Universal Approach to Integrating Reduced Graphene Oxide into Polymer Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymers Preparation
2.2. GO Deposition
2.3. Laser Processing
2.4. Ultrasonication of Prepared Samples
2.5. Heating Model of rGO/Polymer Structures
2.6. High-Speed Camera Recording
2.7. Electrical Measurements
2.8. Optics
2.9. SEM and EDX
2.10. Fe3O4/rGO Experiment
2.11. XPS
2.12. DSC/TGA
3. Results and Discussion
3.1. Thermal Analyses Reveal Polymer Phase Transitions
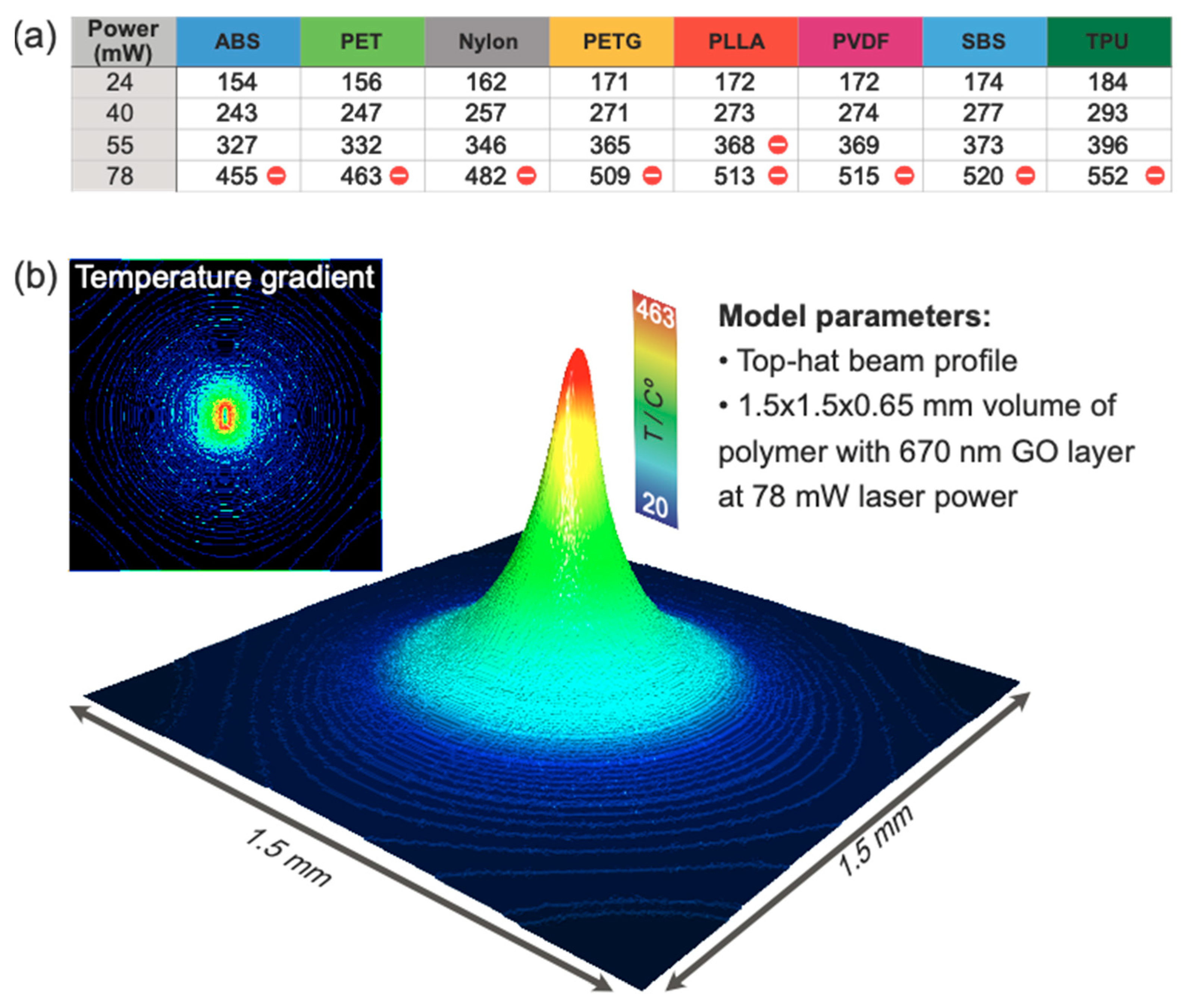
3.2. Numerical Simulation of Laser-Induced Heating
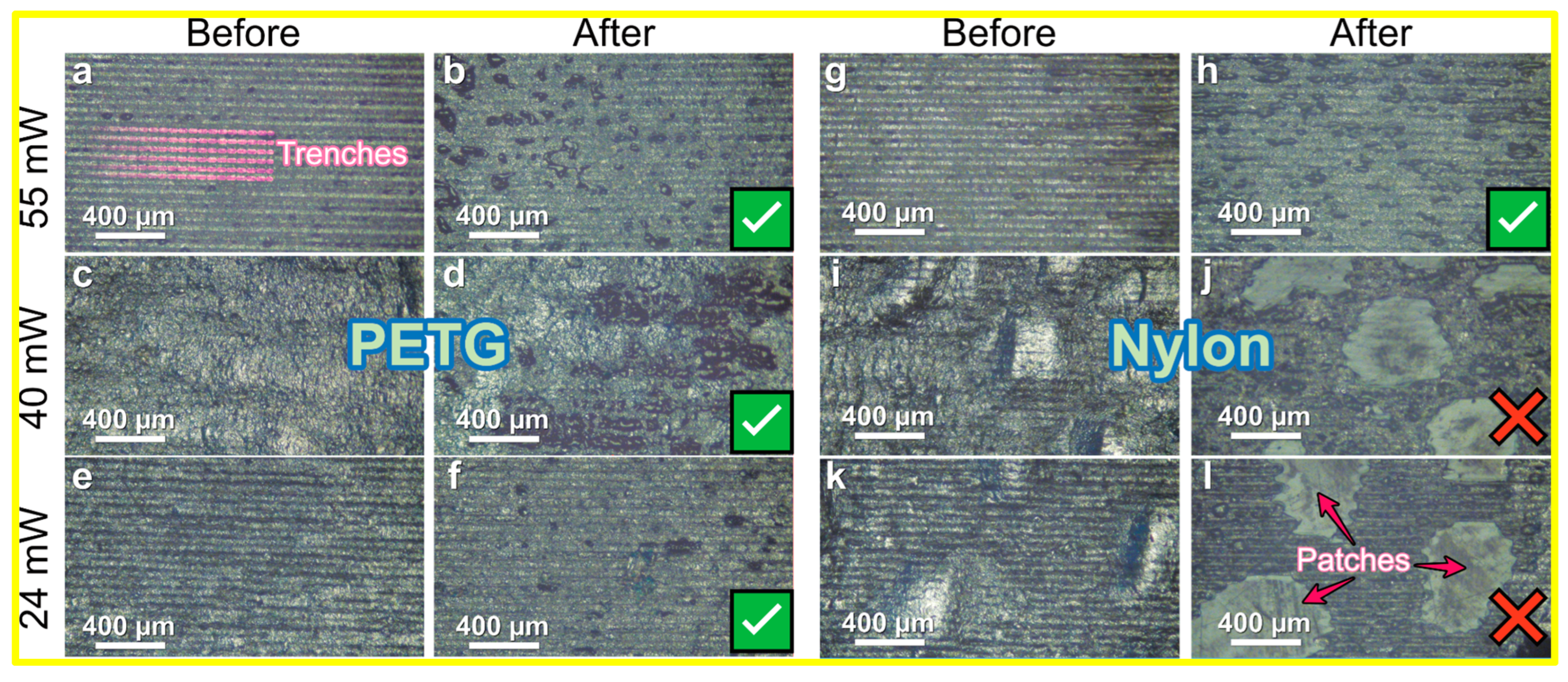
3.3. Optical Microscopy Analysis
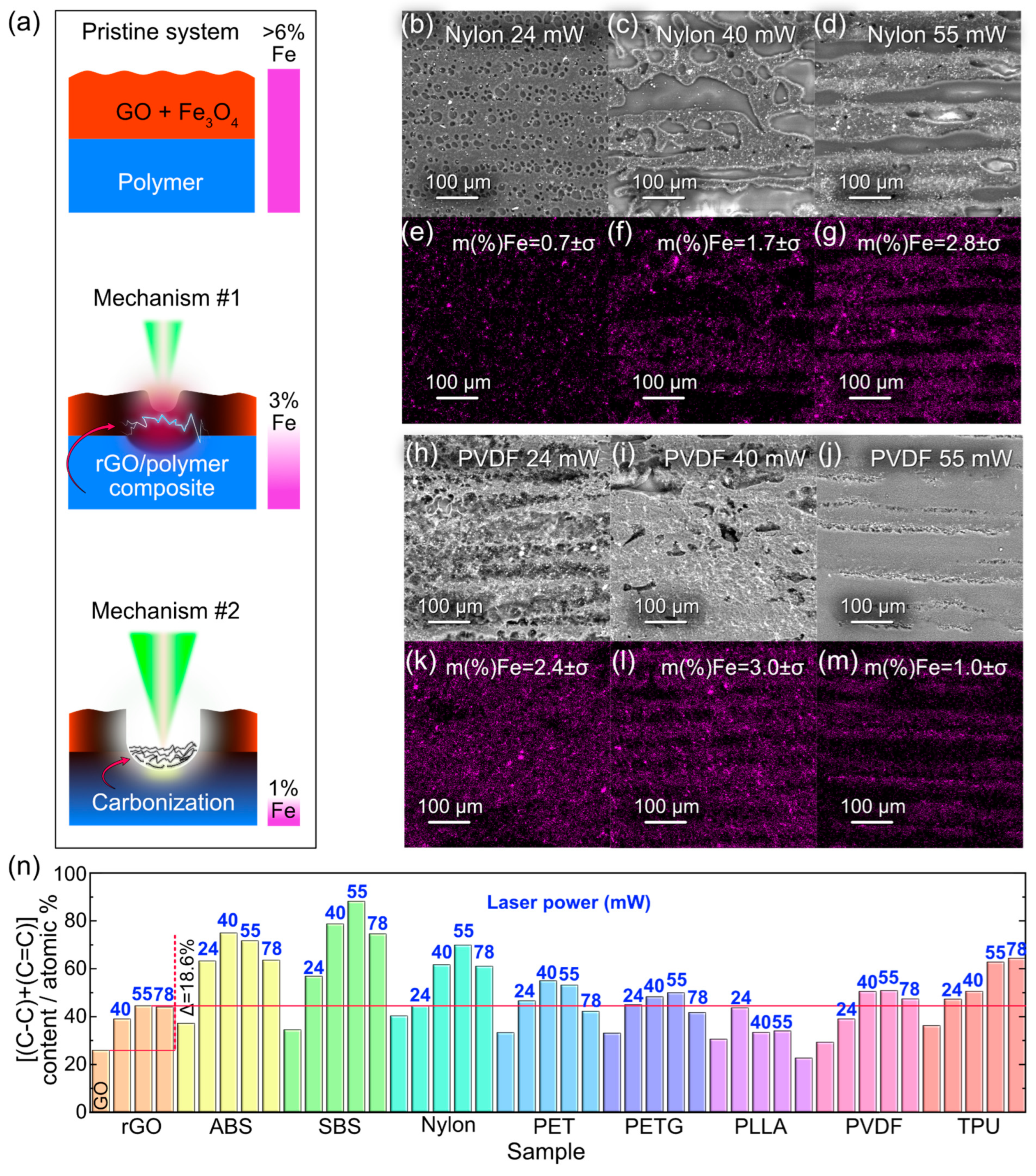
3.4. Elucidating Mechanisms behind the Formation of Electrically Conductive Composite via SEM/EDX and XPS
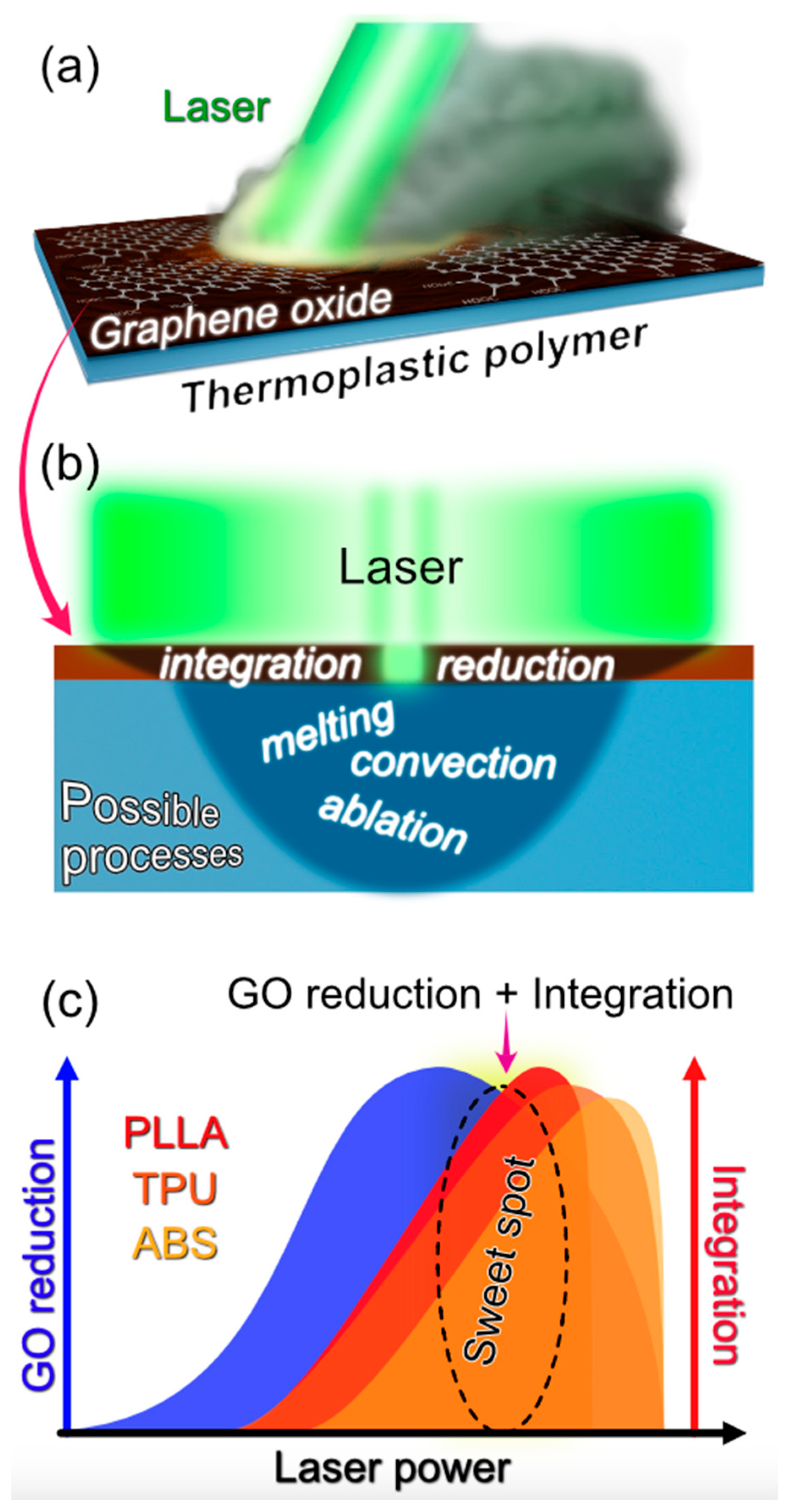
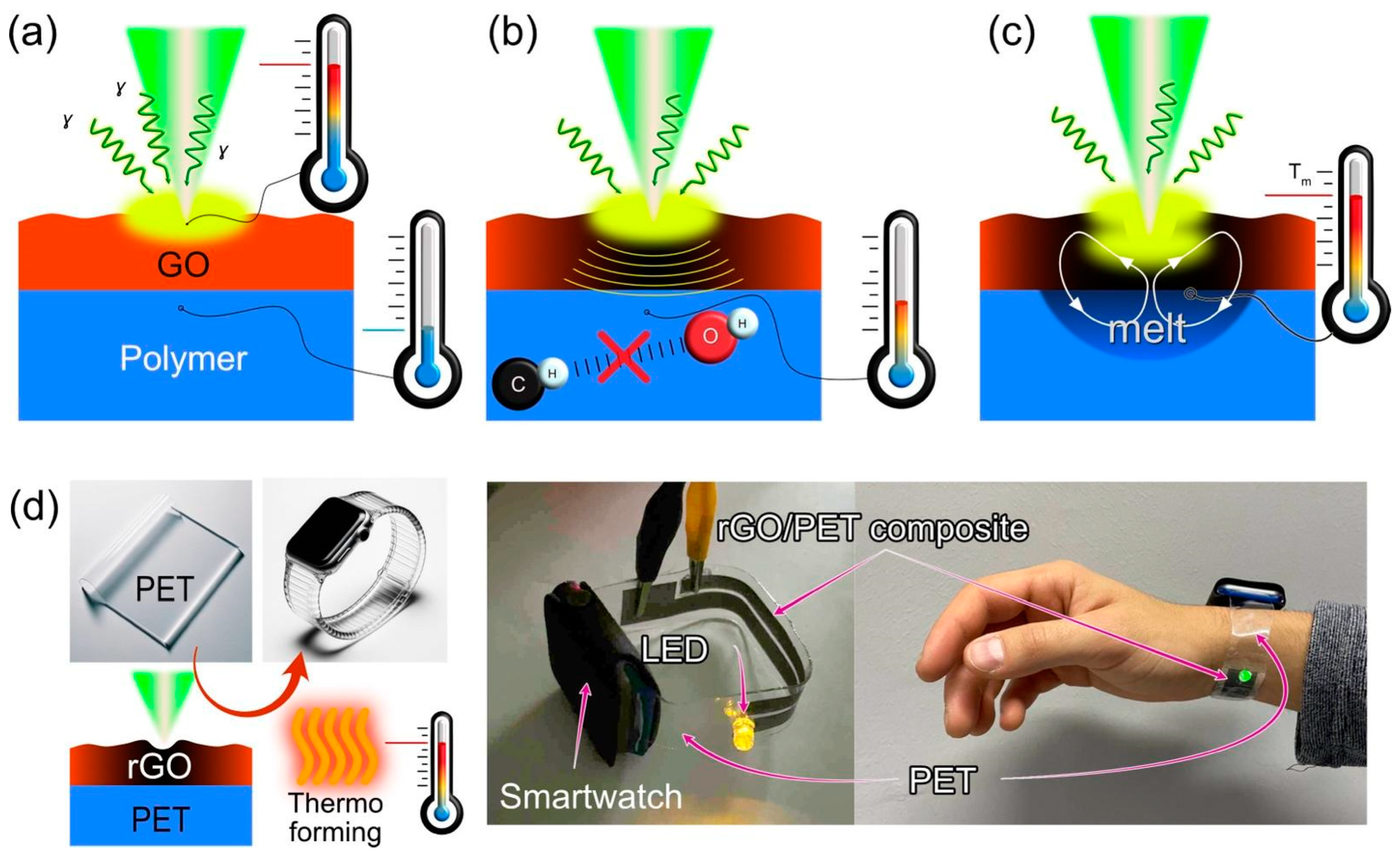
3.5. Laser-Induced Composite Formation Mechanism
- GO Photothermal Heating: Laser irradiation leads to the absorption of light by the GO, initiating its thermal response. This is the primary interaction between light and matter in this context (Figure 7a).
