Hydrothermal Ammonia Carbonization of Rice Straw for Hydrochar to Separate Cd(II) and Zn(II) Ions from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Hydrochar
2.3. Adsorption Experiment
3. Results and Discussion
3.1. Characterization Results
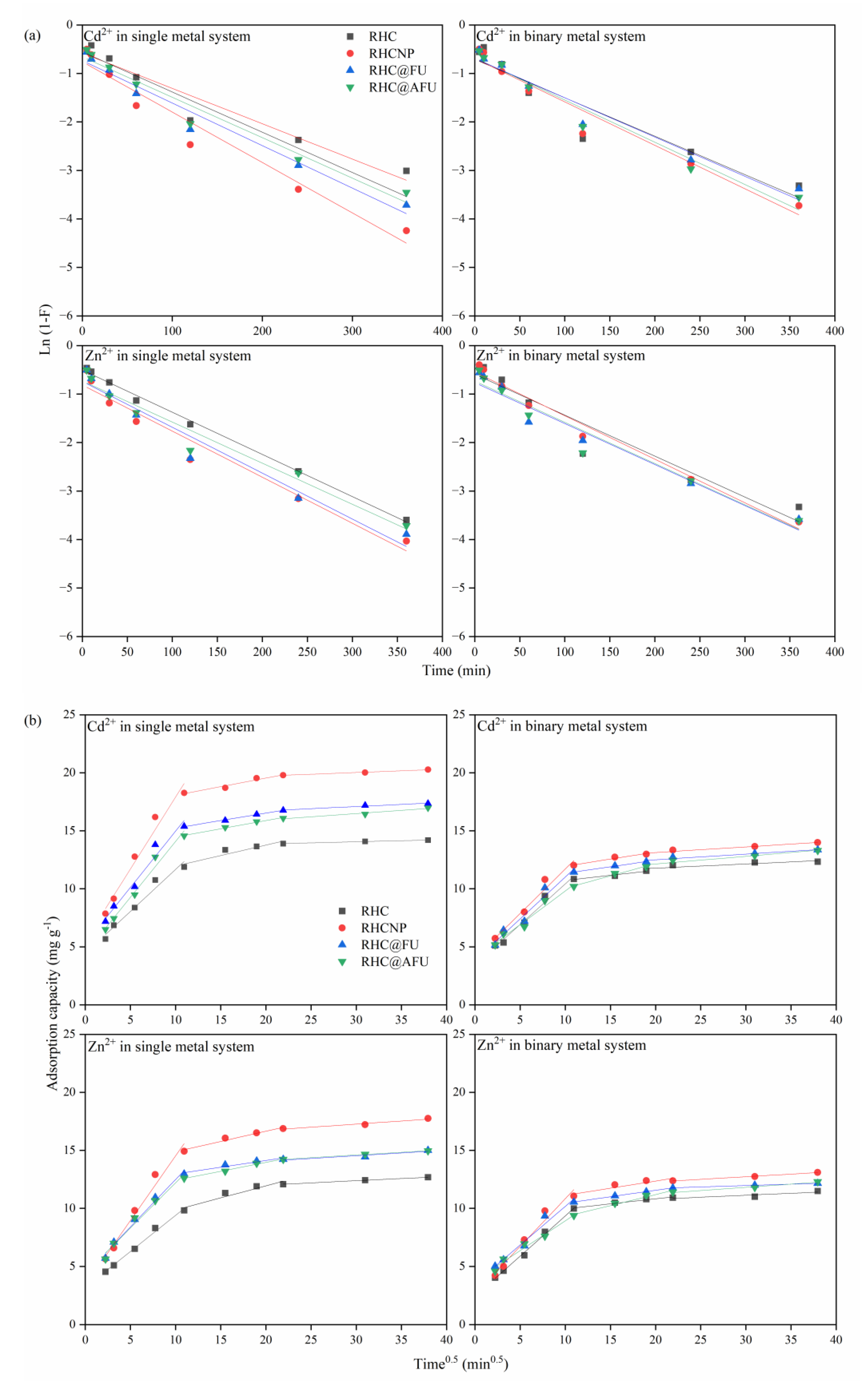
3.2. Adsorption Capacity
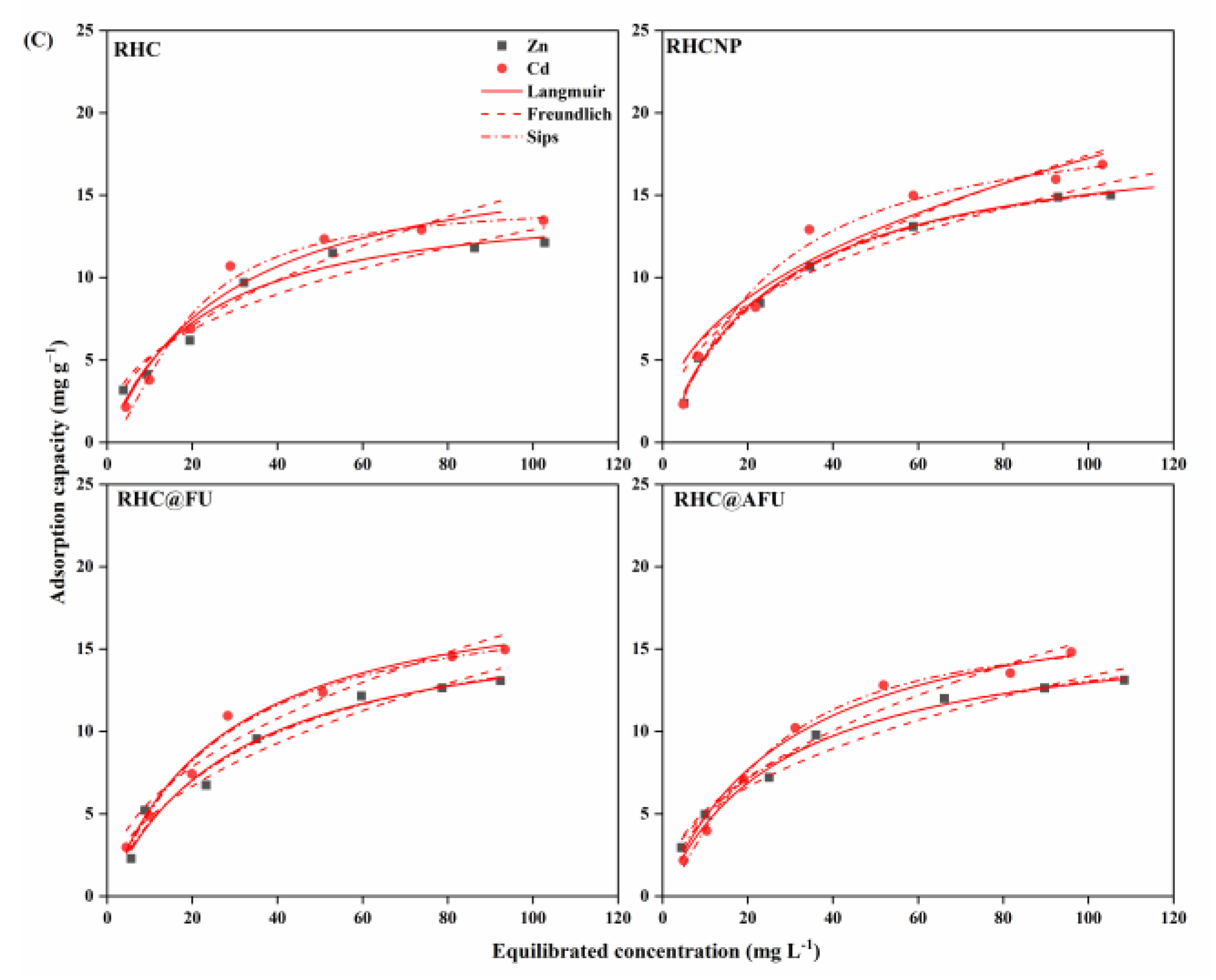
3.3. Adsorption Isotherms
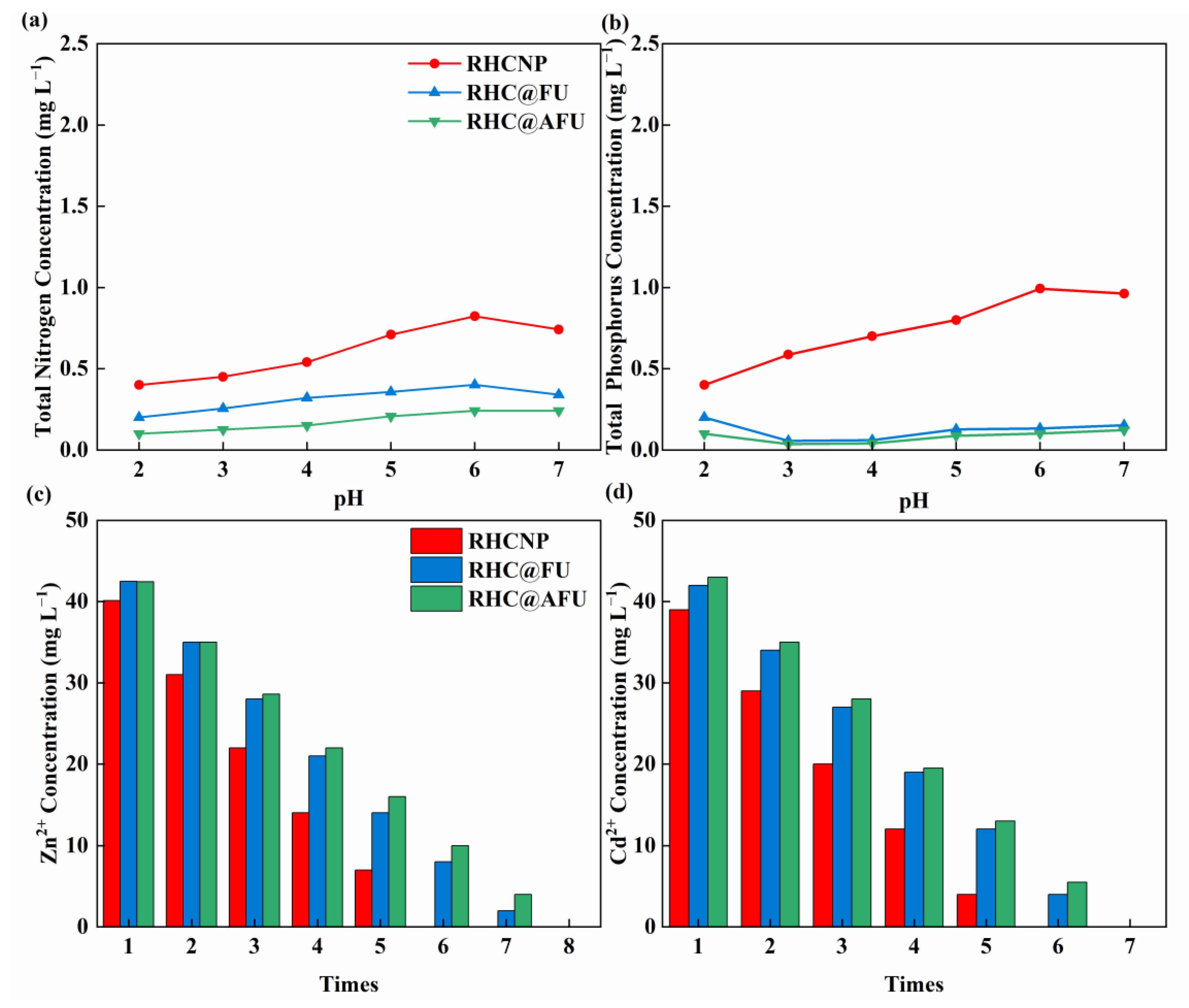
3.4. Effect of Solution pH
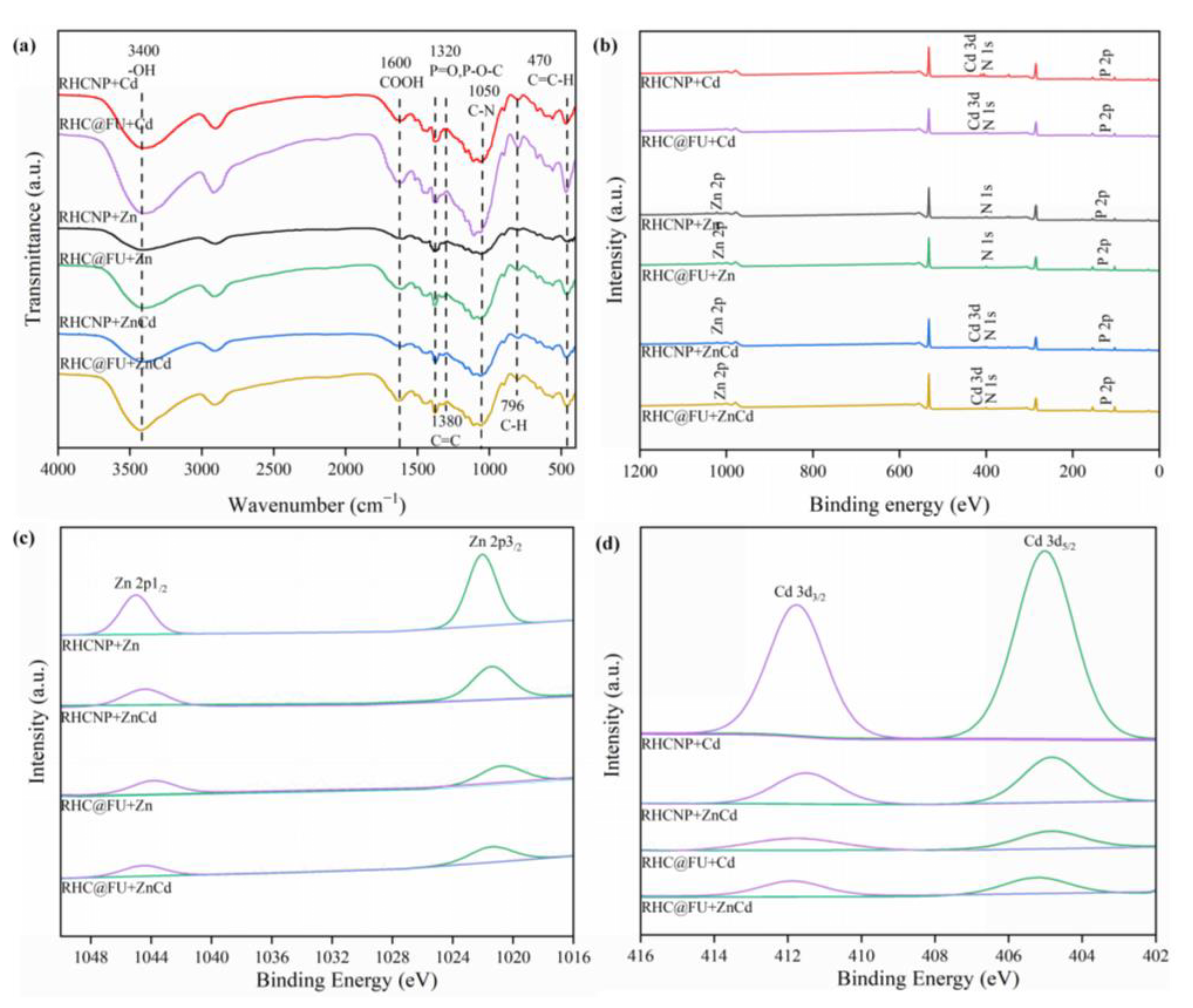
3.5. Mechanisms
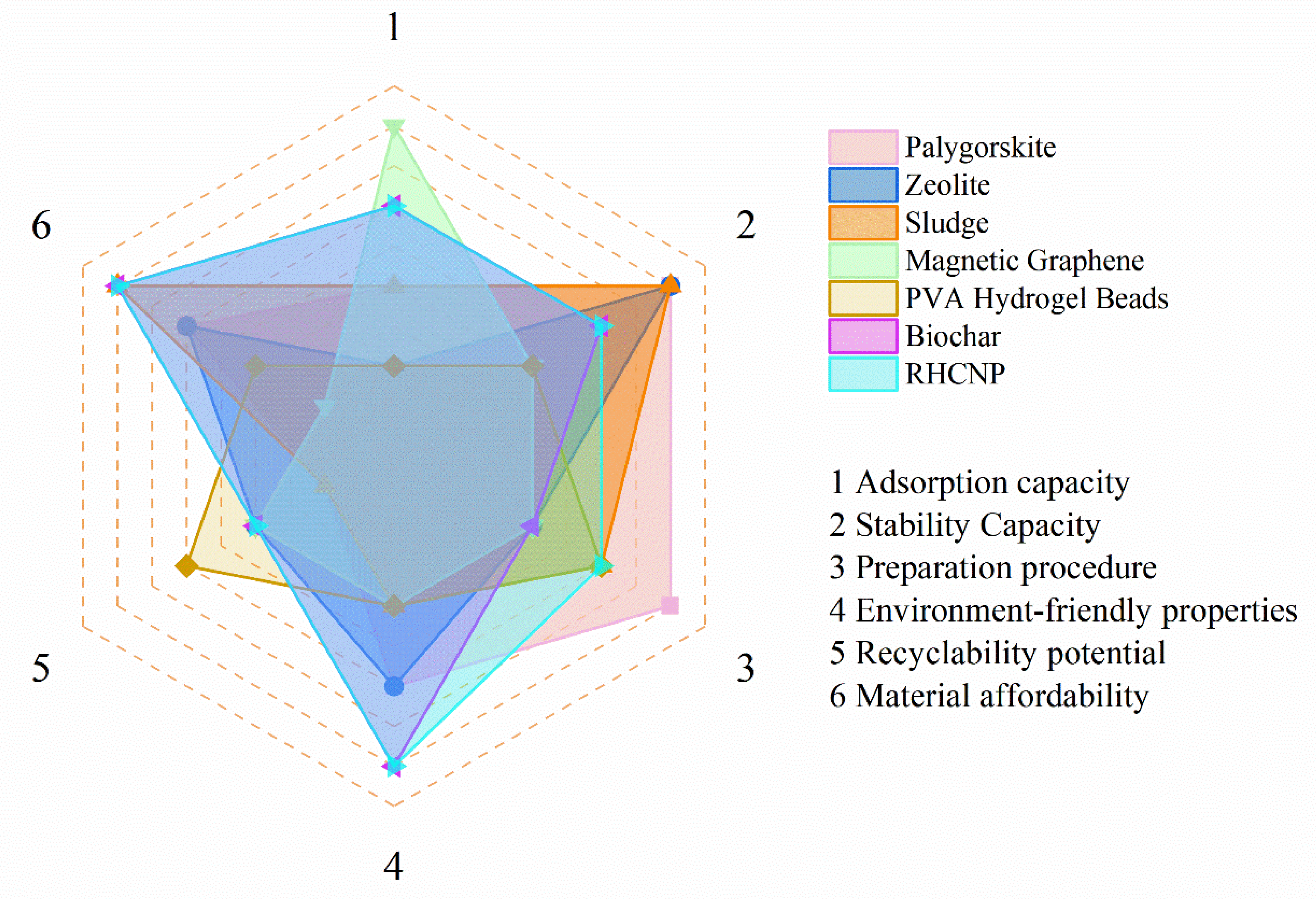
3.6. Research Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, J.; Zhang, C.; Sun, Y.-C.; Zhang, Q.; Lv, M.-W.; Guo, K.; Long, J. Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci. Total Environ. 2019, 689, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Alyasi, H.; Mackey, H.; McKay, G. Novel model analysis for multimechanistic adsorption processes: Case study: Cadmium on nanochitosan. Sep. Purif. Technol. 2021, 274, 117925. [Google Scholar] [CrossRef]
- Smolyakov, B.S.; Sagidullin, A.K.; Chikunov, A.S. Removal of Cd(II), Zn(II), and Cu(II) from aqueous solutions using humic-modified moss (Polytrichum Comm.). J. Environ. Chem. Eng. 2017, 5, 1015–1020. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, L.; Deen, K.M.; Asselin, E.; Ma, B.; Wang, C. Enhanced removal of cadmium from wastewater by electro-assisted cementation process: A peculiar Cd reduction on Zn anode. Chem. Eng. J. 2023, 452, 139692. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd(II), Co(II), Cr(III), Ni(II), Pb(II) and Zn(II) ions from wastewater using polyethyleneimine (PEI) cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef] [PubMed]
- Wijeyawardana, P.; Nanayakkara, N.; Law, D.; Gunasekara, C.; Karunarathna, A.; Pramanik, B.K. Performance of biochar mixed cement paste for removal of Cu, Pb and Zn from stormwater. Environ. Res. 2023, 232, 116331. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Cavali, M.; Junior, N.L.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Filho, P.B.