, 59, 16764–16769. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]
- Gorzkowska-Sobas, A.; Lausund, K.B.; de Koning, M.C.; Petrovic, V.; Chavan, S.M.; Smith, M.W.; Nilsen, O. Utilizing zirconium MOF-functionalized fiber substrates prepared by molecular layer deposition for toxic gas capture and chemical warfare agent degradation. Glob. Chall. 2021, 5, 2100001. [Google Scholar] [CrossRef] [PubMed]
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal-organic frameworks against toxic chemicals. Chem. Rev. 2020, 120, 8130–8160. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jia, S. Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Shcharbin, D.; Halets-Bui, I.; Abashkin, V.; Dzmitruk, V.; Loznikova, S.; Odabaşı, M.; Acet, Ö.; Önal, B.; Özdemir, N.; Shcharbina, N.; et al. Hybrid metal-organic nanoflowers and their application in biotechnology and medicine. Colloids Surf. B 2019, 182, 110354. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Nodoushan, H.; Mojtabavi, S.; Faramarzi, M.A.; Samadi, N. Organic-inorganic hybrid nanoflowers: The known, the unknown, and the future. Adv. Colloid Interface Sci. 2022, 309, 102780. [Google Scholar] [CrossRef]
- Afriat, L.; Roodveldt, C.; Manco, G.; Tawfik, D.S. The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry 2006, 45, 13677–13686. [Google Scholar] [CrossRef]
- Afriat-Jurnou, L.; Jackson, C.J.; Tawfik, D.S. Reconstructing a missing link in the evolution of a recently diverged phosphotriesterase by active-site loop remodeling. Biochemistry 2012, 51, 6047–6055. [Google Scholar] [CrossRef]
- Sirotkina, M.; Efremenko, E.N. Rhodococcus lactonase with organophosphate hydrolase (OPH) activity and His6-tagged OPH with lactonase activity: Evolutionary proximity of the enzymes and new possibilities in their application. Appl. Microbiol. Biotechnol. 2014, 98, 2647–2656. [Google Scholar] [CrossRef]
- Castro, A.A.; Assis, L.C.; Silva, D.R.; Corrêa, S.; Assis, T.; Gajo, G.C.; Soares, F.V.; Ramalho, T.C. Computational enzymology for degradation of chemical warfare agents: Promising technologies for remediation processes. AIMS Microbiol. 2017, 3, 108–135. [Google Scholar] [CrossRef]
- Cavalcante, S. Biodegradation of organophosphorus compounds predicted by enzymatic process using molecular modelling and observed in soil samples through analytical techniques and microbiological analysis: A comparison. Molecules 2019, 25, 58. [Google Scholar] [CrossRef]
- Petrosino, F.; Curcio, S.; Chakraborty, S.; de Luca, G. Enzyme immobilization on polymer membranes: A quantum and molecular mechanics study. Computation 2019, 7, 56. [Google Scholar] [CrossRef]
- Maslova, O.; Aslanli, A.; Stepanov, N.; Lyagin, I.; Efremenko, E. Catalytic characteristics of new antibacterials based on hexahistidine-containing organophosphorus hydrolase. Catalysts 2017, 7, 271. [Google Scholar] [CrossRef]
- Lyagin, I.; Stepanov, N.; Presnov, D.; Trifonov, A.; Efremenko, E. Self-assembling enzymatic nanocomplexes with polypeptides and low-weight organic compounds: Preparation, characterization, and application of new antibacterials. Int. J. Mol. Sci. 2023, 24, 1831. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Charges’ interaction in polyelectrolyte (nano)complexing of His6-OPH with peptides: Unpredictable results due to imperfect or useless concept? Int. J. Biol. Macromol. 2019, 140, 368–376. [Google Scholar] [CrossRef]
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. “Universal” antimicrobial combination of bacitracin and His6-OPH with lactonase activity, acting against various bacterial and yeast cells. Int. J. Mol. Sci. 2022, 23, 9400. [Google Scholar] [CrossRef]
- Aslanli, A.; Lyagin, I.; Stepanov, N.; Presnov, D.; Efremenko, E. Bacterial cellulose containing combinations of antimicrobial peptides with various QQ enzymes as a prototype of an “enhanced antibacterial” dressing: In silico and in vitro data. Pharmaceutic 2020, 12, 1155. [Google Scholar] [CrossRef]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.A.; Lyagin, I.; Lozinsky, V.I.; Efremenko, E.N. Enzymatically functionalized composite materials based on nanocellulose and poly(vinyl alcohol) cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef]
