Epoxy-Encapsulated ZnO–MWCNT Hybrid Nanocomposites with Enhanced Thermoelectric Performance for Low-Grade Heat-to-Power Conversion
Abstract
1. Introduction and background
2. Materials and Methods
2.1. Synthesis of Metallic Zn Nanostructured Networks
2.2. Synthesis and Spray-Coating of MWCNTs
2.3. Oxidation of Zn–MWCNT Nanostructured Networks
2.4. Encapsulation of ZnO Nanowire Network with Epoxy Adhesive
2.5. Morphological and Structural Characterization
2.6. Electrical and Thermoelectric Characterization
2.7. Bending Tests
2.8. Assembly of the Two-Leg Thermoelectric Generator
3. Results and Discussion
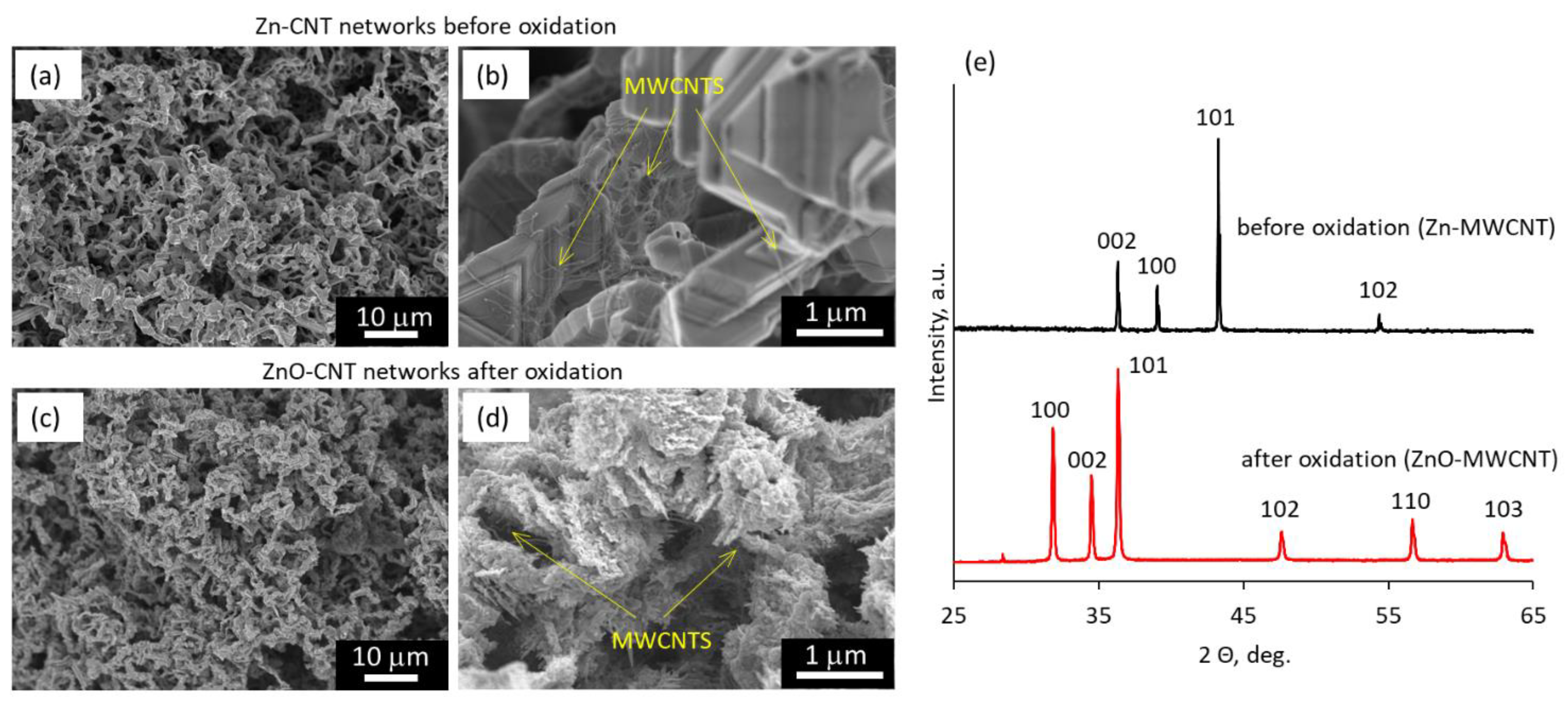
3.1. Fabrication and Morphology of ZnO–MWCNT Hybrid Networks
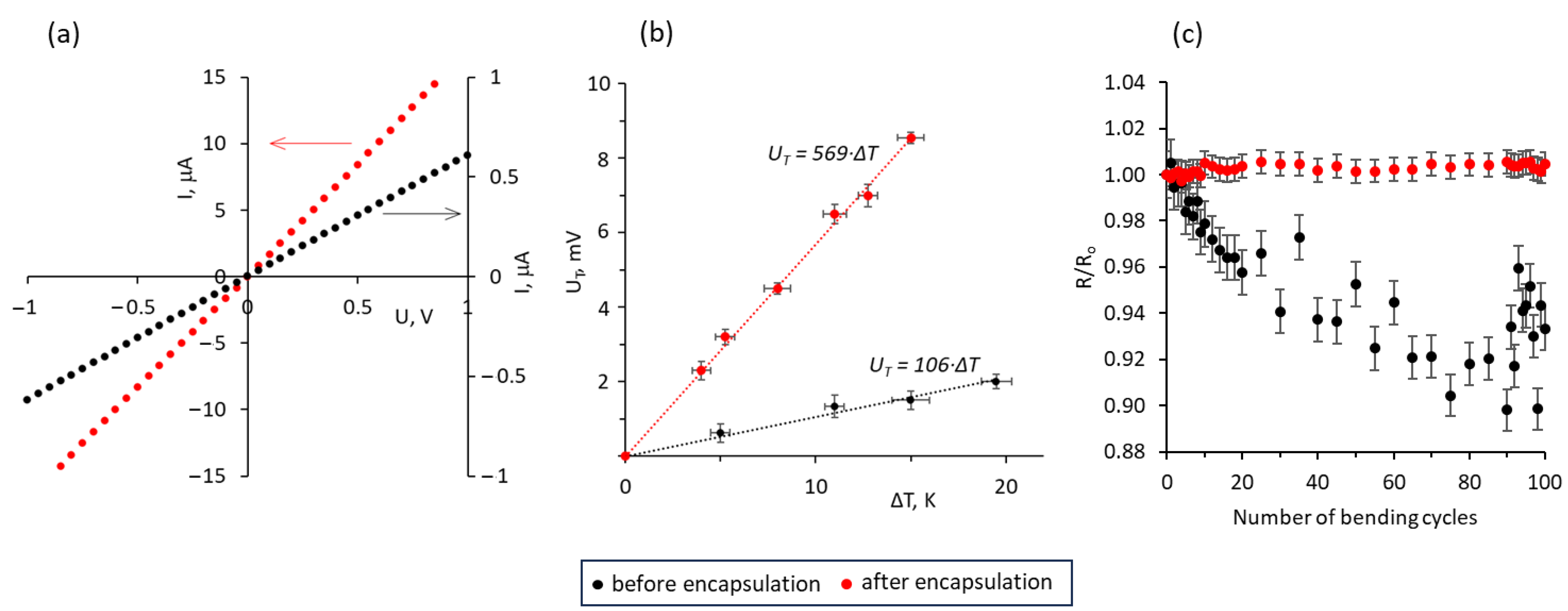
3.2. Electrical and Thermoelectric Properties of ZnO–MWCNTs Hybrid Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Reports Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doǧan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Jeong, M.C.; Oh, B.Y.; Ham, M.H.; Myoung, J.M. Electroluminescence from ZnO nanowires in n-ZnO film/ZnO nanowire array/ p-GaN film heterojunction light-emitting diodes. Appl. Phys. Lett. 2006, 88, 202105. [Google Scholar] [CrossRef]
- Heo, Y.W.; Kang, B.S.; Tien, L.C.; Norton, D.P.; Ren, F.; Roche, J.R.L.A.; Pearton, S.J. UV photoresponse of single ZnO nanowires. Appl. Phys. A Mater. Sci. Process. 2005, 80, 497–499. [Google Scholar] [CrossRef]
- Viter, R.; Kunene, K.; Genys, P.; Jevdokimovs, D.; Erts, D.; Sutka, A.; Bisetty, K.; Viksna, A.; Ramanaviciene, A.; Ramanavicius, A. Photoelectrochemical Bisphenol S Sensor Based on ZnO-Nanoroads Modified by Molecularly Imprinted Polypyrrole. Macromol. Chem. Phys. 2020, 221, 1900232. [Google Scholar] [CrossRef]
- Hsueh, T.J.; Chang, S.J.; Hsu, C.L.; Lin, Y.R.; Chen, I.C. Highly sensitive ZnO nanowire ethanol sensor with Pd adsorption. Appl. Phys. Lett. 2007, 91, 53109–53112. [Google Scholar] [CrossRef]
- Kumar, N.; Dorfman, A.; Hahm, J.I. Ultrasensitive DNA sequence detection using nanoscale ZnO sensor arrays. Nanotechnology 2006, 17, 2875–2881. [Google Scholar] [CrossRef]