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Yuan, D.; Wang, Y.; Liu, J.; Chew, J.W. Investigation of the high U(VI) adsorption properties of phosphoric acid-functionalized heteroatoms-doped carbon materials. Solid State Sci. 2020, 104, 106248. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.; Yang, G.; Lucia, L.A.; Chen, J. Hydrothermal Carbonization of Corncob Residues for Hydrochar Production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- Nassar, A.E.; El-Aswar, E.I.; Rizk, S.A.; Gaber, S.E.-S.; Jahin, H.S. Microwave-assisted hydrothermal preparation of magnetic hydrochar for the removal of organophosphorus insecticides from aqueous solutions. Sep. Purif. Technol. 2023, 306, 122569. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Hu, S.; Gu, J.; Jiang, F.; Hsieh, Y.-L. Holistic Rice Straw Nanocellulose and Hemicelluloses/Lignin Composite Films. ACS Sustain. Chem. Eng. 2016, 4, 728–737. [Google Scholar] [CrossRef]
- Manisha, T.; Amita, S.; Vishakha, A.; Munna, B.; Saswata, G. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar]
- Gao, P.; Yao, D.; Qian, Y.; Zhong, S.; Zhang, L.; Xue, G.; Jia, H. Factors controlling the formation of persistent free radicals in hydrochar during hydrothermal conversion of rice straw. Environ. Chem. Lett. 2018, 16, 1463–1468. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Jin, H.; Sun, E.; Xu, Y.; Guo, R.; Zheng, M.; Huang, H.; Zhang, S. Hydrochar Derived from Anaerobic Solid Digestates of Swine Manure and Rice Straw: A Potential Recyclable Material. Bioresources 2018, 13, 1019–1034. [Google Scholar] [CrossRef]
- Xiao, X.-F.; Chang, Y.-C.; Lai, F.-Y.; Fang, H.-S.; Zhou, C.-F.; Pan, Z.-Q.; Wang, J.-X.; Wang, Y.-J.; Yin, X.; Huang, H.-J. Effects of rice straw/wood sawdust addition on the transport/conversion behaviors of heavy metals during the liquefaction of sewage sludge. J. Environ. Manag. 2020, 270, 110824. [Google Scholar] [CrossRef]