- Transformation of the GO to rGO: The absorbed thermal energy prompts the reduction of the GO to rGO (reduced graphene oxide), enhancing its optical absorption properties and consequently, its heating. This transition is critical as the rGO possesses the electrical conductivity desired for the final composite (Figure 7a,b).
- Heat Transfer to Polymer: The heat generated in the rGO is transferred to the underlying polymer substrate, elevating its temperature, and affecting its physical state (Figure 7b).
- Polymer Melting: Upon reaching its melting temperature, the thermoplastic crystalline polymer transitions from solid to liquid. This phase change is essential for the subsequent integration of the rGO layers into the polymer matrix (Figure 7c). In the case of amorphous polymers, the processing temperature should exceed the glass transition point to provide the polymer in a rubbery state with increased molecular mobility.
- Intermixing of rGO and Molten Polymer: In the liquid state, convective flows (described by the Marangoni effect) facilitate the movement and mixing of the rGO into the molten polymer. This capillary flow is influenced by various factors including temperature-dependent surface tension, viscosity, thermal diffusivity, and the melt pool’s characteristics (Figure 7c).
- Composite Solidification: As the mixture cools, it solidifies, encapsulating the rGO within the polymer matrix. This solidification locks the conductive rGO particles in place, forming a continuous, electrically conductive pathway within the composite. Notably, the solidification of amorphous polymers is defined by glass transition temperature and molecular dynamics around this point (so-called fragility).
- Threshold of Polymer Degradation: If the laser power continues to increase such that the substrate’s temperature reaches its degradation temperature (Td), the polymer undergoes pyrolysis. This leads to carbonization, which can contribute to electrical conductivity but also compromises the structural integrity of the polymer matrix. At this stage, the conductive pathways are more likely due to the carbonized substrate rather than the integrated rGO.
3.6. Application: Thermoforming a Wearable Wristband
4. Conclusions
Supplementary Materials
 symbol; Figure S3: Optical images of rGO/polymer samples processed at different laser power before and after ultrasonication. A red frame is used to indicate non-conductive samples; Figure S4: Resistance of a single rGO/polymer composite lines; Figure S5: COMSOL model of the GO/Polymer composite after 0.25 s of the laser heating at 78 mW power. (a) Side view, (b) Top view, (c) Cross-section from the region indicated in (a), (d) Meshed model; Figure S6: A frame high-speed camera recording during laser processing of GO/polymer with laser power 24, 40, 55, and 78 Mw; Figure S7: Histogram representing the width of different areas of lines taken from high-speed recordings and cross-section images. Each column represents a certain power from left to right (24, 40, 55, and 78 mW); Figure S8: Optical images of laser-induced lines at a power of 24, 40, 55, and 78 mW on polymers before and after 1-minute treatment in an ultrasonic bath; Figure S9: Cross-section optical microscopy of laser-induced lines at a power of 24, 40, 55, and 78 mW. Polymers were broken after 10 min in liquid nitrogen; Table S1: Thermal properties of polymers; Table S2: All polymer parameters used in the COMSOL model; Table S3: The width of the modified area of materials for powers of 24, 40, 55, and 78 mW before and after 1-minute treatment in the ultrasonic bath; Table S4: Fe mass content (%) for the set of polymers processed with different laser power. References [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] are cited in the supplementary materials.