- Peh, C.K.N.; Ke, L.; Ho, G.W. Modification of ZnO nanorods through Au nanoparticles surface coating for dye-sensitized solar cells applications. Mater. Lett. 2010, 64, 1372–1375. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4–11. [Google Scholar] [CrossRef]
- Heo, Y.W.; Tien, L.C.; Kwon, Y.; Norton, D.P.; Pearton, S.J.; Kang, B.S.; Ren, F. Depletion-mode ZnO nanowire field-effect transistor. Appl. Phys. Lett. 2004, 85, 2274–2276. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, H.; Hu, L.; Yang, D.; Wang, L.; Wang, B.; Ji, J.; Liu, G.; Liu, X.; Lin, J.; et al. Flexible piezoelectric nanogenerators based on ZnO nanorods grown on common paper substrates. Nanoscale 2012, 4, 6568–6573. [Google Scholar] [CrossRef]
- Zappa, D.; Dalola, S.; Faglia, G.; Comini, E.; Ferroni, M.; Soldano, C.; Ferrari, V.; Sberveglieri, G. Integration of ZnO and CuO nanowires into a thermoelectric module. Beilstein J. Nanotechnol. 2014, 5, 927–936. [Google Scholar] [CrossRef]
- Araneo, R.; Celozzi, S.; Bini, F.; Notargiacomo, A.; Pea, M.; Rinaldi, A. Thermoelectric characterization of piezoelectric ZnO nanowires. In Proceedings of the IEEE-NANO 2015—International Conference on Nanotechnology, Rome, Italy, 27–30 July 2015; pp. 1142–1146. [Google Scholar] [CrossRef]
- Witkowski, B.S. Applications of ZnO nanorods and nanowires—A review. Acta Phys. Pol. A 2018, 134, 1226–1246. [Google Scholar] [CrossRef]
- Bagga, S.; Akhtar, J.; Mishra, S. Synthesis and applications of ZnO nanowire: A review. AIP Conf. Proc. 2018, 1989, 020004. [Google Scholar] [CrossRef]
- Ren, S.; Bai, Y.F.; Chen, J.; Deng, S.Z.; Xu, N.S.; Wu, Q.B.; Yang, S. Catalyst-free synthesis of ZnO nanowire arrays on zinc substrate by low temperature thermal oxidation. Mater. Lett. 2007, 61, 666–670. [Google Scholar] [CrossRef]
- Campos, A.C.; Paes, S.C.; Correa, B.S.; Cabrera-Pasca, G.A.; Costa, M.S.; Costa, C.S.; Otubo, L.; Carbonari, A.W. Growth of Long ZnO Nanowires with High Density on the ZnO Surface for Gas Sensors. ACS Appl. Nano Mater. 2020, 3, 175–185. [Google Scholar] [CrossRef]