- Shan, R.; Shi, Y.; Gu, J.; Wang, Y.; Yuan, H. Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin. J. Chem. Eng. 2020, 28, 1375–1383. [Google Scholar] [CrossRef]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Deng, H.; Du, Z.; Tian, K.; Zhang, J. Design of nitrogen-phosphorus-doped biochar and its lead adsorption performance. Environ. Sci. Pollut. Res. Int. 2022, 29, 28984–28994. [Google Scholar] [CrossRef] [PubMed]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Li, X.; Shi, J. Simultaneous adsorption of tetracycline, ammonium and phosphate from wastewater by iron and nitrogen modified biochar: Kinetics, isotherm, thermodynamic and mechanism. Chemosphere 2022, 293, 133574. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Geng, N.; Li, X.; Yu, J.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; Lou, Y. Biochar Alleviates Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Foxtail Millet (Setaria italica L.) Cultivated in Cd- and Zn-Contaminated Soil. Front. Plant Sci. 2022, 13, 782963. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Tan, Y.; Ma, X.-Q.; Wu, S.-Y.; Zhang, B. The influence of NaCl during hydrothermal carbonization for rice husk on hydrochar physicochemical properties. Energy 2023, 266, 126463. [Google Scholar] [CrossRef]
- Luo, Y.; Lan, Y.; Liu, X.; Xue, M.; Zhang, L.; Yin, Z.; He, X.; Li, X.; Yang, J.; Hong, Z.; et al. Hydrochar effectively removes aqueous Cr(VI) through synergistic adsorption and photoreduction. Sep. Purif. Technol. 2023, 317, 123926. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Shahab, A.; Zhang, K.; Zeng, H.; Bacha, A.-U.-R.; Nabi, I.; Ullah, H. Comparative study on characterization and adsorption properties of phosphoric acid activated biochar and nitrogen-containing modified biochar employing Eucalyptus as a precursor. J. Clean. Prod. 2021, 303, 127046. [Google Scholar] [CrossRef]
- Soudani, A.; Youcef, L.; Bulgariu, L.; Youcef, S.; Toumi, K.; Soudani, N. Characterizing and modeling of oak fruit shells biochar as an adsorbent for the removal of Cu, Cd, and Zn in single and in competitive systems. Chem. Eng. Res. Des. 2022, 188, 972–987. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms; kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, E.; Rokhmat, M.; Abdullah, M. Reduction of seawater salinity by natural zeolite (Clinoptilolite): Adsorption isotherms, thermodynamics and kinetics. Desalination 2017, 409, 146–156. [Google Scholar] [CrossRef]