symbol; Figure S3: Optical images of rGO/polymer samples processed at different laser power before and after ultrasonication. A red frame is used to indicate non-conductive samples; Figure S4: Resistance of a single rGO/polymer composite lines; Figure S5: COMSOL model of the GO/Polymer composite after 0.25 s of the laser heating at 78 mW power. (a) Side view, (b) Top view, (c) Cross-section from the region indicated in (a), (d) Meshed model; Figure S6: A frame high-speed camera recording during laser processing of GO/polymer with laser power 24, 40, 55, and 78 Mw; Figure S7: Histogram representing the width of different areas of lines taken from high-speed recordings and cross-section images. Each column represents a certain power from left to right (24, 40, 55, and 78 mW); Figure S8: Optical images of laser-induced lines at a power of 24, 40, 55, and 78 mW on polymers before and after 1-minute treatment in an ultrasonic bath; Figure S9: Cross-section optical microscopy of laser-induced lines at a power of 24, 40, 55, and 78 mW. Polymers were broken after 10 min in liquid nitrogen; Table S1: Thermal properties of polymers; Table S2: All polymer parameters used in the COMSOL model; Table S3: The width of the modified area of materials for powers of 24, 40, 55, and 78 mW before and after 1-minute treatment in the ultrasonic bath; Table S4: Fe mass content (%) for the set of polymers processed with different laser power. References [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] are cited in the supplementary materials.Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer Composite Materials: A Comprehensive Review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Bian, J.; Zhou, L.; Wan, X.; Zhu, C.; Yang, B.; Huang, Y. Laser Transfer, Printing, and Assembly Techniques for Flexible Electronics. Adv. Electron. Mater. 2019, 5, 1800900. [Google Scholar] [CrossRef]
- Luong, D.X.; Yang, K.; Yoon, J.; Singh, S.P.; Wang, T.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene Composites as Multifunctional Surfaces. ACS Nano 2019, 13, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, S.; Wang, H.; Heil, T.; Strauss, V. Laser-Induced Carbonization of Natural Organic Precursors for Flexible Electronics. Adv. Electron. Mater. 2020, 6, 2000463. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, e1803621. [Google Scholar] [CrossRef] [PubMed]
- Lavieja, C.; Clemente, M.J.; Oriol, L.; Peña, J.I. Influence of the Wavelength on Laser Marking on ABS Filled with Carbon Black. Polym.-Plast. Technol. Eng. 2017, 56, 1599–1607. [Google Scholar] [CrossRef]
- Zelenska, K.S.; Zelensky, S.E.; Poperenko, L.V.; Kanev, K.; Mizeikis, V.; Gnatyuk, V.A. Thermal Mechanisms of Laser Marking in Transparent Polymers with Light-Absorbing Microparticles. Opt. Laser Technol. 2016, 76, 96–100. [Google Scholar] [CrossRef]
- Wen, L.; Zhou, T.; Zhang, J.; Zhang, A. Local Controllable Laser Patterning of Polymers Induced by Graphene Material. ACS Appl. Mater. Interfaces 2016, 8, 28077–28085. [Google Scholar] [CrossRef]
- Xie, Y.; Wen, L.; Zhang, J.; Zhou, T. Enhanced Local Controllable Laser Patterning of Polymers Induced by Graphene/polystyrene Composites. Mater. Des. 2018, 141, 159–169. [Google Scholar] [CrossRef]
- Park, R.; Kim, H.; Lone, S.; Jeon, S.; Kwon, Y.W.; Shin, B.; Hong, S.W. One-Step Laser Patterned Highly Uniform Reduced Graphene Oxide Thin Films for Circuit-Enabled Tattoo and Flexible Humidity Sensor Application. Sensors 2018, 18, 1857. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Shchadenko, S.; Murastov, G.; Lipovka, A.; Fatkullin, M.; Petrov, I.; Tran, T.-H.; Khalelov, A.; Saqib, M.; Villa, N.E.; et al. Ultra-robust Flexible Electronics by Laser-driven Polymer-nanomaterials Integration. Adv. Funct. Mater. 2021, 31, 2008818. [Google Scholar] [CrossRef]
- Ko, S.H.; Pan, H.; Grigoropoulos, C.P.; Luscombe, C.K.; Fréchet, J.M.J.; Poulikakos, D. All-Inkjet-Printed Flexible Electronics Fabrication on a Polymer Substrate by Low-Temperature High-Resolution Selective Laser Sintering of Metal Nanoparticles. Nanotechnology 2007, 18, 345202. [Google Scholar] [CrossRef]
- In, J.B.; Kwon, H.-J.; Yoo, J.-H.; Allen, F.I.; Minor, A.M.; Grigoropoulos, C.P. Laser Welding of Vertically Aligned Carbon Nanotube Arrays on Polymer Workpieces. Carbon N. Y. 2017, 115, 688–693. [Google Scholar] [CrossRef]
- Murastov, G.; Bogatova, E.; Brazovskiy, K.; Amin, I.; Lipovka, A.; Dogadina, E.; Cherepnyov, A.; Ananyeva, A.; Plotnikov, E.; Ryabov, V.; et al. Flexible and Water-Stable Graphene-Based Electrodes for Long-Term Use in Bioelectronics. Biosens. Bioelectron. 2020, 166, 112426. [Google Scholar] [CrossRef]
