Oligohexamethylene Guanidine Derivative as a Means to Prevent Biological Fouling of a Polymer-Based Composite Optical Oxygen Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
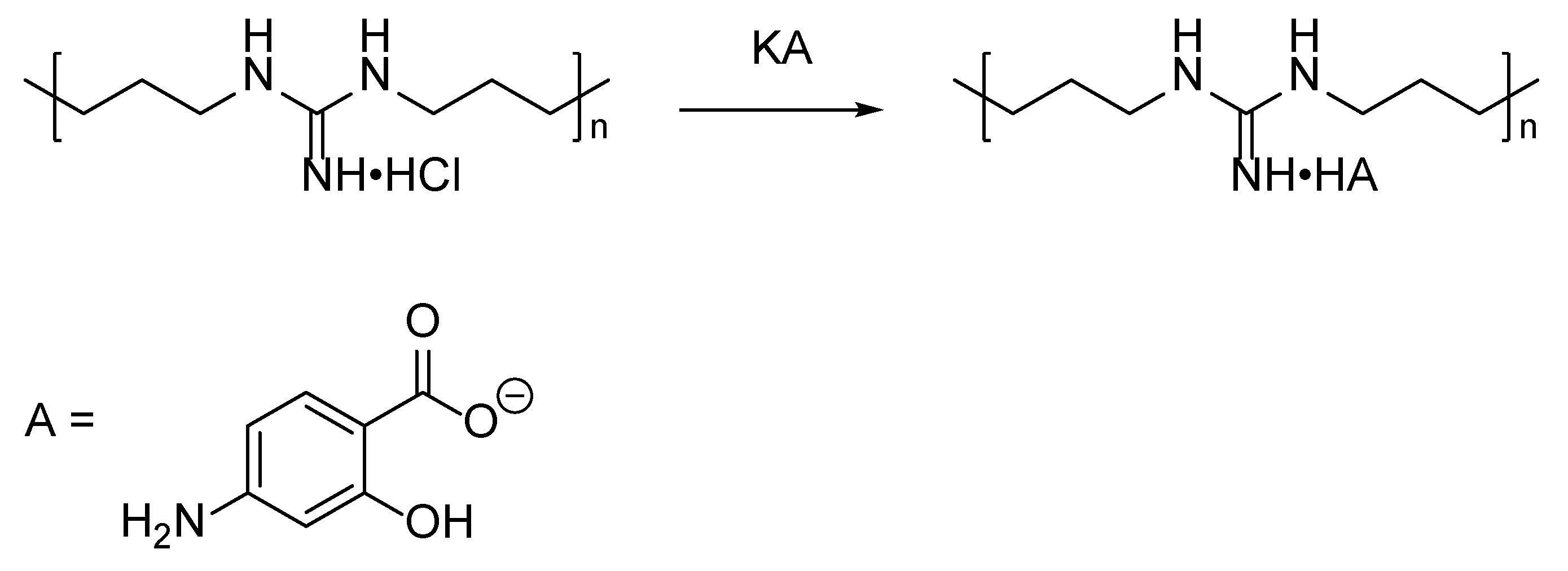
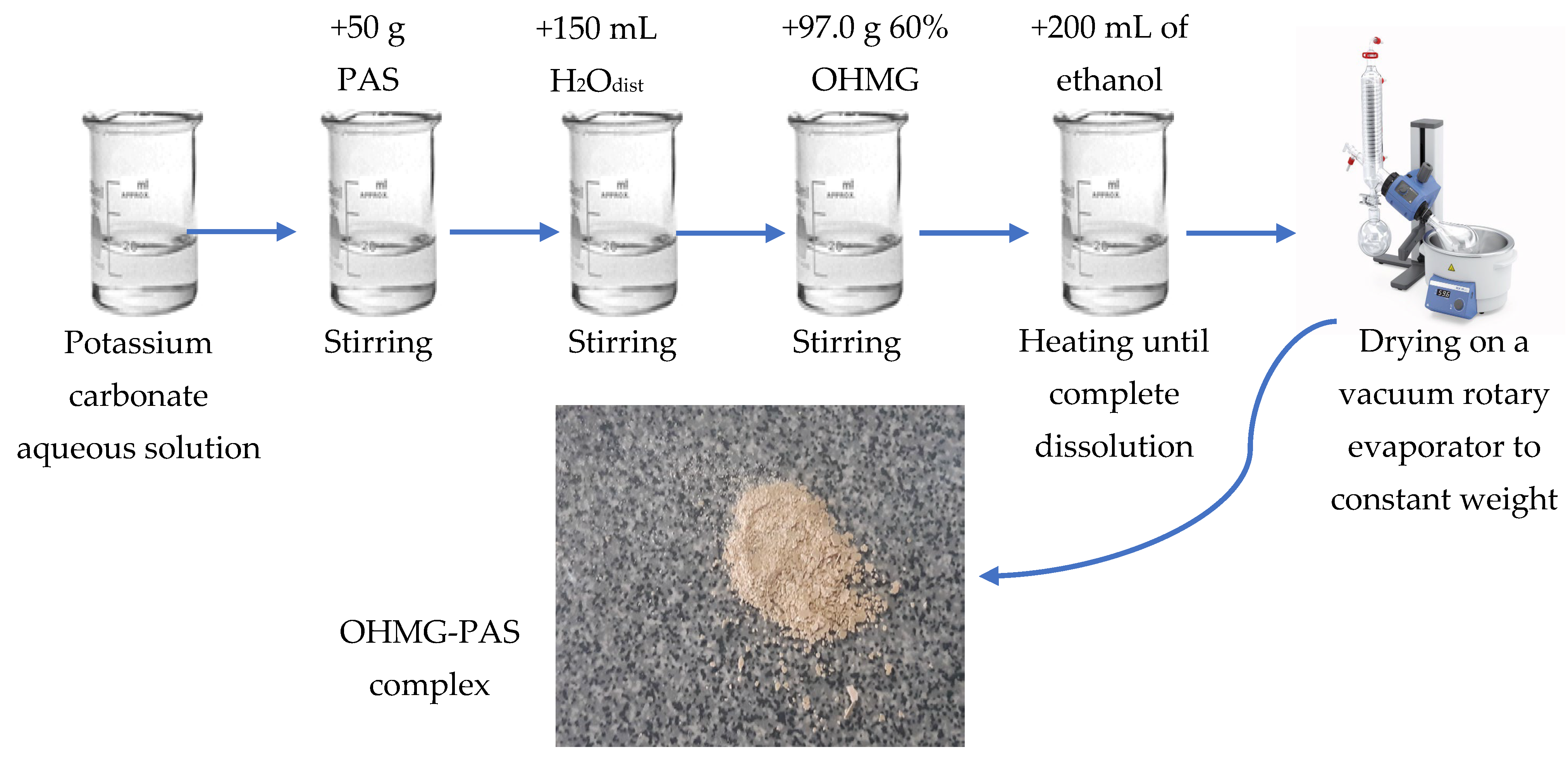
2.2. Synthesis of a Complex of Branched Oligohexamethyleneguanidine and Para-Aminosalicylic Acid
2.3. OHMG-PAS Complex Characterization
2.3.1. NMR Spectroscopy
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Determination of Minimum Inhibitory Concentration (MIC)
2.4. Sensor Fabrication
2.5. Sensor Characterization
2.5.1. Characterization of the Sensor Performance
2.5.2. Determination of the Antimicrobial Activity
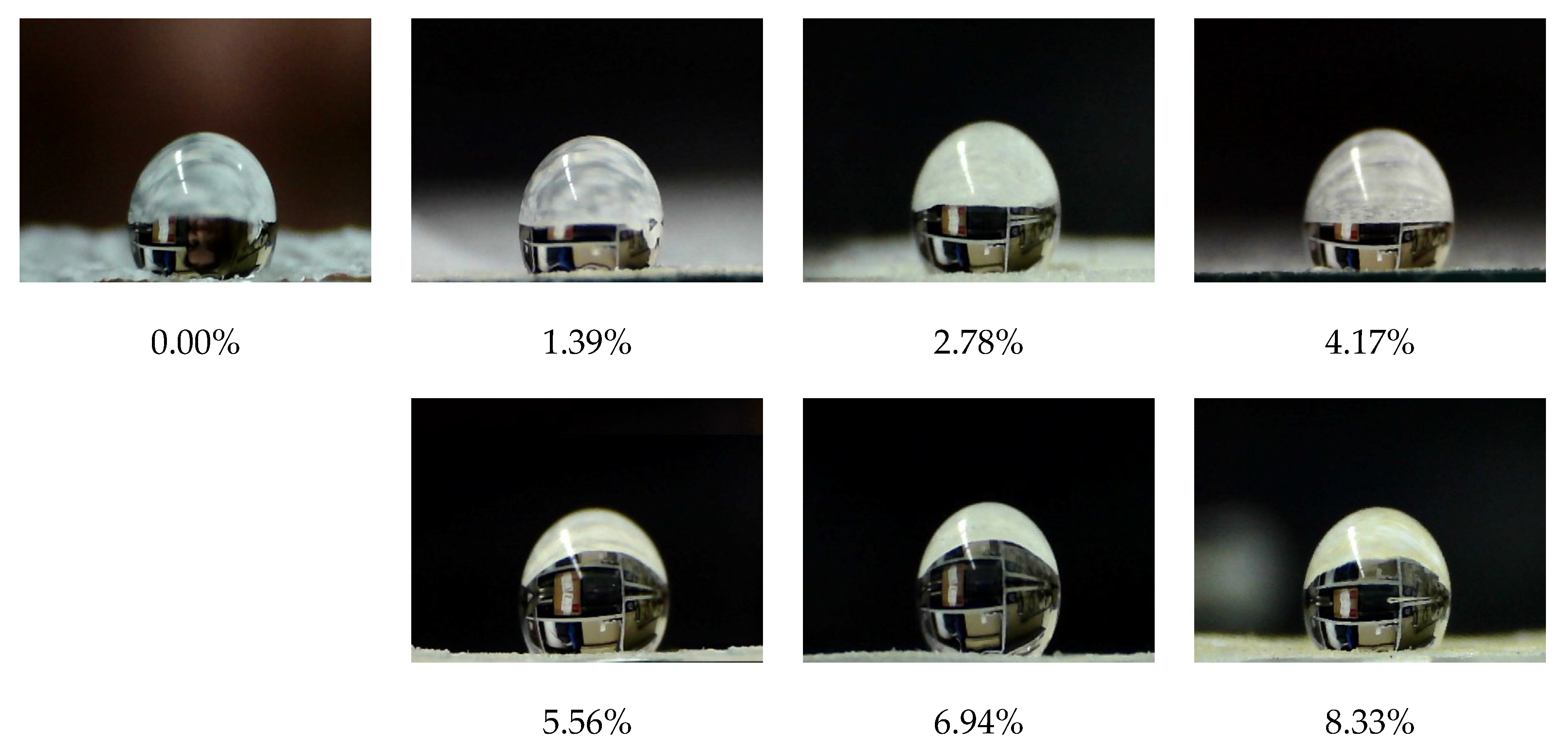
2.5.3. Water Contact Angle and Surface Free Energy
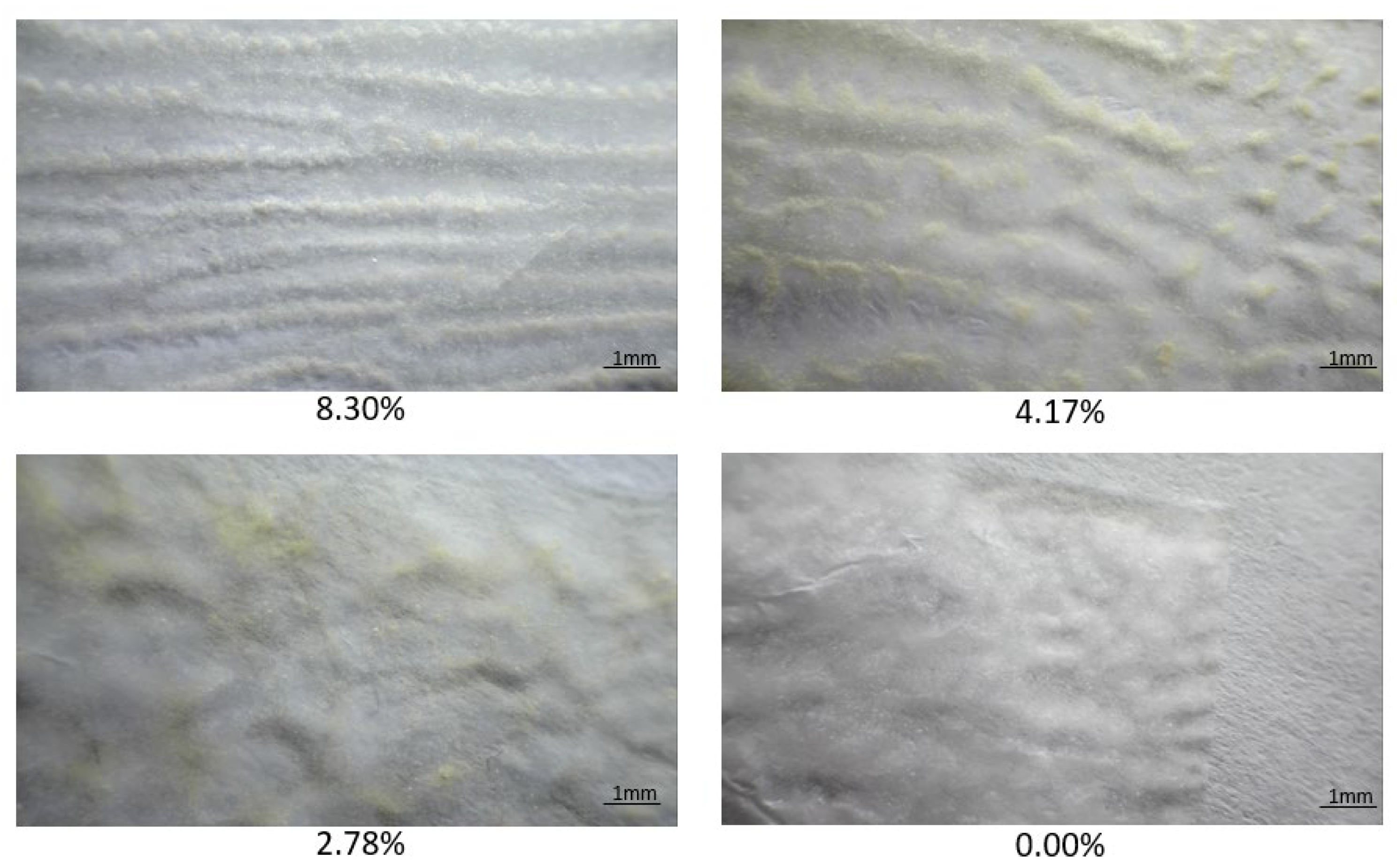
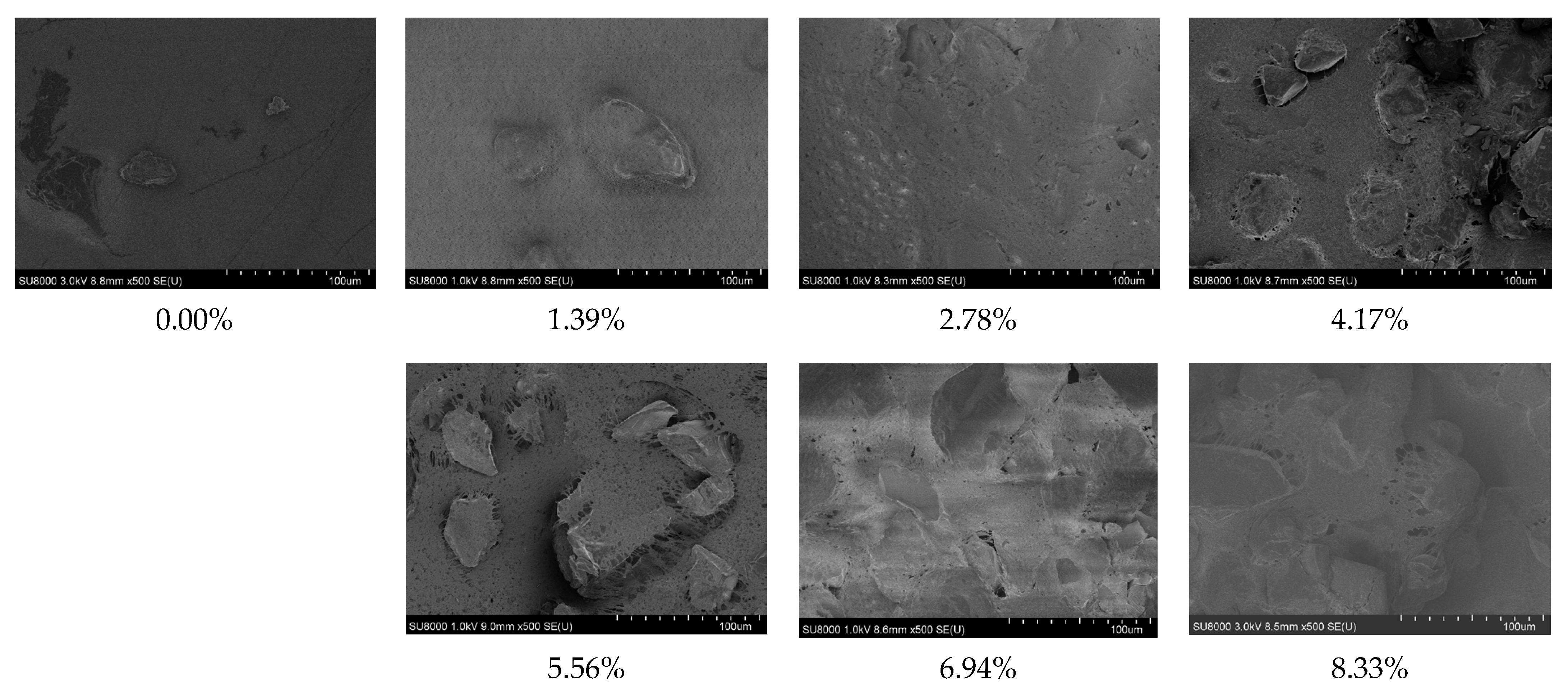
2.5.4. The Morphology of the Surface
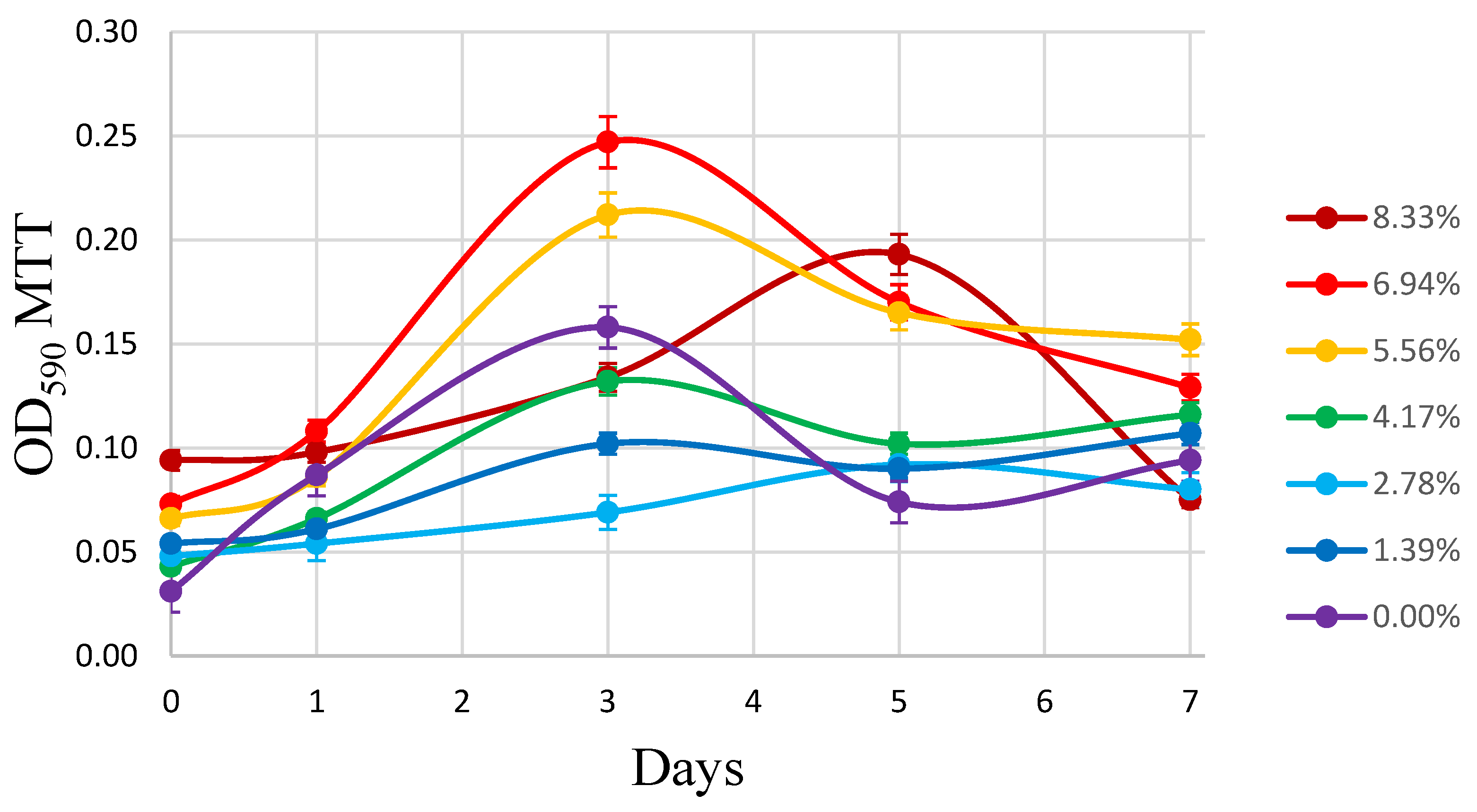
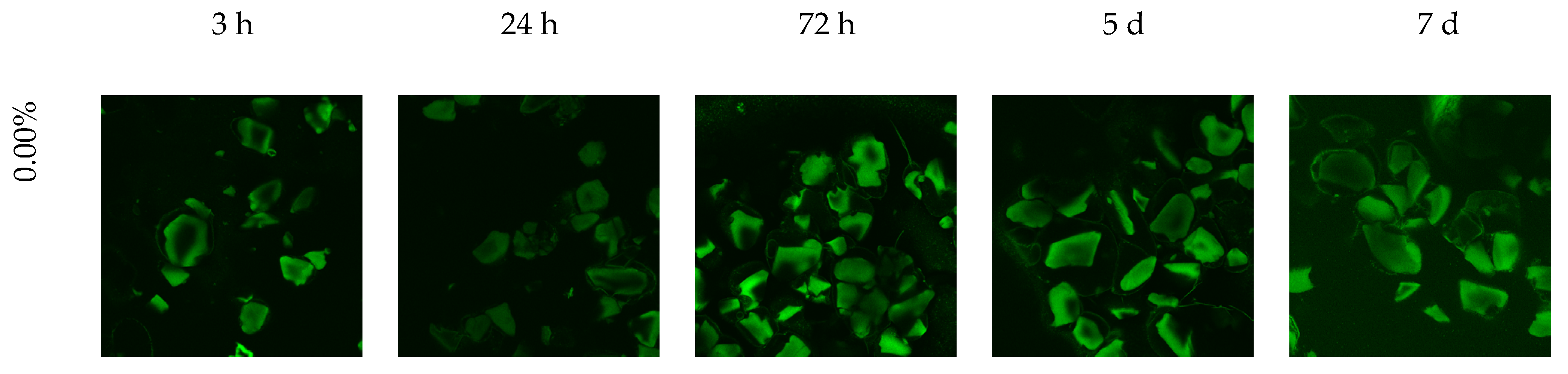
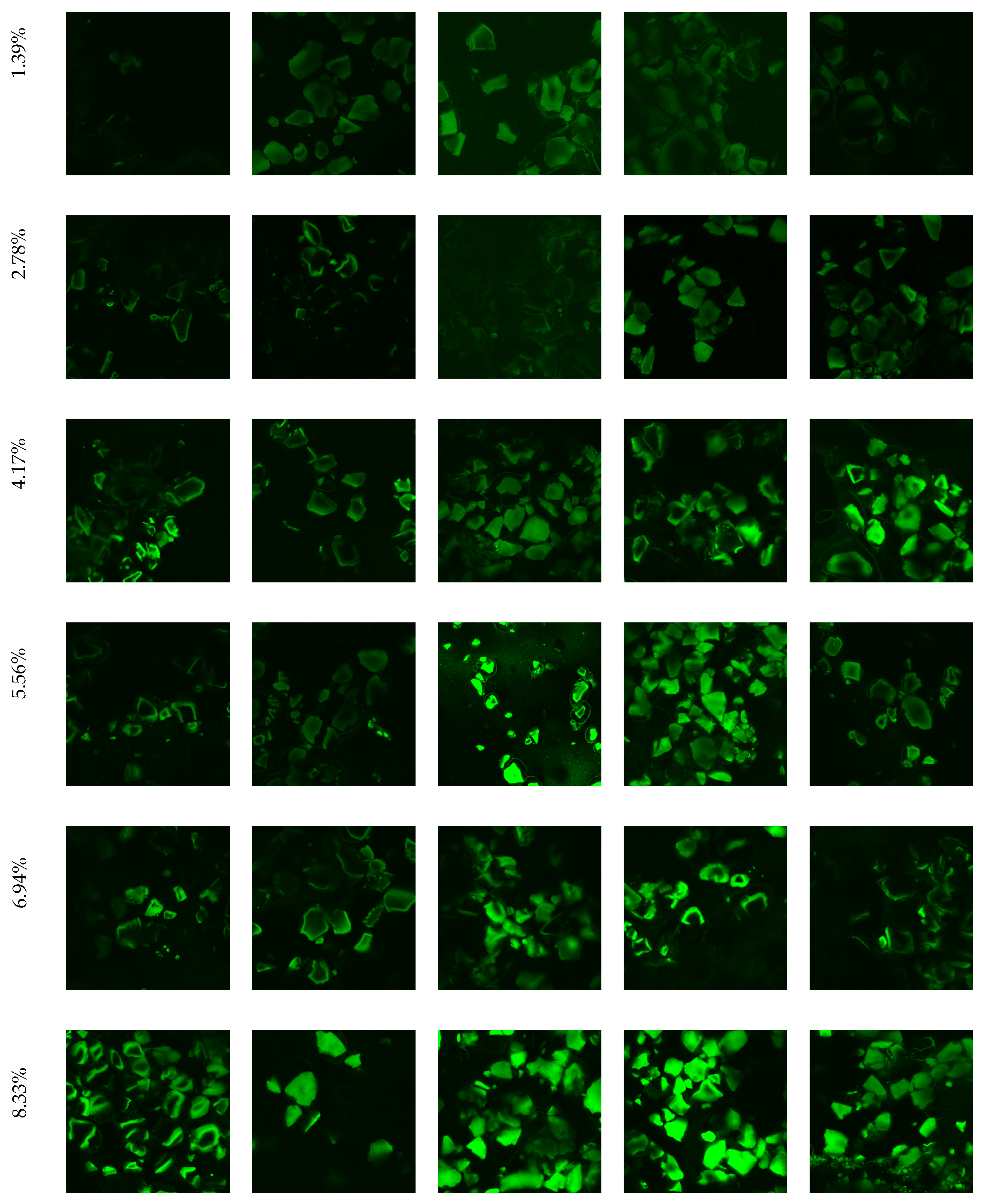