- Bandara, T.; Xu, J.; Potter, I.D.; Franks, A.; Chathurika, J.B.A.J.; Tang, C. Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural wastes. Chemosphere 2020, 254, 126745. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Geng, N.; Li, Y.; Li, X.; Yu, J.; Gao, S.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; et al. Treatment of cadmium and zinc-contaminated water systems using modified biochar: Contaminant uptake, adsorption ability, and mechanism. Bioresour. Technol. 2022, 363, 127817. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Sun, P.; Chen, Y.; Liu, J.; Li, J.; Zheng, T.; Yang, S. The influence of alkali-modified biochar on the removal and release of Zn in bioretention systems: Adsorption and immobilization mechanism. Environ. Pollut. 2022, 310, 119874. [Google Scholar] [CrossRef]
- Inoue, Y.; Guo, H.; Honma, T.; Smith, R.L. Amino-functional biocarbon with CO2-responsive property for removing copper(II) ions from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126304. [Google Scholar] [CrossRef]
- Kong, H.; Li, Q.; Zheng, X.; Chen, P.; Zhang, G.; Huang, Z. Lanthanum modified chitosan-attapulgite composite for phosphate removal from water: Performance, mechanisms and applicability. Int. J. Biol. Macromol. 2023, 224, 984–997. [Google Scholar] [CrossRef]
- Liu, S.; Fan, F.; Ni, Z.; Liu, J.; Wang, S. Sustainable lanthanum-attapulgite/alginate hydrogels with enhanced mechanical strength for selective phosphate scavenging. J. Clean. Prod. 2023, 385, 135649. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Huang, M.; Yan, H.; Yang, H.; Xiao, S.; Li, A. Preparation of chitosan- graft -polyacrylamide magnetic composite microspheres for enhanced selective removal of mercury ions from water. J. Colloid Interface Sci. 2015, 455, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Ma, J.; Xia, P.; Zhao, J. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste–struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, Y.; Pan, J.; Zhang, Y. Use of dewatered sludge as microbial inoculum of a subsurface wastewater infiltration system: Effect on start-up and pollutant removal. Water SA 2017, 43, 595. [Google Scholar] [CrossRef][Green Version]
