Analytical Determination of Cephalosporin Antibiotics Using Coordination Polymer Based on Cobalt Terephthalate as a Sorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Antibiotics
2.3. Synthesis of Coordination Polymer
2.4. Characterization
2.5. Experiments on Equilibrium Adsorption of Antibiotics
2.6. Adsorption Experiments
2.7. Study of Adsorption Kinetics
2.8. An Experiment on The Separation of Antibiotics in a Chromatographic Column
3. Results
3.1. Synthesis and Identification of Cobalt Terephthalate
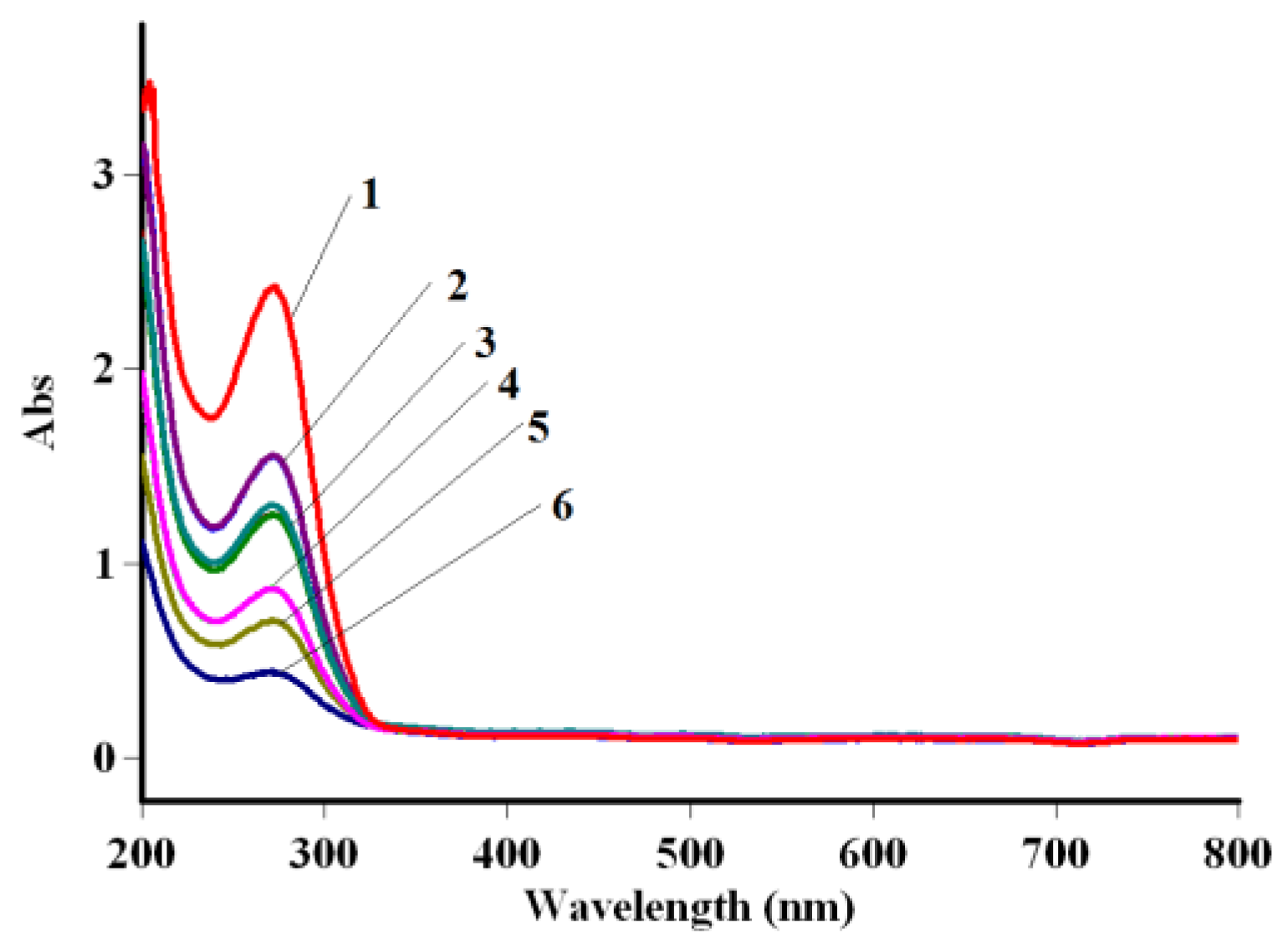
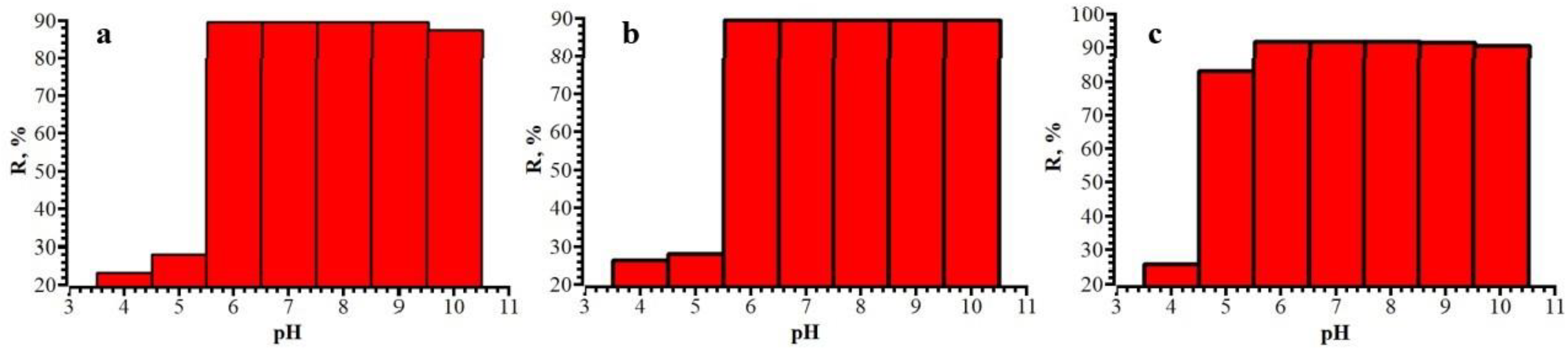
3.2. Solid Phase Extraction of Cephalosporin Antibiotics
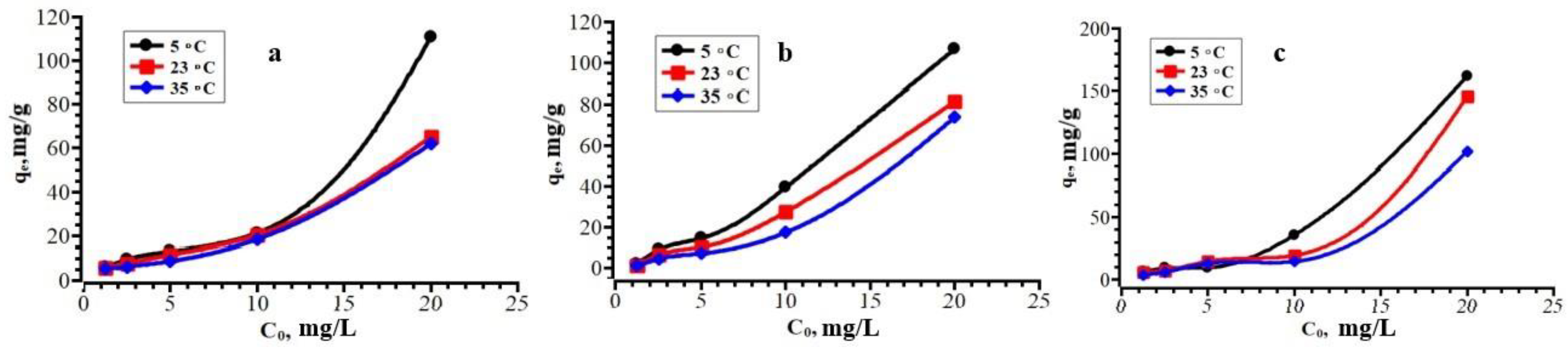
3.3. Adsorption Isotherms
3.4. Adsorption Kinetics
3.5. Thermodynamics of Adsorption
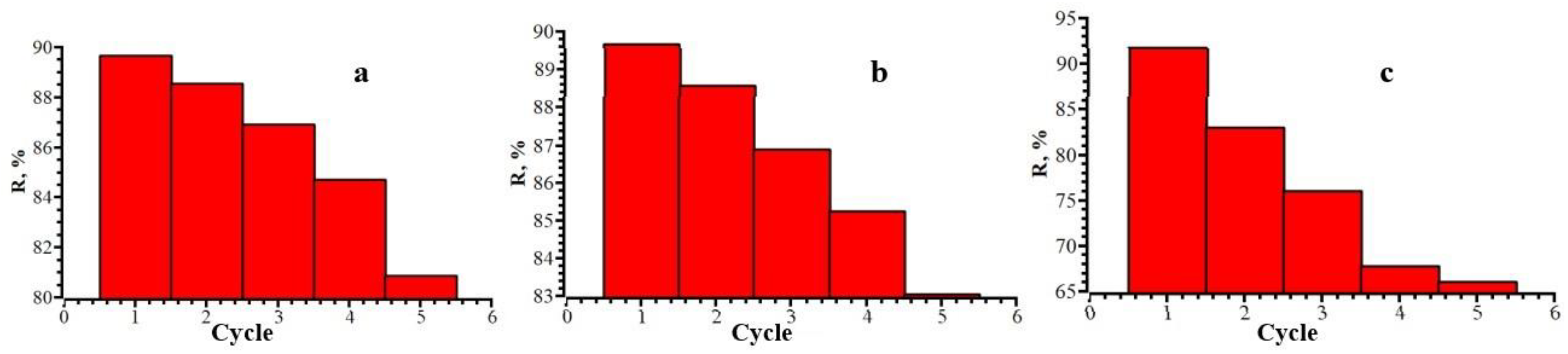
3.6. Reusability
3.7. Separation of a Mixture of Antibiotics on a Chromatographic Column
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- El-Shaboury, S.R.; Saleh, G.A.; Mohamed, F.A.; Rageh, A.H. Analysis of cephalosporin antibiotics. J. Pharm. Biomed. Anal. 2007, 45, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Jones, R.N. Historical overview of the cephalosporin spectrum: Four generations of structural evolution. Antimicrob. Newsl. 1992, 8, 75–82. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Magdaleno, A.; Saenz, M.E.; Juarez, A.B.; Moretton, J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol. Environ. Saf. 2015, 113, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- León, G.; Saura, F.; Hidalgo, A.M.; Miguel, B. Activated Olive Stones as a Low-Cost and Environmentally Friendly Adsorbent for Removing Cephalosporin C from Aqueous Solutions. Int. J. Environ. Res. Public Health. 2021, 18, 4489. [Google Scholar] [CrossRef] [PubMed]
- Junza, A.; Amatya, R.; Barrón, D.; Barbosa, J. Comparative study of the LC–MS/MS and UPLC–MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2601–2610. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Singh, S.; Sharma, S.; Sarma, S.J. Current challenges and advancements towards discovery and resistance of antibiotics. J. Mol. Struct. 2022, 1248, 131380. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Batlles-Delafuente, A.; Molina-Moreno, V.; Molina, J.S.; Belmonte-Ureña, L.J. Removal of pharmaceuticals from wastewater: Analysis of the past and present global research activities. Water 2021, 13, 2353. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, R.; García-Córcoles, M.T.; Çipa, M.; Barrón, D.; Navalón, A.; Zafra-Gómez, A. Determination of quinolone residues in raw cow milk. Application of polar stir-bars and ultra-high performance liquid chromatography–tandem mass spectrometry. Food Addit. Contam. Part A 2018, 35, 1127–1138. [Google Scholar] [CrossRef]