2.5.5. Evaluation of Biofilm Formation
3. Results and Discussion
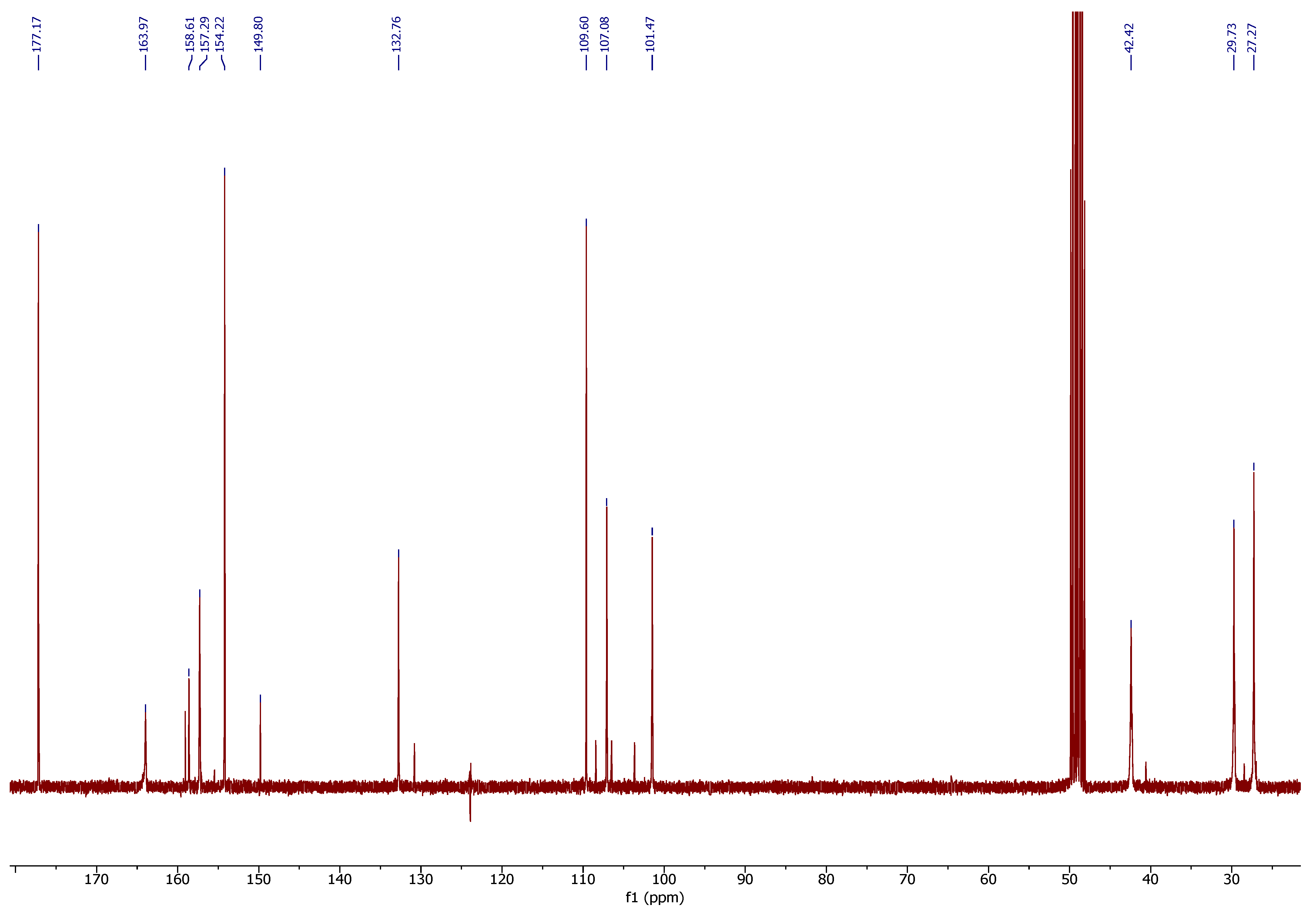
3.1. OHMG-PAS Complex Characterization
3.2. Sensor Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.W.; Islam Bhat, S.; Al Qahtani, H.S.; Aamir, M.; Amin, M.N.; Farhan, M.; Aldabal, S.; Khan, M.S.; Jeelani, I.; Nawaz, A.; et al. Recent Progress, Challenges, and Trends in Polymer-Based Sensors: A Review. Polymers 2022, 14, 2164. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.; Bobrov, A.; Marfin, Y. On the Use of Polymer-Based Composites for the Creation of Optical Sensors: A Review. Polymers 2022, 14, 4448. [Google Scholar] [CrossRef] [PubMed]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent progress in biosensors for environmental monitoring: A review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.A.; Fedorov, Y.V.; Polyakova, A.S.; Fedorova, O.A. Fluorimetric detection of Ag+ cations in aqueous solutions using a polyvinyl chloride sensor film doped with crown-containing 1,8-naphthalimide. Mendeleev Commun. 2021, 31, 517–519. [Google Scholar] [CrossRef]