- Siswoyo, E.; Mihara, Y.; Tanaka, S. Determination of key components and adsorption capacity of a low cost adsorbent based on sludge of drinking water treatment plant to adsorb cadmium ion in water. Appl. Clay Sci. 2014, 97-98, 146–152. [Google Scholar] [CrossRef]
- Du, X.; Cui, S.; Fang, X.; Wang, Q.; Liu, G. Adsorption of Cd(II), Cu(II), and Zn(II) by granules prepared using sludge from a drinking water purification plant. J. Environ. Chem. Eng. 2020, 8, 104530. [Google Scholar] [CrossRef]
- Ain, Q.-U.; Farooq, M.U.; Jalees, M.I. Application of magnetic Graphene oxide for water purification: Heavy metals removal and disinfection. J. Water Process. Eng. 2020, 33, 101044. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J. Hazard. Mater. 2020, 381, 120914. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Li, L.; Wang, Y.; Ma, P.; Chen, M.; Dong, W. Polydopamine-functionalized graphene oxide compounded with polyvinyl alcohol/chitosan hydrogels on the recyclable adsorption of cu(II), Pb(II) and cd(II) from aqueous solution. J. Polym. Res. 2019, 26, 281. [Google Scholar] [CrossRef]
- Cairns, S.; Chaudhuri, S.; Sigmund, G.; Robertson, I.; Hawkins, N.; Dunlop, T.; Hofmann, T. Wood ash amended biochar for the removal of lead, copper, zinc and cadmium from aqueous solution. Environ. Technol. Innov. 2021, 24, 101961. [Google Scholar] [CrossRef]


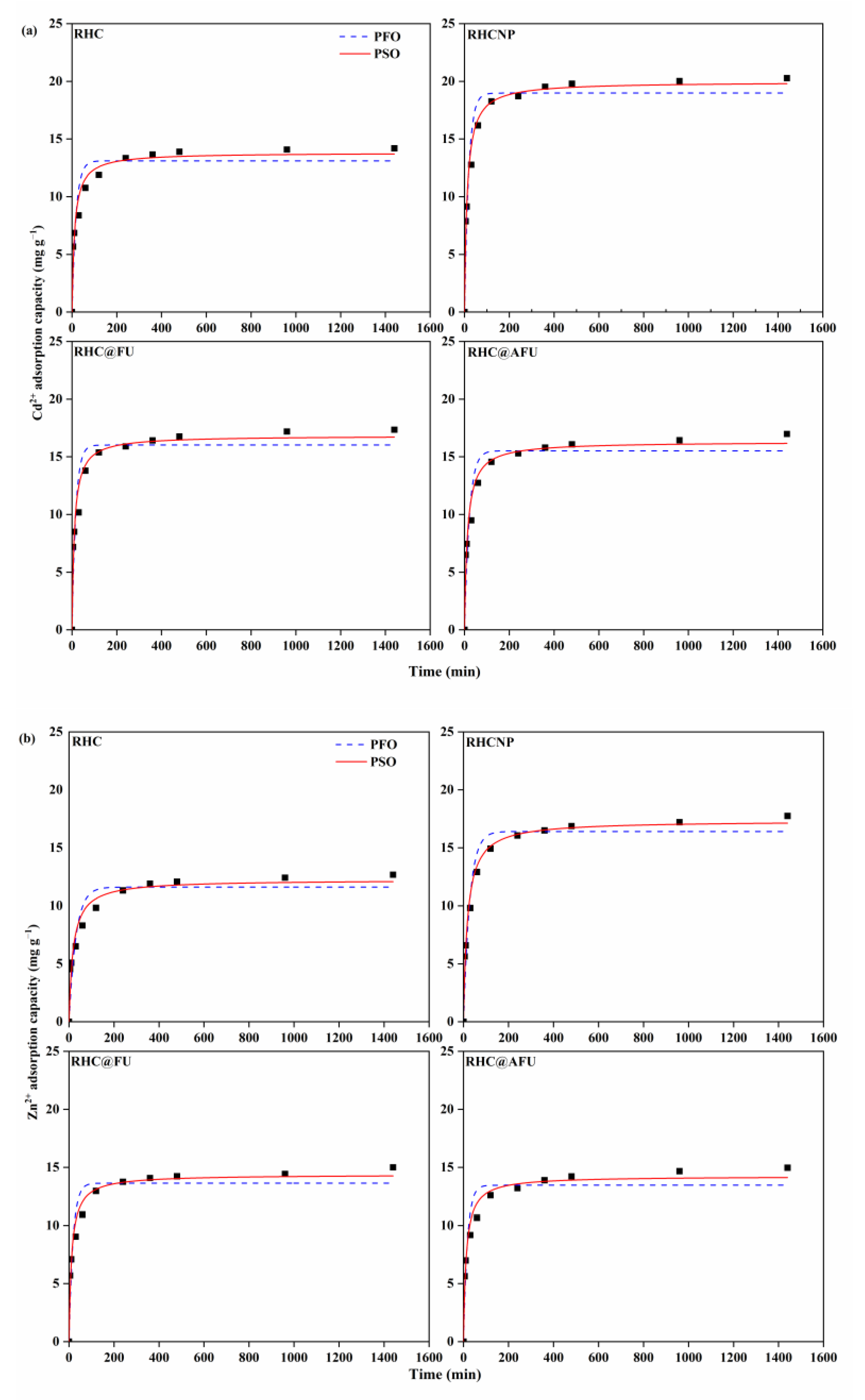
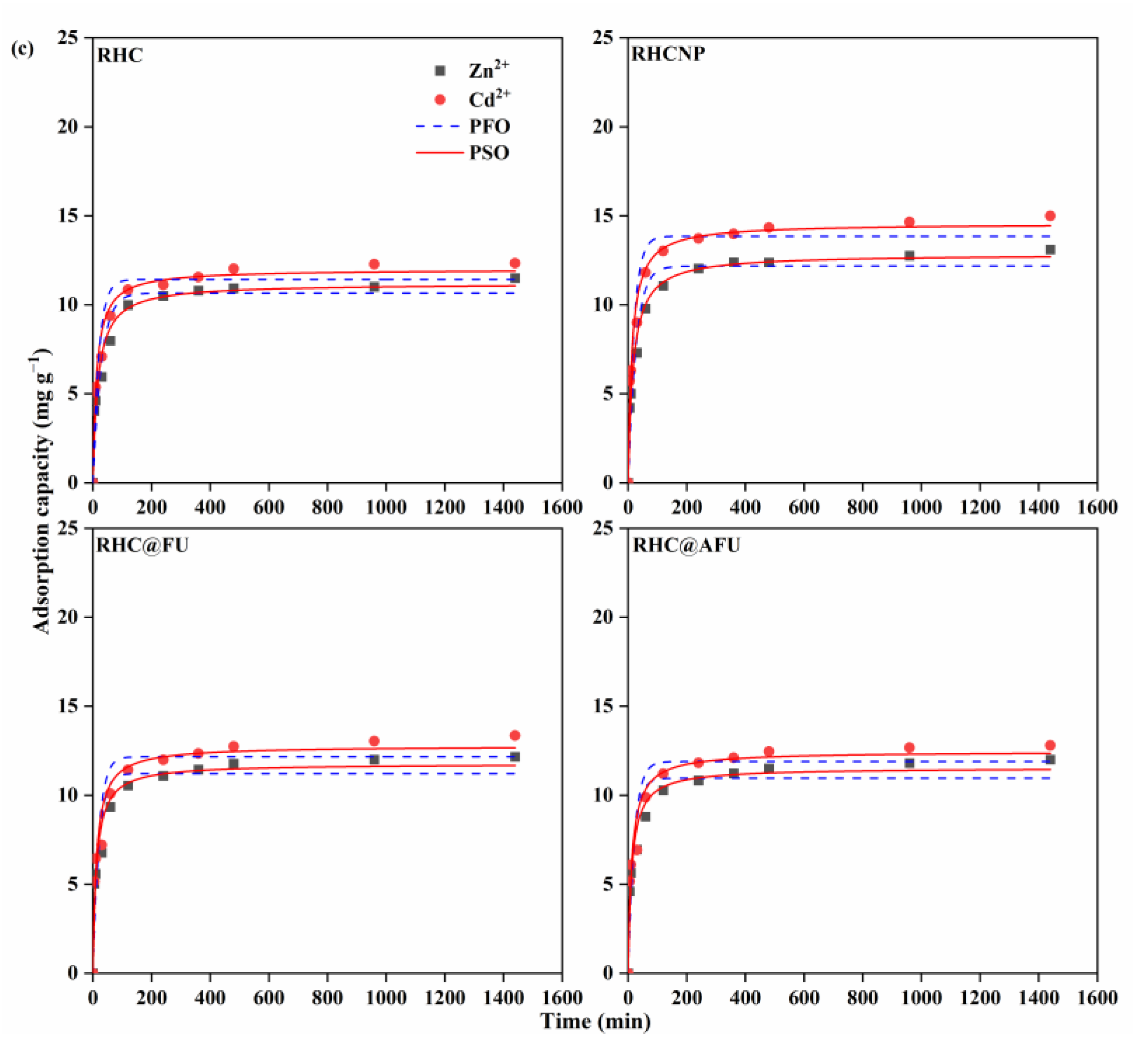



| Metal Ion | Adsorbent | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | qe (mg g−1) | K2 (g mg−1 min−1) | R2 | ||
| Cd2+ | RHC | 13.11 | 0.0553 | 0.899 | 13.82 | 0.0062 | 0.967 |
| RHCNP | 18.99 | 0.0544 | 0.937 | 19.96 | 0.0043 | 0.984 | |
| RHC@FU | 16.02 | 0.0590 | 0.907 | 16.83 | 0.0056 | 0.969 | |