- Florica, C.; Preda, N.; Costas, A.; Zgura, I.; Enculescu, I. ZnO nanowires grown directly on zinc foils by thermal oxidation in air: Wetting and water adhesion properties. Mater. Lett. 2016, 170, 156–159. [Google Scholar] [CrossRef]
- Yu, W.; Pan, C. Low temperature thermal oxidation synthesis of ZnO nanoneedles and the growth mechanism. Mater. Chem. Phys. 2009, 115, 74–79. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Q.; Gao, J.; Zhu, R.; Wang, X.; Xu, J.; Chen, L.; Zhang, J.; Yu, D. Growth mechanism study viain situ epitaxial growth of high-oriented ZnO nanowires. CrystEngComm 2011, 13, 606–610. [Google Scholar] [CrossRef]
- Chao, L.C.; Tsai, S.Y.; Lin, C.N.; Liau, C.C.; Ye, C.C. Vertically aligned Zno nanowires prepared by thermal oxidation of RF magnetron sputtered metallic zinc films. Mater. Sci. Semicond. Process. 2013, 16, 1316–1320. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Su, X.; Zhao, Y. From zinc nanowires to zinc oxide nanowires: A low substrate-temperature approach. J. Phys. D. Appl. Phys. 2005, 38, 1068–1071. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Gundiah, G.; Govindaraj, A.; Rao, C.N.R. A new method for the preparation of metal nanowires by the nebulized spray pyrolysis of precursors. Adv. Mater. 2004, 16, 1842–1845. [Google Scholar] [CrossRef]
- Schroeder, P.; Kast, M.; Halwax, E.; Edtmaier, C.; Bethge, O.; Brückl, H. Morphology alterations during postsynthesis oxidation of Zn nanowires. J. Appl. Phys. 2009, 105, 104307. [Google Scholar] [CrossRef]
- Volkova, M.; Sondors, R.; Bugovecka, L.; Kons, A.; Andzane, J. Enhanced thermoelectric properties of self-assembling ZnO nanowire networks encapsulated in nonconductive polymers. Sci. Rep. 2023, submitted.
- Koumoto, K.; Wang, Y.; Zhang, R.; Kosuga, A.; Funahashi, R. Oxide thermoelectric materials: A nanostructuring approach. Annu. Rev. Mater. Res. 2010, 40, 363–394. [Google Scholar] [CrossRef]