- Lyagin, I.; Stepanov, N.; Frolov, G.; Efremenko, E. Combined modification of fiber materials by enzymes and metal nanoparticles for chemical and biological protection. Int. J. Mol. Sci. 2022, 23, 1359. [Google Scholar] [CrossRef]
- Lyagin, I.; Maslova, O.; Stepanov, N.; Presnov, D.; Efremenko, E. Assessment of composite with fibers as a support for antibacterial nanomaterials: A case study of bacterial cellulose, polylactide and usual textile. Fibers 2022, 10, 70. [Google Scholar] [CrossRef]
- Schneider, Y.K. Bacterial natural product drug discovery for new antibiotics: Strategies for tackling the problem of antibiotic resistance by efficient bioprospecting. Antibiotics 2021, 10, 842. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Nahle, S.; El Khoury, A.; Savvaidis, I.; Chokr, A.; Louka, N.; Atoui, A. Detoxification approaches of mycotoxins: By microorganisms, biofilms and enzymes. Food Contam. 2022, 9, 3. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef]
- Dor, S.; Prusky, D.; Afriat-Jurnou, L. Bacterial quorum-quenching lactonase hydrolyzes fungal mycotoxin and reduces pathogenicity of Penicillium expansum—Suggesting a mechanism of bacterial antagonism. J. Fungi 2021, 7, 826. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Z.; Xu, W.; Xu, W.; Zhang, W.; Guang, C.; Mu, W. Zearalenone lactonase: Characteristics, modification, and application. Appl. Microbiol. Biotechnol. 2022, 106, 6877–6886. [Google Scholar] [CrossRef]
- He, M.; Li, Y.; Pi, F.; Ji, J.; He, X.; Zhang, Y.; Sun, X. A novel detoxifying agent: Using rice husk carriers to immobilize zearalenone-degrading enzyme from Aspergillus niger FS10. Food Control 2016, 68, 271–279. [Google Scholar] [CrossRef]
- Azam, M.S.; Yu, D.; Liu, N.; Wu, A. Degrading ochratoxin A and zearalenone mycotoxins using a multifunctional recombinant enzyme. Toxins 2019, 11, 301. [Google Scholar] [CrossRef]
- Lyagin, I.; Maslova, O.; Stepanov, N.; Efremenko, E. Degradation of mycotoxins in mixtures by combined proteinous nanobiocatalysts: In silico, in vitro and in vivo. Int. J. Biol. Macromol. 2022, 218, 866–877. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Dried-reswollen immobilized biocatalysts for detoxification of organophosphorous compounds in the flow systems. Appl. Biochem. Biotechnol. 2009, 159, 251–260. [Google Scholar] [CrossRef]
| Variants of His-Tagged OPH or PTE [Reference] | Immobilization Technique and Carrier | Properties | Application |
|---|---|---|---|
| Use of metalorganic interactions and frameworks | |||
| OpdA@Ni-NTA-VMSN [23] | Ni2+-nitrilotriacetic acid (NTA)-modified, virus-like, mesoporous silica nanoparticles | The affine capacity of substrates became higher for the enzyme after its oriented immobilization | Hydrolysis of methyl parathion in a plug-flow reactor |
| OpdA@MIL-88A [24] | MOF synthesized on the basis of fumaric acid and FeCl3 | The catalytic activity is 5 times higher as compared to the free form of the enzyme. The enzyme possessed improved organic detergent and solvent tolerance and thermal and storage stability | Degradation of organophosphorus pesticides on grapes and cucumbers |
| BSA-Cu@CaPs-OPH [25] | A hybrid organic–inorganic, calcium phosphate-containing nanocrystal, based on the use of bovine serum albumin modified by Cu2+ ions | Improved multiple usages with up to 56% retainment of activity after 10 working cycles; advanced thermal and pH stability | Hydrolysis of methyl parathion on fruits or vegetables |
| OPH6His/UiO-66-NH2 [26] | MOF, synthesized using amino-terephthalic acid and zirconium chloride with further pre-activation by N,N′-dicyclohexylcarbodiimide, was covalently bonded with the enzyme over the surface via UiO-66-NH2 | Improved storage stability (up to 60 days), reusability (in 9 working cycles), and 37% improved catalytic activity | Degradation of methyl parathion |
| OPH6His@Tb-BTC [27] | Encapsulation of the enzyme in MOF, formed by terbium nitrate and 1,2,4-benzenetricarboxylic acid (BTC) | Improved storage stability and a 30% increase in activity | Sensing of methyl parathion |
| OpdA@Co/C@SiO2@Ni/C [28] | An enzyme created multiple affine coordination bonds with Co and Ni introduced into carbonized hybrid nanocomposite ZIF-67@ SiO2 with a yolk-shell structure | Increased pH, thermal and storage stability, and SDS resistance; long reusability (in 7 working cycles) with 60% of residual activity | Chemo-enzymatic cascade degradation of methyl parathion |