| RHC@AFU | 15.52 | 0.0466 | 0.912 | 16.31 | 0.0047 | 0.970 | |
| Zn2+ | RHC | 11.61 | 0.0321 | 0.888 | 12.24 | 0.0044 | 0.954 |
| RHCNP | 16.41 | 0.0360 | 0.945 | 17.34 | 0.0039 | 0.985 | |
| RHC@FU | 13.63 | 0.0558 | 0.912 | 14.37 | 0.0060 | 0.975 | |
| RHC@AFU | 13.46 | 0.0578 | 0.907 | 14.24 | 0.0598 | 0.972 | |
| Cd2+ (Cd2+ + Zn2+) | RHC | 11.42 | 0.0484 | 0.904 | 11.99 | 0.0067 | 0.964 |
| RHCNP | 13.85 | 0.0494 | 0.934 | 14.57 | 0.0054 | 0.981 | |
| RHC@FU | 12.16 | 0.0502 | 0.894 | 12.77 | 0.0064 | 0.960 | |
| RHC@AFU | 11.89 | 0.0481 | 0.895 | 12.46 | 0.0065 | 0.959 | |
| Zn2+ (Cd2+ + Zn2+) | RHC | 10.66 | 0.0342 | 0.919 | 11.19 | 0.0053 | 0.937 |
| RHCNP | 12.17 | 0.0374 | 0.946 | 12.85 | 0.0046 | 0.985 | |
| RHC@FU | 11.22 | 0.0496 | 0.897 | 11.77 | 0.0070 | 0.961 | |
| RHC@AFU | 10.96 | 0.0515 | 0.903 | 11.54 | 0.0070 | 0.969 |
| Metal Ion | Adsorbent | LFD | IPD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kfd | C | R2 | Ki1 | C | R2 | Ki2 | C | R2 | Ki3 | C | R2 | ||
| (min−1) | (mg L−1) | (mg g−1 min−0.5) | (mg L−1) | (mg g−1 min−0.5) | (mg L−1) | (mg g−1 min−0.5) | (mg L−1) | ||||||
| Cd2+ | RHC | 0.007 | −0.58 | 0.938 | 0.72 | 4.44 | 0.967 | 0.18 | 10.17 | 0.888 | 0.02 | 13.49 | 0.993 |
| RHCNP | 0.010 | −0.76 | 0.955 | 1.24 | 5.52 | 0.971 | 0.15 | 16.58 | 0.965 | 0.03 | 19.14 | 0.990 | |
| RHC@FU | 0.009 | −0.73 | 0.970 | 0.97 | 5.28 | 0.968 | 0.13 | 13.95 | 0.997 | 0.04 | 15.99 | 0.968 | |
| RHC@AFU | 0.008 | −0.66 | 0.967 | 0.96 | 4.46 | 0.980 | 0.14 | 13.08 | 0.991 | 0.06 | 14.82 | 0.962 | |
| Zn2+ | RHC | 0.009 | −0.50 | 0.997 | 0.62 | 3.17 | 0.993 | 0.21 | 7.78 | 0.918 | 0.04 | 11.26 | 1.000 |
| RHCNP | 0.010 | −0.81 | 0.961 | 1.11 | 3.39 | 0.976 | 0.18 | 13.12 | 0.967 | 0.05 | 15.66 | 0.957 | |
| RHC@FU | 0.009 | −0.74 | 0.960 | 0.82 | 4.31 | 0.986 | 0.12 | 11.81 | 0.943 | 0.05 | 13.13 | 0.886 | |
| RHC@AFU | 0.008 | −0.73 | 0.962 | 0.78 | 4.41 | 0.977 | 0.15 | 10.90 | 0.992 | 0.05 | 13.22 | 0.998 | |
| Cd2+ (Cd2+ + Zn2+) | RHC | 0.008 | −0.71 | 0.902 | 0.70 | 3.41 | 0.981 | 0.09 | 9.85 | 0.955 | 0.04 | 11.06 | 0.773 |