- Ji, L. Metal Oxide-Based Thermoelectric Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128111673. [Google Scholar]
- Radingoana, P.M.; Guillemet-Fritsch, S.; Noudem, J.; Olubambi, P.A.; Chevallier, G.; Estournès, C. Thermoelectric properties of ZnO ceramics densified through spark plasma sintering. Ceram. Int. 2020, 46, 5229–5238. [Google Scholar] [CrossRef]
- Sulaiman, S.; Sudin, I.; Al-Naib, U.M.B.; Omar, M.F. Review of the Nanostructuring and Doping Strategies for High-Performance ZnO Thermoelectric Materials. Crystals 2022, 12, 1076. [Google Scholar] [CrossRef]
- Inoue, Y.; Okamoto, M.; Kawahara, T.; Okamoto, Y.; Morimoto, J. Thermoelectric properties of amorphous zinc oxide thin films fabricated by pulsed laser deposition. Mater. Trans. 2005, 46, 1470–1475. [Google Scholar] [CrossRef][Green Version]
- Virtudazo, R.V.R.; Srinivasan, B.; Guo, Q.; Wu, R.; Takei, T.; Shimasaki, Y.; Wada, H.; Kuroda, K.; Bernik, S.; Mori, T. Improvement in the thermoelectric properties of porous networked Al-doped ZnO nanostructured materials synthesized: Via an alternative interfacial reaction and low-pressure SPS processing. Inorg. Chem. Front. 2020, 7, 4118–4132. [Google Scholar] [CrossRef]
- Lupan, O.; Schütt, F.; Postica, V.; Smazna, D.; Mishra, Y.K.; Adelung, R. Sensing performances of pure and hybridized carbon nanotubes-ZnO nanowire networks: A detailed study. Sci. Rep. 2017, 7, 14715. [Google Scholar] [CrossRef]
- Saravanakkumar, D.; Oualid, H.A.; Brahmi, Y.; Ayeshamariam, A.; Karunanaithy, M.; Saleem, A.M.; Kaviyarasu, K.; Sivaranjani, S.; Jayachandran, M. Synthesis and characterization of CuO/ZnO/CNTs thin films on copper substrate and its photocatalytic applications. OpenNano 2019, 4, 100025. [Google Scholar] [CrossRef]
- Taborowska, P.; Wasiak, T.; Sahlman, M.; Lundström, M.; Janas, D. Carbon Nanotube-Based Thermoelectric Modules Enhanced by ZnO Nanowires. Materials 2022, 15, 1924. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Sun, T.; Jiang, M.; Gu, S.; Wang, L.; Yan, H.; Jiang, W. In-situ growth of carbon nanotubes on ZnO to enhance thermoelectric and mechanical properties. J. Adv. Ceram. 