Synthesis and Characterization of a Novel Nanosized Polyaniline
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Synthesis of Nanosized PANI
2.3. Phase 1: Parameters of PANI Synthesis
2.3.1. Initial Molar Ratio of Aniline/APS (r)
2.3.2. Stirring Speed
2.3.3. Synthesis Temperature
2.3.4. Purification Method
2.3.5. Washing Method
2.3.6. Surfactant in Synthesis
2.4. Characterization of PANI Powders and Dispersion
2.4.1. FTIR Analysis of PANI
2.4.2. Particle Size Analysis of PANI
2.4.3. UV-Vis Absorption Spectra of PANI
2.4.4. Morphology of PANI
2.4.5. Conductivity of PANI
3. Results and Discussion
3.1. Synthesis of PANI
3.2. Determination of r
3.2.1. Yield of PANI
3.2.2. FTIR of PANI with Equal and Unequal Molar Ratios of Aniline/APS
3.3. Stirring Speed of PANI Synthesis
3.3.1. Effect of Stirring Speed on the FTIR
3.3.2. Effect of Stirring Speed on Particle Size
3.4. Synthesis of PANI at Different Temperatures
Effect of Synthesis Temperature on Particle Size
3.5. Purification Method for PANI
3.5.1. Effect of PANI Purification Method on the FTIR
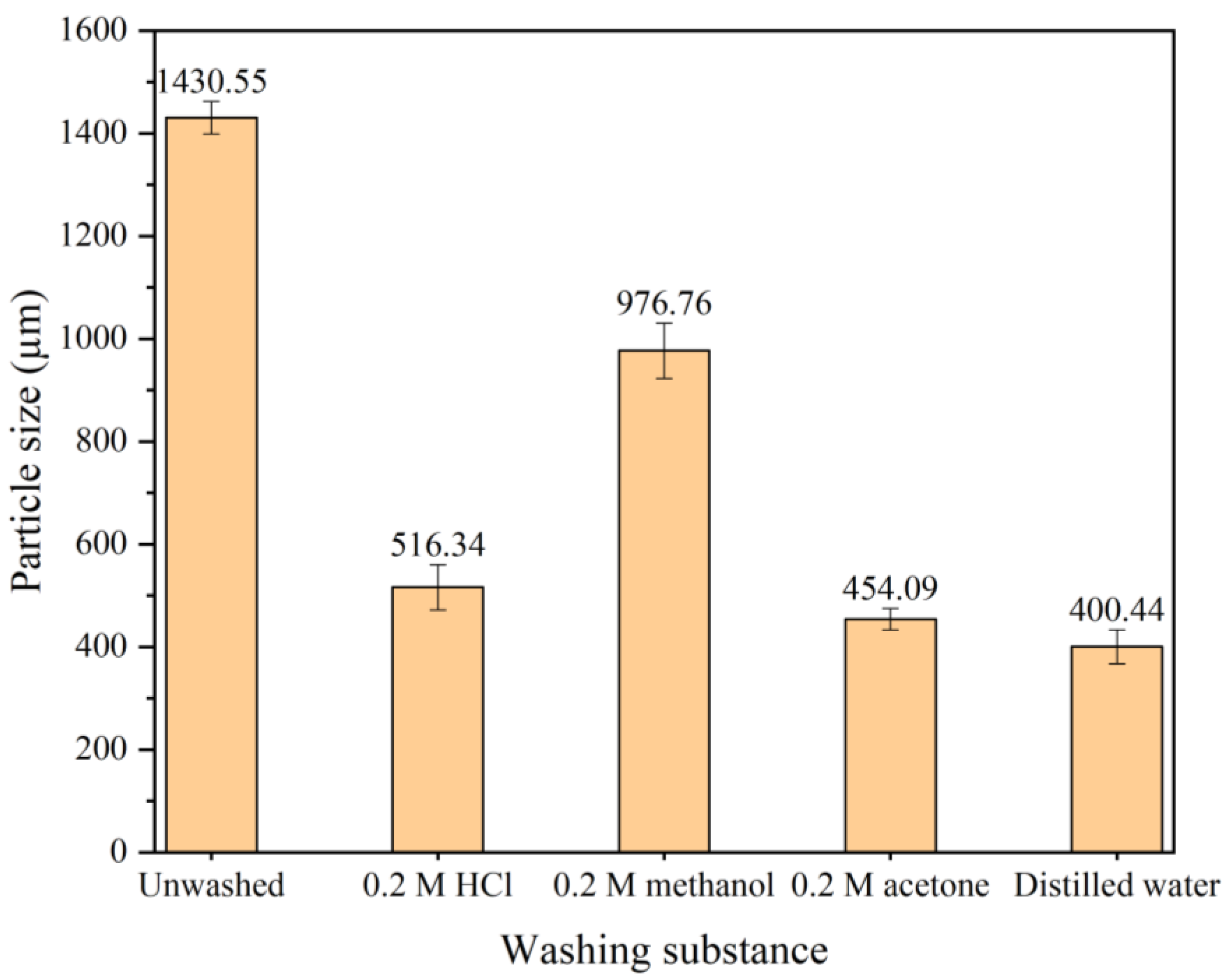
3.5.2. Effect of Purification Method on Particle Size
3.6. Washing Method for PANI
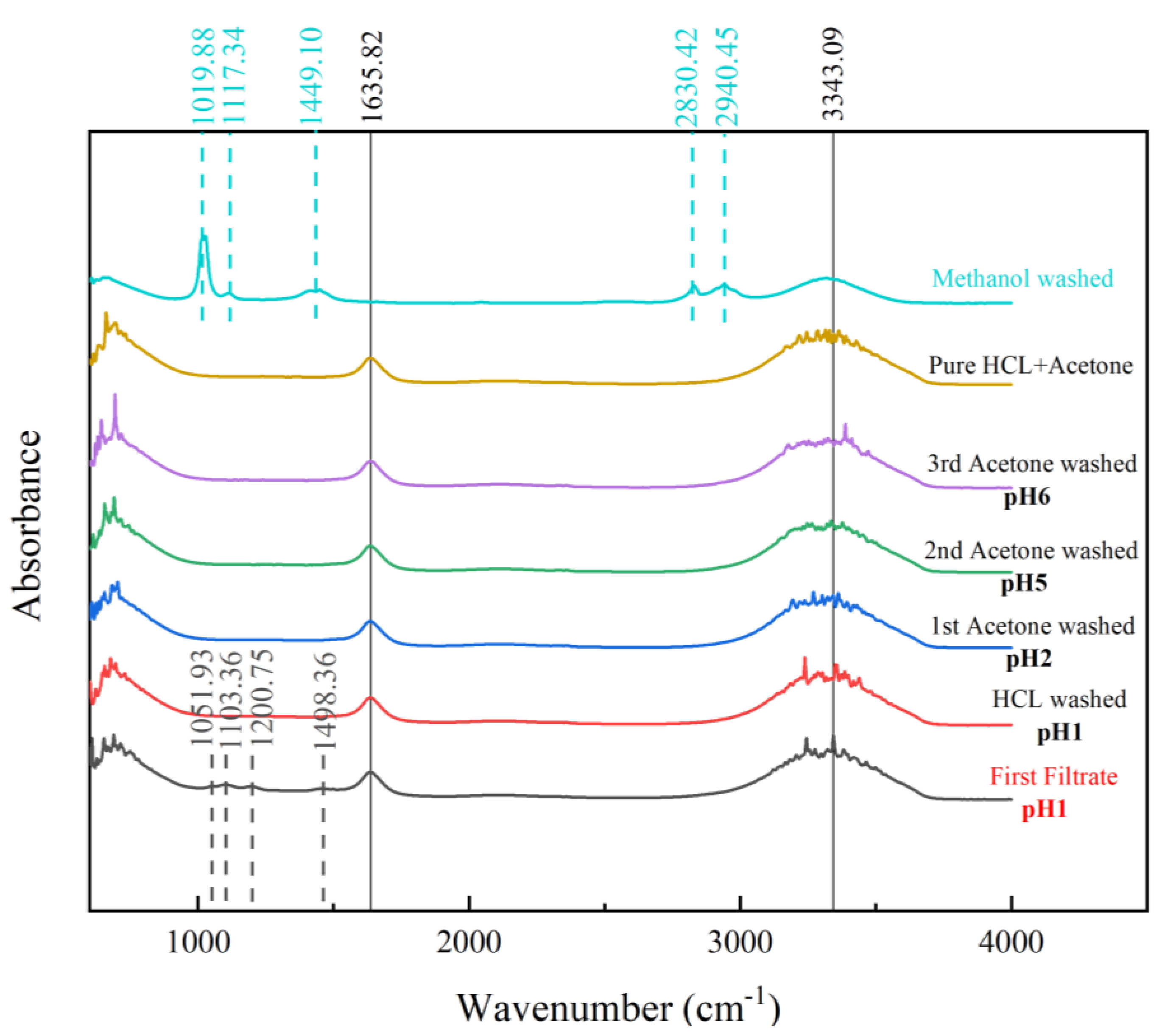
3.6.1. Effect of PANI Washing Method on the FTIR
3.6.2. Effect of PANI Washing Method on Particle Size
3.7. Effect of Different Surfactants in the Synthesis of PANI
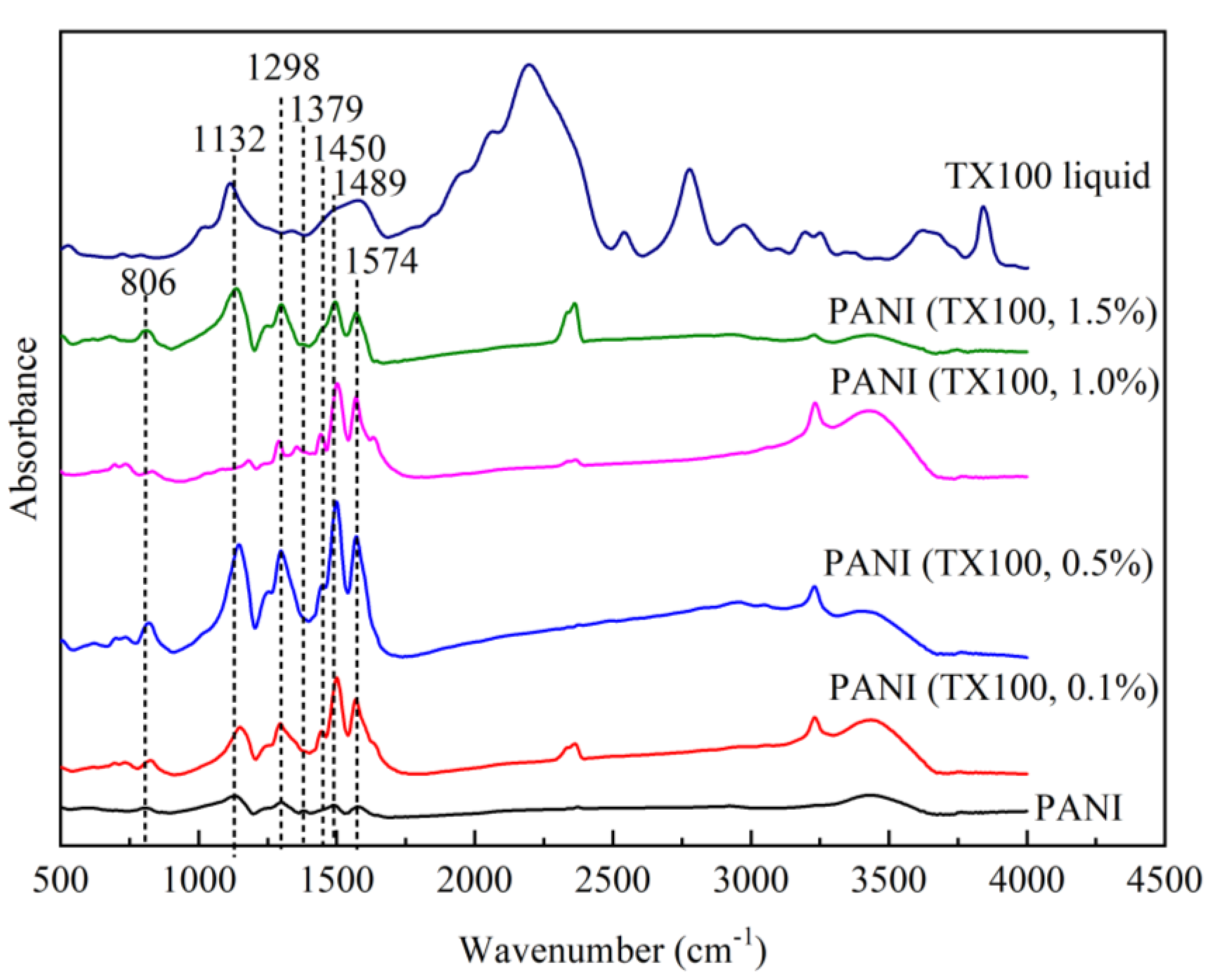
3.7.1. Effect of Different Surfactants in Synthesis on the FTIR Spectra
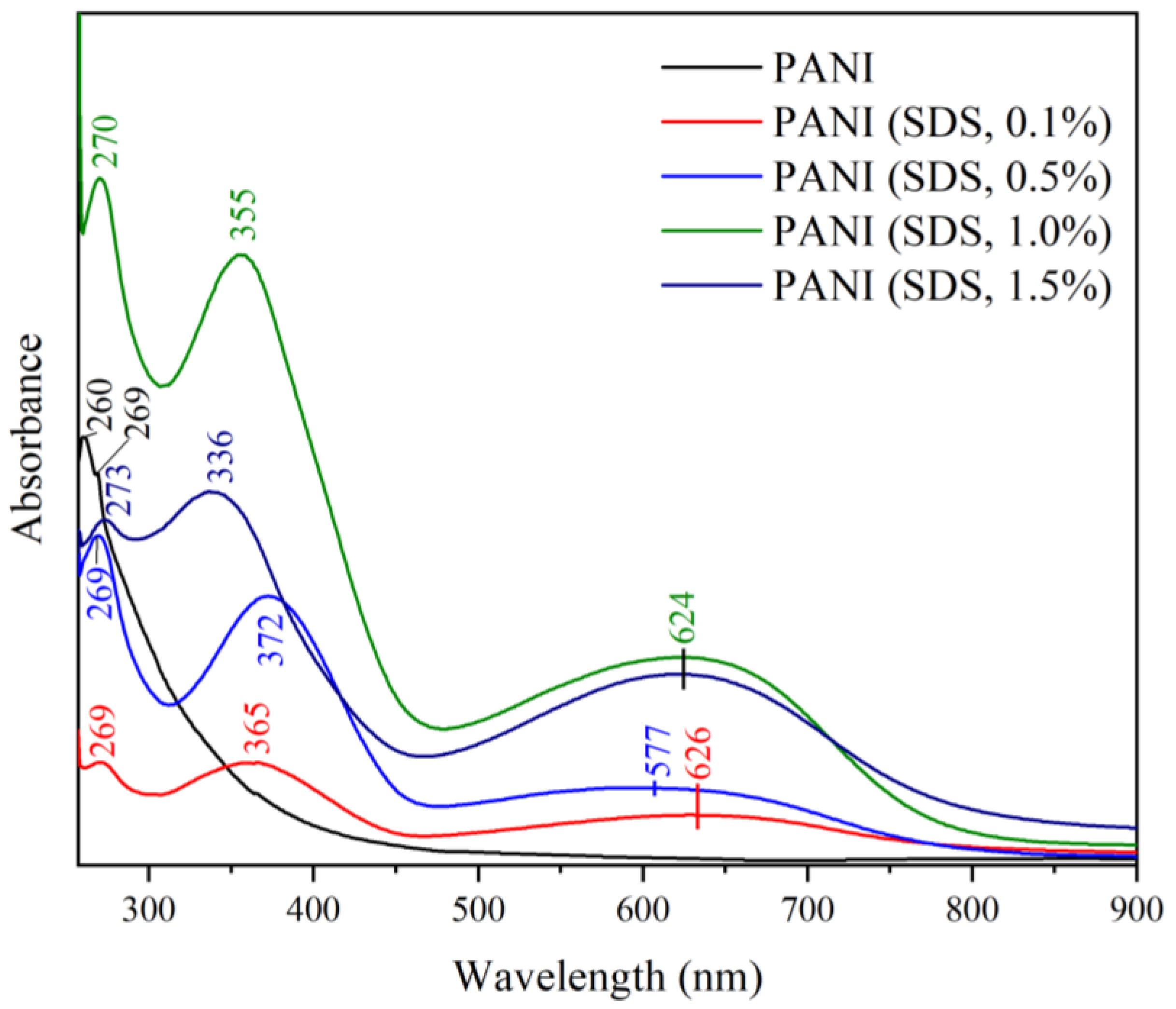
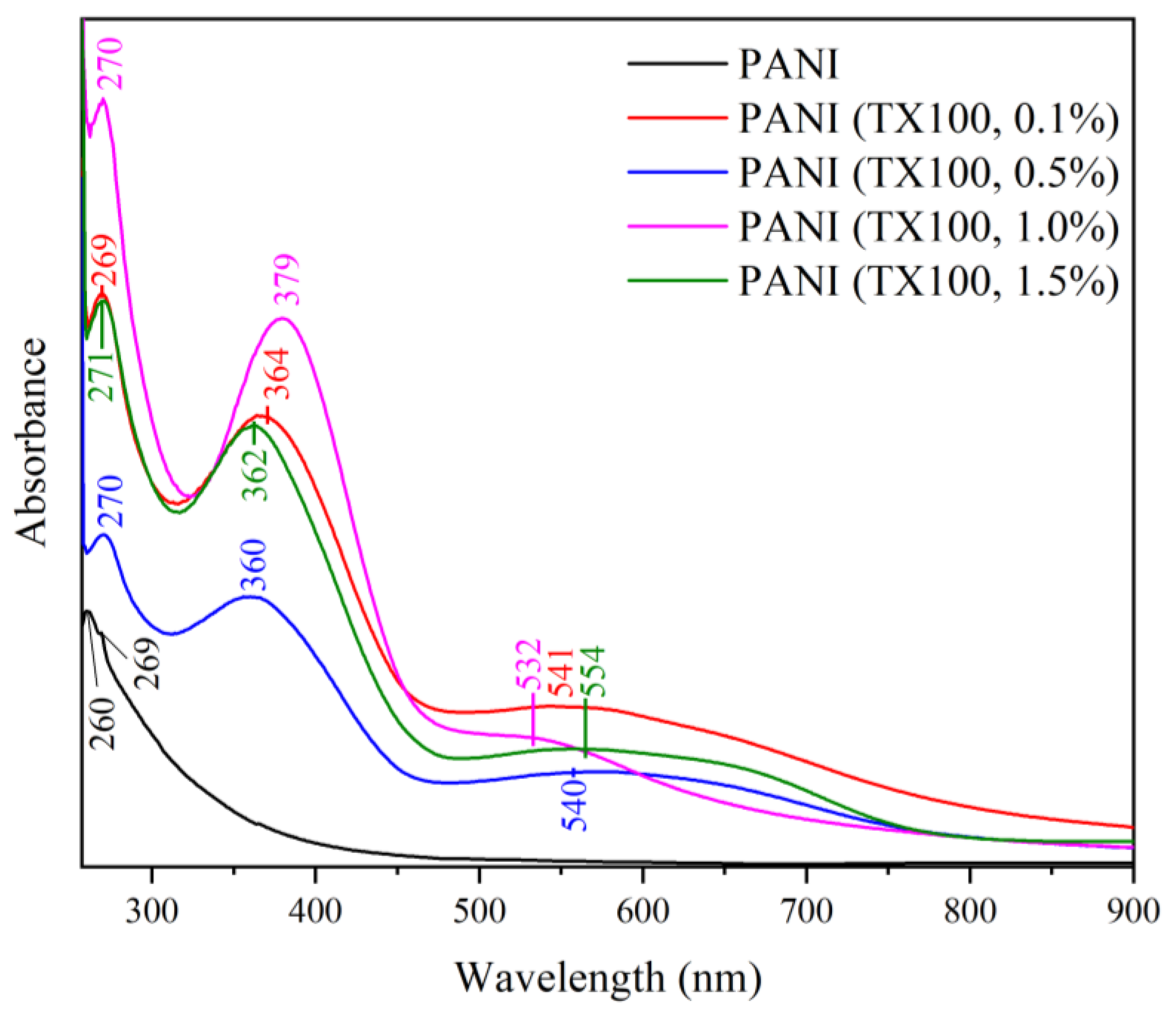