- Gorbatov, S.A.; Kozlov, M.A.; Zlobin, I.E.; Kartashov, A.V.; Zavarzin, I.V.; Volkova, Y.A. Highly selective BODIPY-based fluorescent probe for Zn2+ imaging in plant roots. Mendeleev Commun. 2018, 28, 615–617. [Google Scholar] [CrossRef]
- Gavrilenko, N.A.; Volgina, T.N.; Gavrilenko, M.A. Colorimetric sensor for determination of thiocyanate in fossil and drill waters. Mendeleev Commun. 2017, 27, 529–530. [Google Scholar] [CrossRef]
- Fritzsche, E.; Staudinger, C.; Fischer, J.P.; Thar, R.; Jannasch, H.W.; Plant, J.N.; Blum, M.; Massion, G.; Thomas, H.; Hoech, J.; et al. A validation and comparison study of new, compact, versatile optodes for oxygen, pH and carbon dioxide in marine environments. Mar. Chem. 2018, 207, 63–76. [Google Scholar] [CrossRef]
- Staudinger, C.; Strobl, M.; Breininger, J.; Klimant, I.; Borisov, S.M. Fast and stable optical pH sensor materials for oceanographic applications. Sens. Actuators B Chem. 2019, 282, 204–217. [Google Scholar] [CrossRef]