2022, 11, 1932–1943. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Lan, M.; Zhu, M.; Mori, T.; Wang, Q. Improvement of Thermoelectric Properties of Evaporated ZnO:Al Films by CNT and Au Nanocomposites. J. Phys. Chem. C 2020, 124, 12713–12722. [Google Scholar] [CrossRef]
- Cui, J.; Sun, S.; Lan, M.; Liu, S.; Piao, Y.; Li, G.; Wang, Q. Effect of low-dimensional carbon composite on the thermoelectric properties of vacuum evaporated ZnO: Al films. Thin Solid Films 2023, 766, 139641. [Google Scholar] [CrossRef]
- Dreßler, C.; Löhnert, R.; Gonzalez-Julian, J.; Guillon, O.; Töpfer, J.; Teichert, S. Effect of Carbon Nanotubes on Thermoelectric Properties in Zn0.98Al0.02O. J. Electron. Mater. 2016, 45, 1459–1463. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hussain, S.T.; Ali, S.; Ahmad, N.; Ali, N.; Abbas, S. Structure and electrochemical performance of ZnO/CNT composite as anode material for lithium-ion batteries. J. Mater. Sci. 2013, 48, 5429–5436. [Google Scholar] [CrossRef]
- Klochko, N.P.; Klepikova, K.S.; Khrypunova, I.V.; Zhadan, D.O.; Petrushenko, S.I.; Kopach, V.R.; Dukarov, S.V.; Sukhov, V.M.; Kirichenko, M.V.; Khrypunova, A.L. Flexible thermoelectric module based on zinc oxide thin film grown via SILAR. Curr. Appl. Phys. 2021, 21, 121–133. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- Baghdadi, Y.N.; Youssef, L.; Bouhadir, K.; Harb, M.; Mustapha, S.; Patra, D.; Tehrani-Bagha, A.R. The effects of modified zinc oxide nanoparticles on the mechanical/thermal properties of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49330. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, M. Influence of filler loading on dielectric properties of epoxy-Zno nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 531–542. [Google Scholar] [CrossRef]
- Andzane, J.; Katkov, M.V.; Buks, K.; Sarakovskis, A.; Smits, K.; Erts, D. Synthesis, magnetoresistance, and thermoelectrical properties of environmentally stable n-type nitrogen-doped multiwalled carbon nanotubes. Carbon Trends 2023, 13, 100302. [Google Scholar] [CrossRef]