3.7.2. Effect of Different Surfactants in Synthesis on the UV-Vis Spectra
3.7.3. Effect of Different Surfactants in Synthesis on Particle Size
3.8. Morphologies of PANI Powders
3.9. Effect of Particle Size on the Conductivity of PANI
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Feng, W. Conductive Polymers and Their Composites; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, N.; Seixas, A.; Medeiros, E.; Mélo, T. Evaluation of conductivity of nanostructured polyaniline/cellulose nanocrystals (PANI/CNC) obtained via in situ polymerization. J. Solid State Chem. 2021, 302, 122372. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Mozafari, M. Polyaniline: Future perspectives. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–280. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial Properties of Polyaniline and Polypyrrole Decorated with Zinc-Doped Copper Oxide Microparticles. Polymers 2020, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.; Abdelhamied, M.M.; Abdelreheem, A.M.; Berber, M.R. Flexible Methyl Cellulose/Polyaniline/Silver Composite Films with Enhanced Linear and Nonlinear Optical Properties. Polymers 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.P.S.; Mozafari, M. Polyaniline: An introduction and overview. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Miraftab, R.; Karimi, B.; Bahlakeh, G.; Ramezanzadeh, B. Complementary experimental and quantum mechanics approaches for exploring the mechanical characteristics of epoxy composites loaded with graphene oxide-polyaniline nanofibers. J. Ind. Eng. Chem. 2017, 53, 348–359. [Google Scholar] [CrossRef]