- Fagerquist, C.K.; Lightfield, A.R.; Lehotay, S.J. Confirmatory and Quantitative Analysis of β-Lactam Antibiotics in Bovine Kidney Tissue by Dispersive Solid-Phase Extraction and Liquid Chromatography−Tandem Mass Spectrometry. Anal. Chem. 2005, 77, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Turnipseed, S.B.; Andersen, W.C.; Karbiwnyk, C.M.; Madson, M.R.; Miller, K.E. Multi-class, multi-residue liquid chromatography/tandem mass spectrometry screening and confirmation methods for drug residues in milk. Rapid Commun. Mass Spectrom. 2008, 22, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Zuo, J.; Zhang, M.; Chen, L.; Li, Z. Distribution and persistence of cephalosporins in cephalosporin producing wastewater using SPE and UPLC–MS/MS method. Sci. Total Environ. 2016, 569, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environ. Pollut. 2018, 241, 1153–1166. [Google Scholar] [CrossRef]
- Manzetti, S.; Ghisi, R. The environmental release and fate of antibiotics. Mar. Pollut. Bull. 2014, 79, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.; Muszyńska, B.; Starek, M.; Zmudzki, P.; Opoka, W. Degradation pathway of cephalosporin antibiotics by ˙ in vitro cultures of Lentinula edodes and Imleria badia. Int. Biodeterior. Biodegrad. 2018, 127, 104–112. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Pan, S.F.; Zhu, M.P.; Chen, J.P.; Yuan, Z.H.; Zhong, L.B.; Zheng, Y.M. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane—Implications for antibiotics recovery. Sep. Purif. Technol. 2015, 153, 76–83. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, J.; Li, P.; Wang, K.; Yu, X.; Zhang, M. Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem. Eng. J. 2016, 287, 30–37. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, X.; Li, K.; Gaoa, P.; Chen, J.; Liu, Z.; Zhou, X.; Zhang, Y.; Chen, H.; Li, X.; et al. Enhanced degradation of cephalosporin antibiotics by matrix components during thermally activated persulfate oxidation process. Chem. Eng. J. 2020, 384, 123332. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Jin, H.E.; Jin, S.E.; Maeng, H.J. Recent bioanalytical methods for quantification of third-generation cephalosporins using HPLC and LC-MS(/MS) and their applications in pharmacokinetic studies. Biomed. Chromatogr. 2014, 28, 1565–1587. [Google Scholar] [CrossRef]