| RHCNP | 0.009 | −0.69 | 0.958 | 0.77 | 4.03 | 0.969 | 0.12 | 10.72 | 0.964 | 0.05 | 12.17 | 0.960 | |
| RHC@FU | 0.008 | −0.69 | 0.960 | 0.72 | 3.78 | 0.961 | 0.11 | 10.19 | 0.941 | 0.05 | 11.53 | 0.968 | |
| RHC@AFU | 0.009 | −0.68 | 0.964 | 0.58 | 4.00 | 0.968 | 0.21 | 7.91 | 0.989 | 0.07 | 10.81 | 0.936 | |
| Zn2+ (Cd2+ + Zn2+) | RHC | 0.008 | −0.59 | 0.927 | 0.70 | 2.40 | 0.995 | 0.09 | 9.08 | 0.973 | 0.03 | 10.10 | 0.803 |
| RHCNP | 0.009 | −0.55 | 0.981 | 0.82 | 2.60 | 0.969 | 0.12 | 9.89 | 0.864 | 0.05 | 11.38 | 0.996 | |
| RHC@FU | 0.008 | −0.76 | 0.939 | 0.67 | 3.50 | 0.971 | 0.11 | 9.34 | 0.999 | 0.02 | 11.23 | 0.997 | |
| RHC@AFU | 0.009 | −0.73 | 0.954 | 0.51 | 3.78 | 0.977 | 0.19 | 7.40 | 0.976 | 0.06 | 10.15 | 0.980 | |
| Metal Ion | Adsorbent | Langmuir | Freundlich | Spis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax (mg g−1) | KL (L mg−1) | R2 | n | KF (mg g−1) | R2 | qmax (mg g−1) | n | KLF (L mg−1) | R2 | ||
| Cd2+ | RHC | 21.67 | 0.031 | 0.951 | 0.529 | 2.094 | 0.885 | 17.10 | 1.674 | 0.006 | 0.976 |
| RHCNP | 26.30 | 0.048 | 0.931 | 0.301 | 2.355 | 0.954 | 28.49 | 0.056 | 0.893 | 0.980 | |
| RHC@FU | 24.75 | 0.035 | 0.969 | 0.446 | 2.124 | 0.912 | 20.48 | 1.437 | 0.014 | 0.981 | |
| RHC@AFU | 23.98 | 0.035 | 0.972 | 0.419 | 1.136 | 0.922 | 20.52 | 0.019 | 1.318 | 0.980 | |
| Zn2+ | RHC | 18.71 | 0.049 | 0.989 | 0.359 | 2.619 | 0.949 | 18.17 | 0.053 | 0.957 | 0.989 |
| RHCNP | 24.86 | 0.068 | 0.979 | 0.231 | 2.737 | 0.873 | 21.60 | 0.029 | 1.479 | 0.999 | |
| RHC@FU | 20.06 | 0.047 | 0.993 | 0.362 | 2.512 | 0.963 | 18.53 | 0.034 | 1.170 | 0.995 | |
| RHC@AFU | 19.44 | 0.050 | 0.981 | 0.336 | 2.663 | 0.944 | 20.87 | 0.060 | 0.886 | 0.986 | |
| Cd2+ (Cd2+ + Zn2+) | RHC | 17.89 | 0.035 | 0.967 | 0.588 | 2.101 | 0.909 | 15.66 | 0.018 | 1.317 | 0.974 |
| RHCNP | 21.73 | 0.034 | 0.980 | 0.483 | 2.162 | 0.936 | 21.63 | 0.024 | 1.181 | 0.983 | |
| RHC@FU | 19.47 | 0.036 | 0.984 | 0.627 | 1.310 | 0.945 | 19.12 | 0.038 | 1.006 | 0.986 | |
| RHC@AFU | 19.17 | 0.033 | 0.971 | 0.579 | 2.095 | 0.911 | 18.80 | 0.018 | 1.217 | 0.993 | |