- Schricker, K.; Alhomsi, M.; Bergmann, J.P. Thermal Efficiency in Laser-Assisted Joining of Polymer–Metal Composites. Materials 2020, 13, 4875. [Google Scholar] [CrossRef]
- Schricker, K.; Samfaß, L.; Grätzel, M.; Ecke, G.; Bergmann, J.P. Bonding Mechanisms in Laser-Assisted Joining of Metal-Polymer Composites. J. Adv. Join. Process. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Fatkullin, M.; Rodriguez, R.D.; Petrov, I.; Villa, N.E.; Lipovka, A.; Gridina, M.; Murastov, G.; Chernova, A.; Plotnikov, E.; Averkiev, A.; et al. Molecular Plasmonic Silver Forests for the Photocatalytic-Driven Sensing Platforms. Nanomaterials 2023, 13, 923. [Google Scholar] [CrossRef]
- Lipovka, A.; Petrov, I.; Fatkullin, M.; Murastov, G.; Ivanov, A.; Villa, N.E.; Shchadenko, S.; Averkiev, A.; Chernova, A.; Gubarev, F.; et al. Photoinduced Flexible Graphene/polymer Nanocomposites: Design, Formation Mechanism, and Properties Engineering. Carbon N. Y. 2022, 194, 154–161. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Bruntz, A.; Vidal, L.; Vetter, P.-A.; Roudot, P.; Bua, L.; Ortiz, J.; Zan, H.-W.; Soppera, O. Laser Polymer Tattooing: A Versatile Method for Permanent Marking on Polymer Surfaces. Macromol. Mater. Eng. 2019, 304, 1900402. [Google Scholar] [CrossRef]
- Stewart, R.; Li, L.; Isherwood, R. Melting Mechanisms in Enclosed Laser Processing. Mater. Sci. Eng. A Struct. Mater. 2003, 354, 158–165. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Khalelov, A.; Postnikov, P.S.; Lipovka, A.; Dorozhko, E.; Amin, I.; Murastov, G.V.; Chen, J.-J.; Sheng, W.; Trusova, M.E.; et al. Beyond Graphene Oxide: Laser Engineering Functionalized Graphene for Flexible Electronics. Mater. Horiz. 2020, 7, 1030–1041. [Google Scholar] [CrossRef]
- Singer, J.P.; Kooi, S.E.; Thomas, E.L. Focused Laser-induced Marangoni Dewetting for Patterning Polymer Thin Films. J. Polym. Sci. B Polym. Phys. 2016, 54, 225–236. [Google Scholar] [CrossRef]
- Fico, D.; Rizzo, D.; Casciaro, R.; Esposito Corcione, C. A Review of Polymer-Based Materials for Fused Filament Fabrication (FFF): Focus on Sustainability and Recycled Materials. Polymers 2022, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Sztorch, B.; Brząkalski, D.; Pakuła, D.; Frydrych, M.; Špitalský, Z.; Przekop, R.E. Natural and Synthetic Polymer Fillers for Applications in 3D printing—FDM Technology Area. Eur. J. Mech. A Solids 2022, 3, 508–548. [Google Scholar] [CrossRef]
- Wu, H.; Fahy, W.P.; Kim, S.; Kim, H.; Zhao, N.; Pilato, L.; Kafi, A.; Bateman, S.; Koo, J.H. Recent Developments in Polymers/polymer Nanocomposites for Additive Manufacturing. Prog. Mater Sci. 2020, 111, 100638. [Google Scholar] [CrossRef]
- Ikram, H.; Al Rashid, A.; Koç, M. Additive Manufacturing of Smart Polymeric Composites: Literature Review and Future Perspectives. Polym. Compos. 2022, 43, 6355–6380. [Google Scholar] [CrossRef]
- Vaes, D.; Van Puyvelde, P. Semi-Crystalline Feedstock for Filament-Based 3D Printing of Polymers. Prog. Polym. Sci. 2021, 118, 101411. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Thermal Conductivity of Graphene Oxide: A Molecular Dynamics Study. JETP Lett. 2020, 112, 117–121. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, J.; Wei, N.; Meng, D.; Wang, L.; Ren, G.; Yan, R.; Zhang, N. Thermal Conductivity of Defective Graphene Oxide: A Molecular Dynamic Study. Molecules 2019, 24, 1103. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, T.; Yao, Y.; Li, T.; Hu, L.; Marconnet, A. Thermally Conductive Reduced Graphene Oxide Thin Films for Extreme Temperature Sensors. Adv. Funct. Mater. 2019, 29, 1901388. [Google Scholar] [CrossRef]
- Mahanta, N.K.; Abramson, A.R. Thermal Conductivity of Graphene and Graphene Oxide Nanoplatelets. In Proceedings of the 13th InterSociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May 2012–1 June 2012. [Google Scholar]
- Torrisi, L.; Cutroneo, M.; Torrisi, A.; Silipigni, L. Measurements on Five Characterizing Properties of Graphene Oxide and Reduced Graphene Oxide Foils. Phys. Status Solidi 2022, 219, 2100628. [Google Scholar] [CrossRef]
- Crica, L.E.; Dennison, T.J.; Guerini, E.A.; Kostarelos, K. A Method for the Measurement of Mass and Number of Graphene Oxide Sheets in Suspension Based on Non-Spherical Approximations. 2d Mater. 2021, 8, 035044. [Google Scholar] [CrossRef]
- Zhang, H.; Fonseca, A.F.; Cho, K. Tailoring Thermal Transport Property of Graphene through Oxygen Functionalization. J. Phys. Chem. C Nanomater. Interfaces 2014, 118, 1436–1442. [Google Scholar] [CrossRef]
- McGuinness, M. Polymer Glass Transition Temperature-Material Properties, Impact. Available online: https://www.hzo.com/blog/polymer-glass-transition-temperature-material-properties-impact/ (accessed on 16 October 2023).