- Schmälzlin, E.; Nöhre, M.; Weyand, B. Optical Oxygen Measurements within Cell Tissue Using Phosphorescent Microbeads and a Laser for Excitation; Springer International Publishing: Cham, Switzerland, 2021; pp. 107–129. [Google Scholar]
- Alexandrovskaya, A.Y.; Melnikov, P.V.; Safonov, A.V.; Naumova, A.O.; Zaytsev, N.K. A comprehensive study of the resistance to biofouling of different polymers for optical oxygen sensors. The advantage of the novel fluorinated composite based on core-dye-shell structure. Mater. Today Commun. 2020, 23, 100916. [Google Scholar] [CrossRef]
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors Based on Mechanical and Electrical Detection Techniques. Sensors 2020, 20, 5605. [Google Scholar] [CrossRef] [PubMed]
- Helm, I.; Karina, G.; Jalukse, L.; Pagano, T.; Leito, I. Comparative validation of amperometric and optical analyzers of dissolved oxygen: A case study. Environ. Monit. Assess. 2018, 190, 313. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. BioEssays 2015, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Arlyapov, V.A.; Kamanina, O.A.; Popova, N.M.; Zaitsev, N.K.; Yashtulov, N.A. Optical Oxygen Sensing and Clark Electrode: Face-to-Face in a Biosensor Case Study. Sensors 2022, 22, 7626. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Johansson, S.; Tjell, A.; Werr, G.; Mayr, T.; Tenje, M. In-line analysis of organ-on-chip systems with sensors: Integration, fabrication, challenges, and potential. ACS Biomater. Sci. Eng. 2021, 7, 2926–2948. [Google Scholar] [CrossRef] [PubMed]
- Grist, S.M.; Chrostowski, L.; Cheung, K.C. Optical oxygen sensors for applications in microfluidic cell culture. Sensors 2010, 10, 9286–9316. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, C.; Kolle, C.; McEvoy, A.K.; Dowling, D.L.; Cafolla, A.A.; Cullen, S.J.; MacCraith, B.D. Phase fluorometric dissolved oxygen sensor. Sens. Actuators B Chem. 2001, 74, 124–130. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef]
- Dalfen, I.; Borisov, S.M. Porous matrix materials in optical sensing of gaseous oxygen. Anal. Bioanal. Chem. 2022, 414, 4311–4330. [Google Scholar] [CrossRef]