| Co/MnHF@PTE [29] | An enzyme was involved in the multimetallic nanoflower structure during its formation in a mixture of metal salt solutions and protein: Co-PTE (CoHF@PTE) and Mn-PTE (MnHF@PTE) hybrid | The improved catalytic activity of 4 times due to the use of two metals (Co and Mn) in the frame of the nanoflower-like structure of MOF | Hydrolysis of methyl parathion, VX and soman |
| OPH@H-Au-TiO2 [30] | An enzyme was adsorbed on the hollow-structured nanoparticles, Au-TiO2 | Increased reusability in the destruction of OPCs and very stable activity due to the synergistic combination of photo- and enzymatic catalysis | Degradation of methyl parathion |
| OPH@0.8CoZIF [31] | An enzyme was encapsulated to Zn-doped Co-based ZIF via biomimetic mineralization | The enzyme is involved in a cascade of chemical reactions where the 4-aminophenol is the last product | Conversion of methyl parathion |
| YT-PTE on FE [32] | A mutant enzyme was immobilized by sorption on the Fuller’s Earth (FE) | The temperature and storage stability were improved, but there were no notable changes in the activity of the immobilized enzyme compared to its free form; several bivalent ions (Co, Ni, Cu, Fe, Zn) exhibited significantly higher increases in the activity of the immobilized enzyme | Hydrolysis of paraoxon |
| Use of fusion proteins and bioconjugations | |||
| [scGFP-arPTE][S−] [33] | Fusion proteins, containing green fluorescent protein (GFP) and PTE, were involved in an electrolytic interaction with monomeric S− with further drying and cross-linking to obtain a catalytically active porous film or cross-linking in the presence of cotton fiber to obtain composite textiles | Improved thermal stability and higher catalytic constant (kcat) | Introduction into cotton fibers for the creation of personalized protective composite material useful for the recyclable hydrolysis of paraoxon |
| [arPTE][S+][S−] [34] | Obtainment of bioconjugates through the subsequent introduction of the enzyme into contact with cationic (Ethoquad) and anionic (oxidized IGEPAL) polymer surfactants (S+ or S−), resulting in the formation of such a structure as corona encapsulating the enzyme | Enhancement of the efficiency of catalytic action by 3 times | Hydrolysis of paraoxon |
| [arPTE][S+][S−] –ABC, [arPTE][S+][S−] –PCL [35] | An enzyme, in the presence of cationic or anionic polymer surfactants (S+ or S−), was lyophilized with further melting of the obtained powder and used in co-dispersion with curtain polymers (ABC, acrylonitrile butadiene styrene; PCL, polycaprolactone) for 3D-printing on the surface of stainless steel rings | Improved stability of enzyme action | Hydrolysis of paraoxon-ethyl by composite enzyme plastics developed for use in self-decontaminating surfaces |
| PoOPHM9-CLEPC [36] | An enzyme containing 10 residues of genetically introduced phenylalanine was conjugated with Pluronic F127 to form a cross-linked enzyme–polymer conjugate (CLEPC) | Increased optimal temperature (50 °C) and pH stability in the range of 7–11 | Hydrolysis of malathion |
| OPH@pID-MSN [37] | An enzyme was covalently immobilized on mesoporous silica nanoparticles (MSNs) coated with a zwitterionic polymer containing short hydrophobic chains, which is a product of the ring-opening reaction between poly (isobutylene-alt-maleic anhydride) and N,N-dimethylethylenediamine (pID) | Significantly improved stability | Hydrolysis of methyl parathion |
| Sorption and covalent immobilization on natural carriers | |||
| OPH/PCD [38] | An enzyme was adsorbed onto the microparticles of poly-β-cyclodextrin (PCD) with further freeze-drying | Increased sorption capacity of the substrate and product of the enzymatic hydrolysis; possible regeneration of activity via new enzyme sorption and the self-decontamination of the biocatalyst | Hydrolysis of methyl paraoxon |
| OpdA-PHB [39] | An enzyme was immobilized on non-porous poly(hydroxyl butyrate) (PHB) microspheres | The carrier increased the sorption of the hydrophobic substrate from the medium | Hydrolysis of coumaphos |