- Sunthar, T.P.M.; Marin, E.; Boschetto, F.; Zanocco, M.; Sunahara, H.; Ramful, R.; Kamei, K.; Zhu, W.; Pezzotti, G. Antibacterial and Antifungal Properties of Composite Polyethylene Materials Reinforced with Neem and Turmeric. Antibiotics 2020, 9, 857. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Wang, J.-W.; Wang, G.-Q.; Ren, H. Enhanced dielectric properties of all-organic acrylic resin elastomer-based composite with doped polyaniline. Polym. Bull. 2018, 75, 2901–2916. [Google Scholar] [CrossRef]
- Han, J.; Lu, K.; Yue, Y.; Mei, C.; Huang, C.; Wu, Q.; Xu, X. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crop. Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, G.; Zhang, M.; Luo, Z. Preparation and Characterizing of PANI/PDMS Elastomer for Artificial Muscles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012165. [Google Scholar] [CrossRef]
- Singh, M.; Sharib, S.F.M.; Mok, K.L.; Yatim, A.H.M. Colloidal properties of precipitated calcium carbonate dispersion and its effect on prevulcanised natural rubber latex rheology and film tensile properties. J. Rubber Res. 2019, 22, 43–57. [Google Scholar] [CrossRef]
- Saeb, M.R.; Zarrintaj, P.; Khandelwal, P.; Chauhan, N.P.S. Synthetic route of polyaniline (I): Conventional oxidative polymerization. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–41. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Application of polyaniline and its derivatives. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–272. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Jamdegni, M.; Kaur, A. Role of polarity of surfactants on the morphology of electrochemically synthesized polyaniline nanostructures: Towards faster and efficient electrochromic response. Thin Solid Films 2020, 714, 138373. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A Review on Polyaniline: Synthesis, Properties, Nanocomposites, and Electrochemical Applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Ghorbankhani, A.; Zahedi, A.R. Micro-cellular polymer foam supported polyaniline-nanofiber: Eco-friendly tool for petroleum oil spill cleanup. J. Clean. Prod. 2022, 368, 133240. [Google Scholar] [CrossRef]
- Bhandari, S. Polyaniline: Structure and properties relationship. In Polyaniline Blends, Composites, and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 23–60. [Google Scholar] [CrossRef]
- Jarach, N.; Meridor, D.; Buzhor, M.; Raichman, D.; Dodiuk, H.; Kenig, S.; Amir, E. Hybrid Antibacterial and Electro-conductive Coating for Textiles Based on Cationic Conjugated Polymer. Polymers 2020, 12, 1517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cao, H. Enhancement of the Antibacterial Activity of Natural Rubber Latex Foam by Blending It with Chitin. Materials 2020, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Al-Hada, N.M.; Al-Ghaili, A.M.; Baqer, A.A.; Saleh, M.A.; Kasim, H.; Saion, E.; Liu, J.; Jihua, W. Radiation-induced synthesis, electrical and optical characterization of conducting polyaniline of PANI/ PVA composites. Mater. Sci. Eng. B 2020, 261, 114758. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of enzymatic formation of polyaniline nanoparticles. Polymer 2017, 115, 211–216. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Hong, R.; Gao, J.; Guo, Y.; Gu, C. A novel method for synthesis of polyaniline and its application for catalytic degradation of atrazine in a Fenton-like system. Chemosphere 2018, 197, 576–584. [Google Scholar] [CrossRef]
- Fang, F.F.; Dong, Y.-Z.; Choi, H.J. Effect of oxidants on morphology of interfacial polymerized polyaniline nanofibers and their electrorheological response. Polymer 2018, 158, 176–182. [Google Scholar] [CrossRef]
- Rashid, I.A.; Irfan, M.S.; Gill, Y.Q.; Nazar, R.; Saeed, F.; Afzal, A.; Ehsan, H.; Qaiser, A.A.; Shakoor, A. Stretchable strain sensors based on polyaniline/thermoplastic polyurethane blends. Polym. Bull. 2020, 77, 1081–1093. [Google Scholar] [CrossRef]
- Srinivas, C.H.; Srinivasu, D.; Kavitha, B.; Narsimlu, N.; Kumar, K.S. Synthesis and characterization of nano size conducting polyaniline. IOSR J. Appl. Phys. 2012, 1, 12–15. [Google Scholar] [CrossRef]
- Prasutiyo, Y.J.; Manaf, A.; Hafizah, M.A.E. Synthesis of polyaniline by chemical oxidative polymerization and characteristic of conductivity and reflection for various strong acid dopants. J. Phys. Conf. Ser. 2020, 1442, 012003. [Google Scholar] [CrossRef]
- Aribowo, S.; Hafizah, M.A.E.; Manaf, A.; Andreas, A. Study of aniline polymerization reactions through the particle size formation in acidic and neutral medium. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2018. [Google Scholar] [CrossRef]