- Holstege, D.M.; Puschner, B.; Whitehead, G.; Galey, F.D. Screening and mass spectral confirmation of β-lactam antibiotic residues in milk using LC-MS/MS. J. Agric. Food Chem. 2002, 50, 406–411. [Google Scholar] [CrossRef]
- McWhinney, B.C.; Wallis, S.C.; Hillister, T.; Roberts, J.A.; Lipman, J.; Ungerer, J.P.J. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J. Chromatogr. B 2010, 878, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Ye, N.S. Graphene oxide-reinforced hollow fiber solid-phase microextraction coupled with high-performance liquid chromatography for the determination of cephalosporins in milk samples. Food Anal. Methods 2016, 9, 2452–2462. [Google Scholar] [CrossRef]
- Rigo-Bonnin, R.; Ribera, A.; Arbiol-Roca, A.; Cobo-Sacristán, S.; Padullés, A.; Murillo, Ò.; Shaw, E.; Granada, R.; Pérez-Fernández, X.L.; Tubau, F.; et al. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of beta-lactam antibiotic concentration in human plasma. Clin. Chim. Acta 2017, 468, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, H.; Konoz, E.; Ezabadi, A. Synthesis of DABCO-based ionic liquid functionalized magnetic nanoparticles as a novel sorbent for the determination of cephalosporins in milk samples by dispersive solid-phase extraction followed by ultra-performance liquid chromatography-tandem mass spectrometry. New J. Chem. 2019, 43, 13554–13570. [Google Scholar]
- Roth, T.; Weber, L.; Niestroj, M.; Cipa, F.; Löscher, A.; Mihai, S.; Parsch, H. Simultaneous determination of six antibiotics in human serum by high-performance liquid chromatography with UV detection. Biomed. Chromatogr. 2021, 35, e5010. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, R.; Parshad, V. Separation and Identification of Some Cephalosporin’s on Impregnated TLC Plates. Biomed. Chromatogr. 1996, 10, 258–260. [Google Scholar] [CrossRef]
- Quesada-Molina, C.; Olmo-Iruela, M.; Garcia-Campana, A.M. Analysis of cephalosporin residues in environmental waters by capillary zone electrophoresis with off-line and on-line preconcentration. Anal. Methods 2012, 4, 2341–2347. [Google Scholar] [CrossRef]
- Hancu, G.; Kelemen, H.; Rusu, A.; Gyéresi, Á. Development of a capillary electrophoresis method for the simultaneous determination of cephalosporins. J. Serb. Chem. Soc. 2013, 78, 1413–1423. [Google Scholar] [CrossRef]