- Buks, K.; Andzane, J.; Bugovecka, L.; Katkov, M.V.; Smits, K.; Starkova, O.; Katkevics, J.; Bērziņš, A.; Brauna, L.; Voikiva, V.; et al. Highly Efficient Flexible n-Type Thermoelectric Films Formed by Encapsulation of Bi2Se3-MWCNT Hybrid Networks in Polyvinyl Alcohol. Adv. Mater. Interfaces 2022, 9, 2200318. [Google Scholar] [CrossRef]
- Tsagaropoulos, G.; Eisenberg, A. Dynamic Mechanical Study of the Factors Affecting the Two Glass Transition Behavior of Filled Polymers. Similarities and Differences with Random Ionomers. Macromolecules 1995, 28, 6067–6077. [Google Scholar] [CrossRef]
- Zhang, C.; Mason, R.; Stevens, G. Preparation, characterization and dielectric properties of epoxy and polyethylene nanocomposites. IEEJ Trans. Fundam. Mater. 2006, 126, 1105–1111. [Google Scholar] [CrossRef]
- Pełech, I.; Pełech, R.; Narkiewicz, U.; Kaczmarek, A.; Guskos, N.; Żołnierkiewicz, G.; Typek, J.; Berczyński, P. Magnetic and electrical properties of carbon nanotube/epoxy composites. Mater. Sci. Eng. B 2020, 254, 114507. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Charge Transfer Investigation in the Nanocomposite of ZnO Nanoparticles Modified by Multiwalled Carbon Nanotube. J. Inorg. Organomet. Polym. Mater. 2023. [Google Scholar] [CrossRef]
- Huang, C.; Zhen, W.; Huang, Z.; Luo, D. Thermal and electrical conductivities of epoxy resin-based composites incorporated with carbon nanotubes and TiO2 for a thermoelectric application. Appl. Phys. A Mater. Sci. Process. 2018, 124, 38. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Sondors, R.; Gavars, D.; Spalva, E.; Kons, A.; Volkova, M.; Meija, R.; Andzane, J. Synthesis and enhanced room-temperature thermoelectric properties of CuO–MWCNT hybrid nanostructured composites. Nanoscale Adv. 2023. submitted. [Google Scholar]