- Alfredo Campo, E. Selection of Polymeric Materials: How to Select Design Properties from Different Standards; William Andrew: Norwich, NY, USA, 2008; ISBN 9780815518969. [Google Scholar]
- Murastov, G.V.; Lipovka, A.A.; Fatkullin, M.I.; Rodriguez, R.D.; Sheremet, E.S. Laser Reduction of Graphene Oxide: Tuning Local Material Properties. Phys.-Usp. 2023, 66, 1175–1204. [Google Scholar] [CrossRef]
- Tran, T.X.; Choi, H.; Che, C.H.; Sul, J.H.; Kim, I.G.; Lee, S.-M.; Kim, J.-H.; In, J.B. Laser-Induced Reduction of Graphene Oxide by Intensity-Modulated Line Beam for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2018, 10, 39777–39784. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct Laser Writing of Micro-Supercapacitors on Hydrated Graphite Oxide Films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Ha, I.; Won, P.; Seo, D.-G.; Cho, K.-J.; Ko, S.H. Transparent Wearable Three-Dimensional Touch by Self-Generated Multiscale Structure. Nat. Commun. 2019, 10, 2582. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Li, X.; Zhang, E.; Shi, J.; Ren, J.; Li, C.; Wang, H.; Wu, K. A Fiber-Shaped Temperature Sensor Composed of chitosan/rGO with High Sensitivity and Ultra-Fast Response and Recovery for Real-Time Temperature Monitoring. Prog. Org. Coat. 2024, 186, 107989. [Google Scholar] [CrossRef]
- Getachew, B.A.; Bergsman, D.S.; Grossman, J.C. Laser-Induced Graphene from Polyimide and Polyethersulfone Precursors as a Sensing Electrode in Anodic Stripping Voltammetry. ACS Appl. Mater. Interfaces 2020, 12, 48511–48517. [Google Scholar] [CrossRef]
- Chia, J.W.F.; Sawai, O.; Nunoura, T. Reaction Pathway of Poly(ethylene) Terephthalate Carbonization: Decomposition Behavior Based on Carbonized Product. Waste Manag. 2020, 108, 62–69. [Google Scholar] [CrossRef]
- Wang, J.-M.; Liu, G.-H.; Fang, Y.-L.; Li, W.-K. Marangoni Effect in Nonequilibrium Multiphase System of Material Processing. Rev. Chem. Eng. 2016, 32, 551–585. [Google Scholar] [CrossRef]
- Yousefi, A.; Lafleur, P.G.; Gauvin, R. Kinetic Studies of Thermoset Cure Reactions: A Review. Polym. Compos. 1997, 18, 157–168. [Google Scholar] [CrossRef]
- Bucknall, C.B. Toughened Plastics; Applied Science Publishers: Essex, UK, 1977; ISBN 9780853346951. [Google Scholar]
- Dhotel, A.; Rijal, B.; Delbreilh, L.; Dargent, E.; Saiter, A. Combining Flash DSC, DSC and Broadband Dielectric Spectroscopy to Determine Fragility. J. Therm. Anal. Calorim. 2015, 121, 453–461. [Google Scholar] [CrossRef]
- Hohimer, C.; Christ, J.; Aliheidari, N.; Mo, C.; Ameli, A. 3D Printed Thermoplastic Polyurethane with Isotropic Material Properties. In Proceedings of the Behavior and Mechanics of Multifunctional Materials and Composites 2017, Portland, OR, USA, 26–28 March 2017; Goulbourne, N.C., Ed.; [Google Scholar]
- Angell, C.A. Molecular Mobility in Polyurethane/styrene–acrylonitrile Blends Studied by Dielectric Techniques. Eur. Polym. J. 1999, 35, 923–937. [Google Scholar]
- Peng, G.; Zhao, X.; Zhan, Z.; Ci, S.; Wang, Q.; Liang, Y.; Zhao, M. New Crystal Structure and Discharge Efficiency of Poly(vinylidene Fluoride-Hexafluoropropylene)/poly(methyl Methacrylate) Blend Films. RSC Adv. 2014, 4, 16849–16854. [Google Scholar] [CrossRef]
- SBS Rubber at a Glance. Available online: https://pslc.ws/macrog/sbsg.htm (accessed on 17 October 2023).
- Zhai, H.; Salomon, D. Evaluation of Low-Temperature Properties and the Fragility of Asphalt Binders with Non-Arrhenius Viscosity–temperature Dependence. Transp. Res. Rec. 2005, 1901, 44–51. [Google Scholar] [CrossRef]
- Guo, S.-L.; Chen, B.-L.; Durrani, S.A. Solid-State Nuclear Track Detectors. In Handbook of Radioactivity Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–407. ISBN 9780128143971. [Google Scholar]
- Alaloul, W.S.; John, V.O.; Musarat, M.A. Mechanical and Thermal Properties of Interlocking Bricks Utilizing Wasted Polyethylene Terephthalate. Int. J. Concr. Struct. Mater. 2020, 14, 24. [Google Scholar] [CrossRef]
- Colwill, J.; Simeone, A.; Gould, O.; Woolley, E.; Mulvenna, C. Energy-Efficient Systems for the Sensing and Separation of Mixed Polymers. Procedia CIRP 2017, 62, 512–517. [Google Scholar] [CrossRef]
- Kováčová, M.; Kozakovičová, J.; Procházka, M.; Janigová, I.; Vysopal, M.; Černičková, I.; Krajčovič, J.; Špitalský, Z. Novel Hybrid PETG Composites for 3D Printing. Appl. Sci. 2020, 10, 3062. [Google Scholar] [CrossRef]
- Valvez, S.; Silva, A.P.; Reis, P.N.B. Optimization of Printing Parameters to Maximize the Mechanical Properties of 3D-Printed PETG-Based Parts. Polymers 2022, 14, 2564. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Rahmatabadi, D.; Soltanmohammadi, K.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. Assessment of Controllable Shape Transformation, Potential Applications, and Tensile Shape Memory Properties of 3D Printed PETG. J. Jpn. Res. Inst. Adv. Copper-Base Mater. Technol. 2022, 18, 4201–4215. [Google Scholar] [CrossRef]