- Wang, X.; An, J.; Li, J.; Ye, N. A capillary coated with a metal-organic framework for the capillary electrochromatographic determination of cephalosporins. Microchim. Acta 2017, 184, 1345–1351. [Google Scholar] [CrossRef]
- Tůma, P.; Jaček, M.; Sommerová, B.; Dlouhý, P.; Jarošíková, R.; Husáková, J.; Wosková, V.; Fejfarová, V. Monitoring of amoxicilline and ceftazidime in the microdialysate of diabetic foot and serum by capillary electrophoresis with contactless conductivity detection. Electrophoresis 2021, 43, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Elbalkiny, H.T.; Yehia, A.; Riad, S.M.; Elsaharty, T.S. Removal and tracing of cephalosporins in industrial wastewater by SPE-HPLC: Optimization of adsorption kinetics on mesoporous silica nanoparticles. J. Anal. Sci. Technol. 2019, 10, 21. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 1001–1006. [Google Scholar] [CrossRef]
- Vasiliu, S.; Bunia, I.; Racovita, S.; Neagu, V. Adsorption of cefotaxime sodium salt on polymer coated ion exchange resin microparticles: Kinetics, equilibrium and thermodynamic studies. Carbohydr. Polym. 2011, 85, 376–387. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Chemistry of Polymeric Metal Chelates; Springer: Cham, Switzerland, 2018; pp. 633–760. [Google Scholar]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design and synthesis of coordination polymers with chelated units and their application in nanomaterials science. RSC Adv. 2017, 7, 42242–42288. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wang, J.; Guo, J.; Yan, M.-H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D rare cubane-like tetramer Cu(II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloid Surf. A 2023, 656, 130475. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, S.; Gao, Y.; Bi, J. Removal of aqueous pharmaceuticals by magnetically functionalized Zr-MOFs: Adsorption Kinetics, Isotherms, and regeneration. J. Colloid Interface Sci. 2022, 615, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, T.; Sun, Z.; Hu, X. Preparation of copper based metal organic framework materials and its effective adsorptive removal of ceftazidime from aqueous solutions. Appl. Surf. Sci. 2020, 532, 147411. [Google Scholar] [CrossRef]