- Hafizah, M.A.E.; Riyadi, A.F.; Manaf, A.; Andreas, A. Particle size reduction of polyaniline assisted by anionic emulsifier of sodium dodecyl sulphate (SDS) through emulsion polymerization. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019; Volume 515. [Google Scholar] [CrossRef]
- Lorenzen, A.L.; Rossi, T.S.; Riegel-Vidotti, I.C.; Vidotti, M. Influence of cationic and anionic micelles in the (sono)chemical synthesis of stable Ni(OH)2 nanoparticles: “In situ” zeta-potential measurements and electrochemical properties. Appl. Surf. Sci. 2018, 455, 357–366. [Google Scholar] [CrossRef]
- Banjar, M.F.; Suphi, H.D.; Sarizan, M.I.; Yahaya, A.N.A.; Khalil, N.A.; Singh, M.; Zulkifli, M. Fundamental study of colloidal stability and dispersion of novel nanosized conductive polyaniline (PANI)/prevulcanised latex (PVL) film for antimicrobial applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1195, 012055. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Mahitha, P.; Sneha, K.; Advaith, P.; Sultan, K.R.; Sajith, M.; Jose, B.; Hema, S.; Sambhudevan, S.; Shankar, B. Conducting polyaniline based rubber nanocomposites—Synthesis and characterization studies. Mater. Today Proc. 2019, 33, 1429–1433. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Khalili, R.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Mozafari, M. Polyaniline/metal oxides nanocomposites. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–141. [Google Scholar] [CrossRef]
- ASTM E168-16; Standard Practices for General Techniques of Infrared Quantitative Analysis. ASTM: West Conshohocken, PN, USA, 2016.