| Zn2+ (Cd2+ + Zn2+) | RHC | 15.04 | 0.047 | 0.964 | 0.475 | 2.539 | 0.925 | 15.23 | 0.049 | 0.978 | 0.964 |
| RHCNP | 19.10 | 0.036 | 0.966 | 0.448 | 2.384 | 0.966 | 18.82 | 0.035 | 1.022 | 0.994 | |
| RHC@FU | 17.44 | 0.034 | 0.973 | 0.622 | 2.104 | 0.956 | 18.52 | 0.038 | 0.038 | 0.974 | |
| RHC@AFU | 16.64 | 0.035 | 0.962 | 0.556 | 2.298 | 0.958 | 18.07 | 0.053 | 0.817 | 0.990 | |
| Adsorbents | Metal Ion | Initial Concentration (mg g−1) | Adsorbent Dose (g L−1) | Equilibrium Time (h) | qmax (mg g−1) | Reference |
|---|---|---|---|---|---|---|
| Agricultural wastes biochars | Cd2+ | 0–100 | 1 | - | 6.28 | [35] |
| KMnO4 and hematite modified biochar | Cd2+ | 0–100 | 2.5 | 2 | 34.25 | [36] |
| N-doping biochar | Cd2+ | 0–150 | 1 | 8 | 8.72 | [21] |
| RHCNP | Cd2+ | 0–100 | 0.5 | 1 | 26.30 | This work |
| Cement-biochar composite | Zn2+ | 10 | 0.5 | 4 | 19.0 | [6] |
| KOH modified biochar | Zn2+ | 0–100 | 3 | 8 | 97.68 | [37] |
| KMnO4 and hematite modified biochar | Zn2+ | 0–100 | 2.5 | 2 | 17.92 | [36] |
| RHCNP | Zn2+ | 0–100 | 0.5 | 1 | 24.86 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wei, X.; Kong, H.; Zheng, X.; Guo, H. Hydrothermal Ammonia Carbonization of Rice Straw for Hydrochar to Separate Cd(II) and Zn(II) Ions from Aqueous Solution. Polymers 2023, 15, 4548. https://doi.org/10.3390/polym15234548
Wang J, Wei X, Kong H, Zheng X, Guo H. Hydrothermal Ammonia Carbonization of Rice Straw for Hydrochar to Separate Cd(II) and Zn(II) Ions from Aqueous Solution. Polymers. 2023; 15(23):4548. https://doi.org/10.3390/polym15234548
Chicago/Turabian StyleWang, Jiarui, Xiaocheng Wei, Hao Kong, Xiangqun Zheng, and Haixin Guo. 2023. "Hydrothermal Ammonia Carbonization of Rice Straw for Hydrochar to Separate Cd(II) and Zn(II) Ions from Aqueous Solution" Polymers 15, no. 23: 4548. https://doi.org/10.3390/polym15234548
APA StyleWang, J., Wei, X., Kong, H., Zheng, X., & Guo, H. (2023). Hydrothermal Ammonia Carbonization of Rice Straw for Hydrochar to Separate Cd(II) and Zn(II) Ions from Aqueous Solution. Polymers, 15(23), 4548. https://doi.org/10.3390/polym15234548