- Qin, M.; Shi, Y.; Lu, D.; Deng, J.; Shi, G.; Zhou, T. High-performance Hf/Ti-doped defective Zr-MOFs for cefoperazone adsorption: Behavior and mechanisms. Appl. Surf. Sci. 2022, 595, 153494. [Google Scholar] [CrossRef]
- Hu, X.; Sun, T.; Jia, L.; Wei, J.; Sun, Z. Preparation of metal-organic framework based carbon materials and its application to adsorptive removal of cefepime from aqueous solution. J. Hazard. Mater. 2020, 390, 122190. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(II)-based metal–organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. CrystEngComm 2022, 24, 7157–7165. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al(III)/In(III)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Zhinzhilo, V.A.; Bryantseva, J.D.; Uflyand, I.E.; Kharisov, B.I. ZrIV metal-organic framework based on terephthalic acid and 1,10-phenanthroline as adsorbent for solid-phase extraction of tetracycline antibiotics. Mendeleev Commun. 2022, 32, 661–663. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Sun, Y.; Liu, D. Highly Effective Removal of Ofloxacin from Water with Copper-Doped ZIF-8. Molecules 2022, 27, 4312. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Framework as an Efficient Adsorbent for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O.M. Rod Packings and Metal−Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef]
- Miles, D.O.; Jiang, D.; Burrows, A.D.; Halls, J.E.; Marken, F. Conformal transformation of [Co(bdc)(DMF)] (Co-MOF-71, bdc = 1,4-benzenedicarboxylate, DMF = N,N-dimethylformamide) into porous electrochemically active cobalt hydroxide. Electrochem. Commun. 2013, 27, 9–13. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, T.; Hu, J.; Tan, Y. Determination of cephalosporin antibiotics in water samples by optimised solid phase extraction and high performance liquid chromatography with ultraviolet detector. Int. J. Environ. Anal. Chem. 2011, 91, 1267–1281. [Google Scholar] [CrossRef]
- Ferreira, M.M.C.; Kiralj, R. QSAR study of β-lactam antibiotic efflux by the bacterial multidrug resistance pump AcrB. J. Chemom. 2004, 18, 242–252. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, Z.; Aisha, A.A.; Aazam, E.S. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J. Mol. Liq. 2019, 283, 287–298. [Google Scholar] [CrossRef]