- La Gala, A.; Fiorio, R.; Ceretti, D.V.A.; Erkoç, M.; Cardon, L.; D’hooge, D.R. A Combined Experimental and Modeling Study for Pellet-Fed Extrusion-Based Additive Manufacturing to Evaluate the Impact of the Melting Efficiency. Materials 2021, 14, 5566. [Google Scholar] [CrossRef]
- Tarrazó-Serrano, D.; Castiñeira-Ibáñez, S.; Sánchez-Aparisi, E.; Uris, A.; Rubio, C. MRI Compatible Planar Material Acoustic Lenses. Appl. Sci. 2018, 8, 2634. [Google Scholar] [CrossRef]
- Clark, S.; Yap, T.; Tehrani, M. Validation of a Finite Element Model for Fused Filament Fabrication Additive Manufacturing. In Proceedings of the ASME 2021 International Mechanical Engineering Congress and Exposition, Virtual, 1–5 November 2021. [Google Scholar]
- Flaata, T.; Michna, G.J.; Letcher, T. Thermal Conductivity Testing Apparatus for 3D Printed Materials. In Proceedings of the ASME 2017 Summer Heat Transfer Conference HT2017, Washington, DC, USA, 9–14 July 2017. [Google Scholar]
- Haleem, A.; Kumar, V.; Kumar, L. Mathematical Modelling & Pressure Drop Analysis of Fused Deposition Modelling Feed Wire. Int. J. Eng. Technol. 2017, 9, 2885–2894. [Google Scholar]
- Spinelli, G.; Kotsilkova, R.; Ivanov, E.; Georgiev, V.; Naddeo, C.; Romano, V. Thermal and Dielectric Properties of 3D Printed Parts Based on Polylactic Acid Filled with Carbon Nanostructures. Macromol. Symp. 2022, 405, 2100244. [Google Scholar] [CrossRef]
- Xie, K.; He, Y.; Cai, J.; Hu, W. Thermal Conductivity of Nylon 46, Nylon 66 and Nylon 610 Characterized by Flash DSC Measurement. Thermochim. Acta 2020, 683, 178445. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustain. Sci. Pract. Policy 2020, 12, 6755. [Google Scholar] [CrossRef]
- Ngo, I.-L.; Jeon, S.; Byon, C. Thermal Conductivity of Transparent and Flexible Polymers Containing Fillers: A Literature Review. Int. J. Heat Mass Transf. 2016, 98, 219–226. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Kim, K.; Kim, C.-K.; Kang, E. Reinforcement of Nylon 6,6/nylon 6,6 Grafted Nanodiamond Composites by in Situ Reactive Extrusion. Sci. Rep. 2016, 6, 37010. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hausberger, A.; Berer, M.; Pinter, G.; Grün, F.; Schwarz, T. Fretting Behavior of Thermoplastic Polyurethanes. Lubricants 2019, 7, 73. [Google Scholar] [CrossRef]
- Pinedo, B.; Hadfield, M.; Tzanakis, I.; Conte, M.; Anand, M. Thermal Analysis and Tribological Investigation on TPU and NBR Elastomers Applied to Sealing Applications. Tribol. Int. 2018, 127, 24–36. [Google Scholar] [CrossRef]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.N.; Iguchi, C.Y.; Gregorio, R., Jr. Thermal Properties of Poly(vinilidene Fluoride) in the Temperature Range from 25 to 210 °C. Polym. Test. 2008, 27, 204–208. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat Transport and Membrane Distillation Coefficients in Direct Contact Membrane Distillation. J. Memb. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P.; Adamovsky, S.; Minakov, A.; Schick, C. Crystallization of Poly(vinylidene Fluoride) during Ultra-Fast Cooling. Thermochim. Acta 2007, 461, 153–157. [Google Scholar] [CrossRef]
- Kou, C.; Wu, X.; Xiao, P.; Liu, Y.; Wu, Z. Physical, Rheological, and Morphological Properties of Asphalt Reinforced by Basalt Fiber and Lignin Fiber. Materials 2020, 13, 2520. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, S.; Wang, C.; Li, Y.; Ma, Z.; Peng, L. A Study on Evaluation and Application of Snowmelt Performance of Anti-Icing Asphalt Pavement. Appl. Sci. 2017, 7, 583. [Google Scholar] [CrossRef]
- Pan, P.; Wu, S.; Hu, X.; Liu, G.; Li, B. Effect of Material Composition and Environmental Condition on Thermal Characteristics of Conductive Asphalt Concrete. Materials 2017, 10, 218. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abyzova, E.; Petrov, I.; Bril’, I.; Cheshev, D.; Ivanov, A.; Khomenko, M.; Averkiev, A.; Fatkullin, M.; Kogolev, D.; Bolbasov, E.; et al. Universal Approach to Integrating Reduced Graphene Oxide into Polymer Electronics. Polymers 2023, 15, 4622. https://doi.org/10.3390/polym15244622
Abyzova E, Petrov I, Bril’ I, Cheshev D, Ivanov A, Khomenko M, Averkiev A, Fatkullin M, Kogolev D, Bolbasov E, et al. Universal Approach to Integrating Reduced Graphene Oxide into Polymer Electronics. Polymers. 2023; 15(24):4622. https://doi.org/10.3390/polym15244622
Chicago/Turabian StyleAbyzova, Elena, Ilya Petrov, Ilya Bril’, Dmitry Cheshev, Alexey Ivanov, Maxim Khomenko, Andrey Averkiev, Maxim Fatkullin, Dmitry Kogolev, Evgeniy Bolbasov, and et al. 2023. "Universal Approach to Integrating Reduced Graphene Oxide into Polymer Electronics" Polymers 15, no. 24: 4622. https://doi.org/10.3390/polym15244622
APA StyleAbyzova, E., Petrov, I., Bril’, I., Cheshev, D., Ivanov, A., Khomenko, M., Averkiev, A., Fatkullin, M., Kogolev, D., Bolbasov, E., Matkovic, A., Chen, J.-J., Rodriguez, R. D., & Sheremet, E. (2023). Universal Approach to Integrating Reduced Graphene Oxide into Polymer Electronics. Polymers, 15(24), 4622. https://doi.org/10.3390/polym15244622