- Reddy, K.R.; Hemavathi, B.; Balakrishna, G.R.; Raghu, A.V.; Naveen, S.; Shankar, M.V. Organic conjugated polymer-based functional nanohybrids: Synthesis methods, mechanisms and its applications in electrochemical energy storage supercapacitors and solar cells. In Polymer Composites with Functionalized Nanoparticles: Synthesis, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–379. [Google Scholar] [CrossRef]
- Barbero, C.A.; Acevedo, D.F. Mechanochemical Synthesis of Polyanilines and Their Nanocomposites: A Critical Review. Polymers 2022, 15, 133. [Google Scholar] [CrossRef]
- Batista, A.F.; Rodrigues-Siqueli, A.C.; de Oliveira, A.P.S.; Petraconi, G.; Baldan, M.R. Facile synthesis of polyaniline catalyzed by carbon fiber for supercapacitor applications. Synth. Met. 2022, 289, 117116. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Saad, E.A.; El-Khatib, A.M.; Soliman, M.A.; Allam, E.A.; Fekry, N.A. Green solid synthesis of polyaniline-silver oxide nanocomposite for the adsorptive removal of ionic divalent species of Zn/Co and their radioactive isotopes 65Zn/ 60Co. Environ. Sci. Pollut. Res. 2018, 25, 22120–22135. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, S.; Jiang, W. Electrochemical Properties of PANI as Single Electrode of Electrochemical Capacitors in Acid Electrolytes. Sci. World J. 2013, 2013, 940153. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, S.; Rani, M.S.A.; Mohammad, M.; Mohammed, N.H.; Su’Ait, M.S.; Ibrahim, M.A.; Asim, N.; Razali, H. Investigation on size and conductivity of polyaniline nanofiber synthesised by surfactant-free polymerization. J. Mater. Res. Technol. 2021, 14, 255–261. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M. Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog. Polym. Sci. 2010, 35, 1420–1481. [Google Scholar] [CrossRef]
- Sajith, M.; Jose, B.; Sambhudevan, S.; Sreekala, C.O.; Shankar, B. Effect of matrix type and doping on polyaniline based natural rubber nanocomposites. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2019. [Google Scholar] [CrossRef]
- Lin, C.-W.; Mak, W.H.; Chen, D.; Wang, H.; Aguilar, S.; Kaner, R.B. Catalytic Effects of Aniline Polymerization Assisted by Oligomers. ACS Catal. 2019, 9, 6596–6606. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Corti, D.S.; Franses, E.I. Effect of Triton X-100 on the stability of titania nanoparticles against agglomeration and sedimentation: A masked depletion interaction. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 296–304. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Optimisation and Stability Assessment of Solid Lipid Nanoparticles Using Particle Size and Zeta Potential. J. Phys. Sci. 2014, 25, 59–75. [Google Scholar]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Janeczek, H.; Schab-Balcerzak, E.; Libera, M. Effect of polyaniline content and protonating dopants on electroconductive composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. A facile method for the synthesis of polyaniline nanospheres and the effect of doping on their electrical conductivity. Polym. Int. 2011, 60, 1291–1295. [Google Scholar] [CrossRef]
- Yang, J.; Ding, Y.; Chen, G.; Li, C. Synthesis of conducting polyaniline using novel anionic Gemini surfactant as micellar stabilizer. Eur. Polym. J. 2007, 43, 3337–3343. [Google Scholar] [CrossRef]