- Weber, T.; Chakravorti, R. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C.; Juang, R.S. Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics. J. Taiwan Inst. Chem. Eng. 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order model for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Li, C.; Chen, T.; Xu, W.; Lou, X.; Pan, L.; Chen, Q.; Hu, B. Mesoporous nanostructured Co3O4 derived from MOF template: A high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 5585–5591. [Google Scholar] [CrossRef]
- Zhao, G.X.; Huang, X.B.; Tang, Z.W.; Huang, Q.F.; Niu, F.L.; Wang, X.K. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Dutta, N.; Dutta Saikia, M. Adsorption equilibrium of 7-aminodeacetoxy cephalosporanic acid—Cephalexin mixture onto carbon and polymeric resins. Ind. J. Chem. Tech. 2005, 12, 296–303. [Google Scholar]
- Lishai, N.; Savitskaya, T.; Tsyhankova, N.; Hrynshpan, D.; Ivashekevich, O. Adsorption of cefotaxim sodium on activated carbon of various origin. J. Belarusian State Univ. Chem. 2020, 1, 95–107. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Mohamed, R.H.A.; Saad, S.R. Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater. Sci. Eng. C. 2020, 108, 110199. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Estrada, M.; Ramírez-García, J.J.; Solache-Ríos, M.J.; Gallegos-Pérez, J.L. Kinetic and Equilibrium Sorption Studies of Ceftriaxone and Paracetamol by Surfactant-Modified Zeolite. Water Air Soil Pollut. 2018, 229, 123. [Google Scholar] [CrossRef]








| Analyte | Acronym | CAS No. | Molecular Structure | Molecular Weight | logKow a | pKa b |
|---|---|---|---|---|---|---|
| Ceftriaxone | CRO | 73384-59-5 | C18H18N8O7S3 | 554.58 | 0.78 | 3, 3.2, 4.1 |
| Cefotaxime | CTX | 63527-52-6 | C16H17N5O7S2 | 455.46 | 0.64 | 3.8 |
| Cefazolin | CZO | 25953-19-9 | C14H14N8O4S3 | 454.51 | −0.58 | 2.1 |
| Model | Sorbate | Index | T, °C | ||
|---|---|---|---|---|---|
| 5 | 23 | 35 | |||
| Langmuir | CZO | qmax | 308.6 | 321.5 | 300.3 |
| KL | 0.28 | 0.49 | 0.48 | ||
| R2 | 0.945 | 0.846 | 0.619 | ||
| CTX | qmax | 496.0 | 396.8 | 243.2 | |
| KL | 0.151 | 0.268 | 0.457 | ||
| R2 | 0.770 | 0.903 | 0.947 | ||
| CRO * | qmax | 520.8 | 429.2 | 458.7 | |
| KL | 0.062 | 0.056 | 0.067 | ||
| R2 | 0.707 | 0.761 | 0.847 | ||
| Freindlich | CZO | 1/n | 0.55 | 0.57 | 0.55 |
| KF | 2.56 | 2.18 | 1.88 | ||
| R2 | 0.992 | 0.974 | 0.899 | ||
| CTX | 1/n | 0.67 | 0.68 | 0.69 | |
| KF | 3.1 | 4.1 | 4.8 | ||
| R2 | 0.970 | 0.972 | 0.941 | ||
| CRO * | 1/n | 0.69 | 0.58 | 0.58 | |
| KF | 1.87 | 2.18 | 2.18 | ||
| R2 | 0.925 | 0.943 | 0.943 | ||
| Sorbate | t, °C | qe, mg/g | R2 | k1, min−1 | k2, g/mg min | |
|---|---|---|---|---|---|---|