| SpOpdA-SPS-S [40] | An enzyme was immobilized via the covalent binding of His-tag as SpyTag (Sp) to such fusion proteins as SpyCatcher (SPS)-coated poly(hydroxy alkanoates) (PHAs) spheres (S) | Improved catalytic activity and stability | Hydrolysis of coumaphos |
| Conjugation using DNA molecules | |||
| QD-DNA-PTE [41] | An enzyme was attached, via the DNA-containing linker, to PEGylated quantum dots (QDs) | Enhanced efficiency and rate of catalytic reaction (kcat) | Hydrolysis of paraoxon |
| DNA cage-QD-PTE [42] | Molecules of DNA were conjugated into a cage by His5-peptide and modified by quantum dots (QDs) of ZeS with further immobilization of PTE | Enhanced catalytic activity by 12.5 times | Hydrolysis of paraoxon |
| PTEpAzF -DNA [43] | An enzyme was conjugated to a DNA scaffold modified by dibenzocyclooctyl via a site-specific incorporated azido-group to the enzyme molecule structure | Improved catalytic constants | Hydrolysis of paraoxon |
| Formation of complexes | |||
| OPT−PIMs [44] | An enzyme (OPT) was involved in the formation of pollen-inspired microparticles (PIMs), prepared based on complexation between CaCO3, gelatin, and the enzyme | Improved stability at 50 °C and low pH 4.8 (citric acid/sodium citrate buffer) | Detoxification of pollen contaminated by paraoxon or malathion |
| His6-OPH/PLE50, His6-OPH/PLD50 [45] | Enzyme was involved in polyelectrolyte complexes with poly-l-glutamic acid (PLE50) or poly-l-aspartic acid (PLD50) | Increased stability of catalytic action in the soil | Destruction of chlorpyrifos in different types of soil |
| His6OPH/PEG113PLE10, His6OPH/PEG113PLE50, His6OPH/PEG113PLE100, His6OPH/PEG113PLD50, His6OPH/PEG22PLE50, His6OPH/PLE50PEG113PLE50 His6OPH/Hydroxyethyl starch His6OPH/Succinylated gelatin [46] | An enzyme was involved in polyelectrolyte complexes with PEGylated poly-l-glutamic acid (PLE10-100), poly-l-aspartic acid (PLD50) of a different polymerization degree, hydroxyethyl starch, or succinylated gelatin | 20–40% increased catalytic efficiency of enzyme action | Hydrolysis of methyl parathion and paraoxon |
| PCL-RHP-OPH [47] | An enzyme was immobilized through electrospinning in poly-ε-caprolactone fibrous mats in the form of a lyophilized complex dissolved in toluene with random heteropolymers (RHPs) | Enhanced stability and reusability (40% residual activity after everyday use for 3 months) | Hydrolysis of methyl parathion and paraoxon |
| Forms of His6-Tagged OPH [Reference] | Method of Enzyme Immobilization | Antimicrobial Agent | Antimicrobial Effect |
|---|---|---|---|
| Polyelectrolyte complexes of His6-OPH | |||
| His6-OPH/PLD50 [64] | The formation of EPC with poly-l-aspartic acid (PLD50) in the presence of an antibiotic | Antibiotic is one of the following: ampicillin gentamicin kanamycin rifampicin | The best result for the catalytic activity of His6-OPH/PLD50 was obtained with the β-lactam antibiotic ampicillin |
| His6-OPH/PLD50 [65] | The formation of EPC with poly-l-aspartic acid (PLD50), with the further introduction of polymyxin B and emodin | Polymyxin B and inhibitor of quorum sensing | The triple nanoparticles composed of the QS effector, QQ enzyme, and antibiotic demonstrated a significantly improved antimicrobial effect |
| His6-OPH/AMP [66] | The formation of EPC with an antimicrobial peptide (AMP) | AMP is one of following: indolicidin temporin A polymyxin B polymyxin E hepcidin dermcidin bactenecin 2A cecropin A cecropin B kappacin A enkelytin CAP-18 | A positive effect was obtained only for the His6-OPH/AMP when indolicidin or temporin A was applied as the AMP partner for the enzyme |
| His6-OPH/Bacitracin [67] | The formation of EPC with an antimicrobial peptide, such as bacitracin | Bacitracin | An increase of 3.5–8.5 times the antibiotic effect was in relation to gram-negative bacterial cells and yeasts |
| Polyelectrolyte complexes of His6-OPH in/on various materials | |||
| His6-OPH/AMP/BC [68] | The sorption of EPC with an antimicrobial peptide (AMP) onto bacterial cellulose (BC) fiber samples | AMP is one of following: polymyxin B colistin oritavancin dermcidin temporin A indolicidin | A prototype of new dressing material with enhanced antibacterial activity owing to the use of His6-OPH in a complex with polymyxin B or colistin for sorption onto BC |