- Swaruparani, H.; Basavaraja, S.; Basavaraja, C.; Huh, D.S.; Venkataraman, A. A new approach to soluble polyaniline and its copolymers with toluidines. J. Appl. Polym. Sci. 2010, 117, 1350–1360. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Mikulik, D.; Ricci, M.; Tutuncuoglu, G.; Matteini, F.; Vukajlovic, J.; Vulic, N.; Alarcon-Llado, E.; i Morral, A.F. Conductive-probe atomic force microscopy as a characterization tool for nanowire-based solar cells. Nano Energy 2017, 41, 566–572. [Google Scholar] [CrossRef]












| Chemical | Usage | CAS No. | Brand |
|---|---|---|---|
| Aniline, >99.5% | Monomer | 62-53-3 | Sigma-Aldrich, St. Louis, MI, USA |
| Ammonium persulfate | Oxidizing agent | 7787-54-0 | R&M Chemicals, Calgary, AB, Canada |
| Hydrochloric acid, Fuming 37% | Doping agent | 7647-01-0 | R&M Chemicals |
| Sodium dodecyl sulfate, >99.5% | Anionic surfactant | 151-21-3 | Bendosen, Alen, Norway |
| Triton X-100, >99.5% | Nonionic surfactant | 9036-19-5 | Sigma-Aldrich |
| Acetone, >99.5% | Washing agent | 67-64-1 | Sigma-Aldrich |
| Methanol, >99.5% | Washing agent | 67-56-1 | Sigma-Aldrich |
| N-methylpyrrolidone, >99.5% | Solvent for analysis | 872-50-4 | Sigma-Aldrich |
| Chemicals | Chemicals |
|---|---|
| Initial molar ratio of aniline/APS (r) | 1 (0.2 M:0.2 M) |
| Stirring speed during the synthesis period | 800 rpm |
| Synthesis temperature | 0–2 °C |
| Purification method | Filtration |
| Chemicals used for washing | Washing agent in the following order: 0.2 M HCl 0.2 M acetone Distilled water |
| Surfactant use | Not applied |
| Sample | Initial Molar Ratio (r) | Molar Concentration (M) | Weight of Aniline (g) | Weight of PANI (g) | % Yield | |
|---|---|---|---|---|---|---|
| Aniline | APS | |||||
| Equal molar | 1 | 0.2 | 0.2 | 5.10 | 4.61 | 90.39 |
| Unequal molar | 3 | 0.3 | 0.1 | 6.12 | 2.12 | 34.72 |
| Sample | Population | Range (µm) | Median Diameter (µm) |
|---|---|---|---|
| PANI 600 rpm | 1 | 2.41–29.91 | 10.10 |
| 2 | 29.91–344.21 | 152.45 | |
| PANI 1200 rpm | 1 | 4.47–44.94 | 15.17 |
| 2 | 44.94–394.24 | 152.45 |
| Purification Method | Weight of Aniline (g) | Weight of PANI (g) | %Yield |
|---|---|---|---|
| Heating | 10.50 | 10.06 | 95.99 |
| Filtration | 8.03 | 76.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banjar, M.F.; Joynal Abedin, F.N.; Fizal, A.N.S.; Muhamad Sarih, N.; Hossain, M.S.; Osman, H.; Khalil, N.A.; Ahmad Yahaya, A.N.; Zulkifli, M. Synthesis and Characterization of a Novel Nanosized Polyaniline. Polymers 2023, 15, 4565. https://doi.org/10.3390/polym15234565
Banjar MF, Joynal Abedin FN, Fizal ANS, Muhamad Sarih N, Hossain MS, Osman H, Khalil NA, Ahmad Yahaya AN, Zulkifli M. Synthesis and Characterization of a Novel Nanosized Polyaniline. Polymers. 2023; 15(23):4565. https://doi.org/10.3390/polym15234565
Chicago/Turabian StyleBanjar, Mohd Faizar, Fatin Najwa Joynal Abedin, Ahmad Noor Syimir Fizal, Norazilawati Muhamad Sarih, Md. Sohrab Hossain, Hakimah Osman, Nor Afifah Khalil, Ahmad Naim Ahmad Yahaya, and Muzafar Zulkifli. 2023. "Synthesis and Characterization of a Novel Nanosized Polyaniline" Polymers 15, no. 23: 4565. https://doi.org/10.3390/polym15234565
APA StyleBanjar, M. F., Joynal Abedin, F. N., Fizal, A. N. S., Muhamad Sarih, N., Hossain, M. S., Osman, H., Khalil, N. A., Ahmad Yahaya, A. N., & Zulkifli, M. (2023). Synthesis and Characterization of a Novel Nanosized Polyaniline. Polymers, 15(23), 4565. https://doi.org/10.3390/polym15234565