| Pseudo First Order | Pseudo Second Order | |||||
| CZO | 5 | 193.10 | 0.823 | 0.877 | 0.8 | 1.61 |
| 23 | 192.80 | 0.920 | 0.884 | 0.8 | 1.80 | |
| 35 | 191.20 | 0.983 | 0.971 | 0.83 | 1.41 | |
| CTX | 5 | 193.10 | 0.936 | 0.993 | 0.8 | 0.68 |
| 23 | 192.80 | 0.941 | 0.994 | 0.57 | 1.76 | |
| 35 | 191.20 | 0.983 | 0.992 | 0.75 | 1.10 | |
| CRO | 2 | 193.10 | 0.881 | 0.882 | 0.975 | 0.56 |
| 23 | 192.80 | 0.876 | 0.861 | 0.925 | 0.76 | |
| 35 | 191.20 | 0.880 | 0.891 | 0.900 | 0.31 | |
| Sorbate | T, K | Kc | ΔG0, kJ/mol | ΔH0, kJ/mol | ΔS0, J/mol K |
|---|---|---|---|---|---|
| CZO | 278 | 2.535 | −2.150 | −2.123 | 9.321 |
| 296 | 2.387 | −2.141 | |||
| 308 | 1.260 | −0.563 | |||
| CTX | 278 | 2.560 | −2.173 | −1.844 | 11.230 |
| 296 | 2.387 | −2.141 | |||
| 308 | 1.377 | −0.819 | |||
| CRO | 275 | 1.363 | −0.717 | −0.332 | 4.416 |
| 296 | 1.162 | −0.369 | |||
| 308 | 1.234 | −0.538 |
| Factor | Index |
|---|---|
| Degree of extraction from the column, % | CZO—96.7 CRO—93.4 CTX—98.5 |
| R2 | CZO—0.965 CRO—0.974 CTX—0.987 |
| The degree of resolution from the mixture, arb. units | CZO—1.345 CRO—0.5 CTX—0.5 |
| Range of measured mass concentrations, mg/L | 3.25–100 |
| qmax, mg/g | CZO—365.2 CRO—396.8 CTX—361.1 |
| Repeatability | 0.0047 |
| Accuracy index ± Δ, % (error characteristic) | 2.7 ± 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernomorova, M.A.; Myakinina, M.S.; Zhinzhilo, V.A.; Uflyand, I.E. Analytical Determination of Cephalosporin Antibiotics Using Coordination Polymer Based on Cobalt Terephthalate as a Sorbent. Polymers 2023, 15, 548. https://doi.org/10.3390/polym15030548
Chernomorova MA, Myakinina MS, Zhinzhilo VA, Uflyand IE. Analytical Determination of Cephalosporin Antibiotics Using Coordination Polymer Based on Cobalt Terephthalate as a Sorbent. Polymers. 2023; 15(3):548. https://doi.org/10.3390/polym15030548
Chicago/Turabian StyleChernomorova, Maria A., Marina S. Myakinina, Vladimir A. Zhinzhilo, and Igor E. Uflyand. 2023. "Analytical Determination of Cephalosporin Antibiotics Using Coordination Polymer Based on Cobalt Terephthalate as a Sorbent" Polymers 15, no. 3: 548. https://doi.org/10.3390/polym15030548
APA StyleChernomorova, M. A., Myakinina, M. S., Zhinzhilo, V. A., & Uflyand, I. E. (2023). Analytical Determination of Cephalosporin Antibiotics Using Coordination Polymer Based on Cobalt Terephthalate as a Sorbent. Polymers, 15(3), 548. https://doi.org/10.3390/polym15030548