- Andzane, J.; Buks, K.; Bitenieks, J.; Bugovecka, L.; Kons, A.; Merijs-meri, R.; Svirksts, J.; Zicans, J.; Erts, D. p-Type PVA/MWCNT-Sb2Te3 Composites for Application in Different Types of Flexible Thermoelectric Generators in Combination with n-Type PVA/MWCNT-Bi2Se3 Composites. Polymers 2022, 14, 5130. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.L.; Cruz, I.F.; Silva, J.; Oliveira, G.N.P.; Ferreira-Teixeira, S.; Lopes, A.M.L.; Araújo, J.P.; Fonseca, J.; Pereira, C.; Pereira, A.M. Printed Flexible μ-Thermoelectric Device Based on Hybrid Bi2Te3/PVA Composites. ACS Appl. Mater. Interfaces 2019, 11, 8969–8981. [Google Scholar] [CrossRef]
- Choi, J.H.; Hyun, C.M.; Jo, H.; Son, J.H.; Lee, J.E.; Ahn, J.H. Thermoelectric elastomer fabricated using carbon nanotubes and nonconducting polymer. Jpn. J. Appl. Phys. 2017, 56, 8–11. [Google Scholar] [CrossRef]
- Francioso, L.; De Pascali, C.; Farella, I.; Martucci, C.; Cret, P.; Siciliano, P.; Perrone, A. Flexible thermoelectric generator for ambient assisted living wearable biometric sensors. J. Power Sources 2011, 196, 3239–3243. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Mei, D.; Shi, Y.; Chen, Z. Design of a wearable thermoelectric generator for harvesting human body energy. Lect. Notes Electr. Eng. 2017, 399, 55–66. [Google Scholar] [CrossRef]
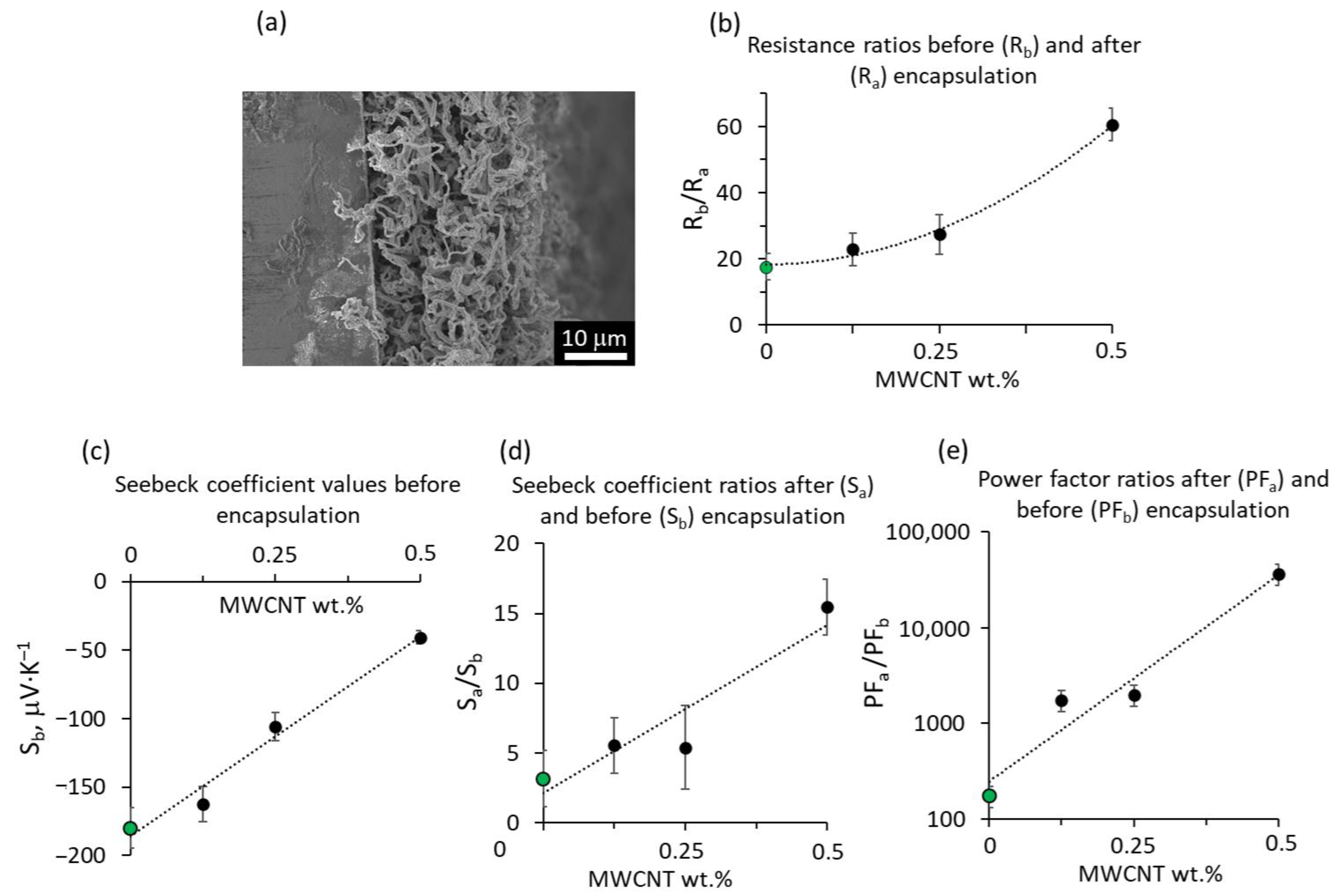

| MWCNT wt.% in the ZnO-MWCNT Hybrid Network | Resistance before Encapsulation, Rb, ×106 Ω | Resistance after Encapsulation, Ra, ×106 Ω | Rb/Ra |
|---|---|---|---|
| 0 | 0.8 ± 0.02 | 0.044 ± 0.002 | 18 |
| 0.125 | 8.4 ± 0.2 | 0.368 ± 0.003 | 23 |
| 0.25 | 1.63 ± 0.03 | 0.059 ± 0.002 | 28 |
| 0.5 | 3.27 ± 0.03 | 0.054 ± 0.001 | 60 |
| Nanostructured Network | MWCNT wt.% | Seebeck Coefficient before Encapsulation, Sb, μV·K–1 | Seebeck Coefficient after Encapsulation, Sa μV·K–1 |
|---|---|---|---|
| ZnO, this work | 0 | −180 ± 15 | −570 ± 50 |
| ZnO–MWCNT, this work | 0.125 | −160 ± 10 | −900 ± 100 |
| ZnO–MWCNT, this work | 0.25 | −105 ± 10 | −570 ± 40 |
| ZnO–MWCNT, this work | 0.5 | −40 ± 5 | −625 ± 55 |
| ZnO [12] | 0 | −150 ± 40 | - |
| Ni-CNTs/ZnO [35] | 2.0 | −260 ± 10 | - |
| SWCNTs with 15 wt.% ZnO nanowires [34] | 85 | −24 | - |