| His6-OPH/PVA-CG/BC [69] | The entrapment of EPC with an antimicrobial agent (β-lactam antibiotic or antimicrobial peptide) into the poly(vinyl alcohol) cryogel (PVA-CG) with inclusions of bacterial cellulose (BC) | The antimicrobial agent is one of the following: meropenem temporin A indolicidin | A combination of His6-OPH with meropenem or temporin A before its inclusion into the PVA-CG gave the best catalytic stability and antimicrobial activity |
| His6-OPH/PLE50/Polymyxin His6-OPH/PEG-PLE50/Me NPs [70] | The sorption of EPC with PEGylated or non-PEGylated poly-l-glutamic acid (PLE50) on the fiber materials, containing antibiotic or metal nanoparticles (Me NPs) | Antibiotic is one of the following: polymyxin B polymyxin E metal nanoparticles of Zn or Ta | The prototypes of new fiber materials for chemical-biological protection with improvement by 1.5–2.1 times and 2.9 times the antimicrobial effect and hydrolytic activity against OPC, respectively |
| His6-OPH/PLE50/Ta NPs/fiber material [71] | The introduction of EPC with poly-l-glutamic acid (PLE50) into the fibrous materials (bacterial cellulose polylactide or nonwoven fiber material containing 70% viscose and 30% polyester) modified by poly-ε-caprolactone or polyhydroxybutyrate and functionalized by tantalum nanoparticles (Ta NPs) | Tantalum nanoparticles | A notable improvement in the antibacterial effect was confirmed in composite materials with polyhydroxybutyrate and Ta NPs. |
| Type of Stabilization/Immobilization | Main Advantages | Main Disadvantages | References |
|---|---|---|---|
| Metal chelating interactions of His6-OPH | Simple procedure realization; Combination with enzyme purification and isolation from cell debris; Stable long-term usage; High enough catalytic activity due to unshielded of active sites for interaction with substrates | Chaotropic agents and pH of the medium can influence metal chelating interactions of the His6-tag of the enzyme with a used carrier; the activity of obtained biocatalysts depends on the chelating modifier of the carrier and used metal | [2,16,23,82] |
| Covalent binding of His6-OPH | Stable long-term functioning; possible washings of carriers with immobilized enzymes | Notable (30–40%) decrease in catalytic activity as compared to the native enzyme | [2,37,40] |
| Involvement of His6-OPH in interactions with MOF | Various forms of MOFs can be applied; A possible combination of catalytic and biocatalytic reactions | Steric hindrances; decrease in catalytic activity due to diffusion limitations | [24,26,27,29] |
| Sorption of His6-OPH on carriers | Very simple procedure of biocatalyst obtaining; High enough level of catalytic activity as compared to the original enzyme | Desorption depends on various factors; low enough levels of stabilization | [32,38,39,68,70] |
| Polyelectrolyte complexation of His6-OPH | Simple procedure; High catalytic activity | Possible shielding of active cites of the enzyme; decomplexation with the release of His6-OPH | [8,9,33,34,35,45,64,65,66,67,68,70,71] |
| Involvement of His6-OPH in bioconjugation | Simple procedure; High stability of obtained biocatalysts | Possible blocking of active sites; decrease in catalytic activity compared to original enzyme | [33,34,35,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, E.; Lyagin, I.; Aslanli, A.; Stepanov, N.; Maslova, O.; Senko, O. Carrier Variety Used in Immobilization of His6-OPH Extends Its Application Areas. Polymers 2023, 15, 591. https://doi.org/10.3390/polym15030591
Efremenko E, Lyagin I, Aslanli A, Stepanov N, Maslova O, Senko O. Carrier Variety Used in Immobilization of His6-OPH Extends Its Application Areas. Polymers. 2023; 15(3):591. https://doi.org/10.3390/polym15030591
Chicago/Turabian StyleEfremenko, Elena, Ilya Lyagin, Aysel Aslanli, Nikolay Stepanov, Olga Maslova, and Olga Senko. 2023. "Carrier Variety Used in Immobilization of His6-OPH Extends Its Application Areas" Polymers 15, no. 3: 591. https://doi.org/10.3390/polym15030591
APA StyleEfremenko, E., Lyagin, I., Aslanli, A., Stepanov, N., Maslova, O., & Senko, O. (2023). Carrier Variety Used in Immobilization of His6-OPH Extends Its Application Areas. Polymers, 15(3), 591. https://doi.org/10.3390/polym15030591