| ZnO: Al films deposited on MWCNT substrates [36,37] | 0.1 g of MWCNTs | −130; −145 | - |
| Zn0.98Al0.02O mixed with MWCNTs [38] | 0.1 | −80 | - |
| Epoxy–MWCNT-TiO2 [50] | 6–8 | - | −15–25 |
| Type of the Thermoelectric Generator | CNT wt.% | U0, mV·K–1 | Pd, nW·cm–2·K–1 |
|---|---|---|---|
| Epoxy-encapsulated ZnO–MWCNT-PDMS-encapsulated CuO–MWCNT (with 0.9 wt.% MWCNT), this work | 0.5 | 0.13 | 7.5 |
| SWCNT-ZnO (n- and p-doped) [34] | 85 | 0.03 | - |
| ZnO thin films grown on polyimide substrates [40] | - | 0.07 | 6 |
| Mixed in PVA MWCNT-Sb2Te3 and MWCNT-Bi2Se3 [53] | 25–30 | 0.07–0.14 | 0.06–0.13 |
| One-type legs PVA/Bi2Te3 [54] | - | 0.1 | 0.02 |
| One-type legs MWCNT/PDMS [55] | - | 0.002 | - |
| Sb2Te3-Bi2Te3 thin films sputtered on the flexible substrate [56] | - | 0.1 | 0.04 |
| Bi2Te3-based printed wearable TEG [57] | - | 4 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, M.; Sondors, R.; Spalva, E.; Bugovecka, L.; Kons, A.; Meija, R.; Andzane, J. Epoxy-Encapsulated ZnO–MWCNT Hybrid Nanocomposites with Enhanced Thermoelectric Performance for Low-Grade Heat-to-Power Conversion. Polymers 2023, 15, 4540. https://doi.org/10.3390/polym15234540
Volkova M, Sondors R, Spalva E, Bugovecka L, Kons A, Meija R, Andzane J. Epoxy-Encapsulated ZnO–MWCNT Hybrid Nanocomposites with Enhanced Thermoelectric Performance for Low-Grade Heat-to-Power Conversion. Polymers. 2023; 15(23):4540. https://doi.org/10.3390/polym15234540
Chicago/Turabian StyleVolkova, Margarita, Raitis Sondors, Elmars Spalva, Lasma Bugovecka, Artis Kons, Raimonds Meija, and Jana Andzane. 2023. "Epoxy-Encapsulated ZnO–MWCNT Hybrid Nanocomposites with Enhanced Thermoelectric Performance for Low-Grade Heat-to-Power Conversion" Polymers 15, no. 23: 4540. https://doi.org/10.3390/polym15234540
APA StyleVolkova, M., Sondors, R., Spalva, E., Bugovecka, L., Kons, A., Meija, R., & Andzane, J. (2023). Epoxy-Encapsulated ZnO–MWCNT Hybrid Nanocomposites with Enhanced Thermoelectric Performance for Low-Grade Heat-to-Power Conversion. Polymers, 15(23), 4540. https://doi.org/10.3390/polym15234540