- Dalfen, I.; Burger, T.; Slugovc, C.; Borisov, S.M.; Klimant, I. Materials for optical oxygen sensing under high hydrostatic pressure. Sens. Actuators B Chem. 2022, 352, 131037. [Google Scholar] [CrossRef]
- Machado, M.G.C.; Pound-Lana, G.; de Oliveira, M.A.; Lanna, E.G.; Fialho, M.C.P.; de Brito, A.C.F.; Barboza, A.P.M.; Aguiar-Soares, R.D.d.O.; Mosqueira, V.C.F. Labeling PLA-PEG nanocarriers with IR780: Physical entrapment versus covalent attachment to polylactide. Drug Deliv. Transl. Res. 2020, 10, 1626–1643. [Google Scholar] [CrossRef]
- Chae, K.H.; Hong, D.W.; Lee, M.K.; Son, O.J.; Rhee, J. Il Anti-fouling sol-gel-derived sensing membrane entrapped with polymer containing phosphorylcholine groups for an optical O2 sensor application. Sens. Actuators B Chem. 2012, 173, 636–642. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Rosace, G.; Cardiano, P.; Urzì, C.; De Leo, F.; Galletta, M.; Ielo, I.; Plutino, M.R. Potential roles of fluorine-containing sol-gel coatings against adhesion to control microbial biofilm. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012021. [Google Scholar] [CrossRef]
- Bartoš, D.; Rewers, M.; Wang, L.; Sørensen, T.J. Incorporating fluorescent nanomaterials in organically modified sol–gel materials—Creating single composite optical pH sensors. Sens. Diagn. 2022, 1, 185–192. [Google Scholar] [CrossRef]
- Du, X.H.; Jiang, Z.; Liu, Z.; Xu, C. BODIPY-linked conjugated porous polymers for dye wastewater treatment. Microporous Mesoporous Mater. 2022, 332, 111711. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials 2021, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lu, S.; Qu, Z.; Feng, X. Tuning the sensitivity and dynamic range of optical oxygen sensing films by blending various polymer matrices. Biosensors 2022, 12, 5. [Google Scholar] [CrossRef]
- Ivanov, I.S.; Shatalov, D.O.; Kedik, S.A.; Sedishev, I.P.; Beliakov, S.V.; Trachuk, K.N.; Komarova, V.V. An effective method for preparation of high purity oligohexamethylene guanidine salts. Tonkie Khimicheskie Tekhnologii 2020, 15, 31–38. [Google Scholar] [CrossRef]
- Akhmedova, D.A.; Shatalov, D.O.; Ivanov, I.S.; Aydakova, A.V.; Herbst, A.; Greiner, L.; Kaplun, A.P.; Zhurbenko, A.S.; Kedik, S.A. The use of microfluidic hardware in the synthesiof oligohexamethylene guanidine derivatives. Tonkie Khimicheskie Tekhnologii 2021, 16, 307–317. [Google Scholar] [CrossRef]
- Beliakov, S.V.; Shatalov, D.O.; Kedik, S.A.; Aydakova, A.V.; Zasypkina, N.A. Preliminary in vitro and in vivo evaluation of specific activity of branched oligohexamethyleneguanidine hydrochloride. Drug Dev. Ind. Pharm. 2020, 46, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Kedik, S.A.; Bocharova, O.A.; An, H.K.; Panov, A.V.; Sedishev, I.P.; Zhavoronok, E.S.; Timofeeva, G.I.; Suslov, V.V.; Beksaev, S.G. Structure and molecular-weight characteristics of oligo(hexamethyleneguanidine) hydrochlorides. Pharm. Chem. J. 2011, 44, 568–573. [Google Scholar] [CrossRef]
- Senchikhin, I.N.; Zhavoronok, E.S.; Matveev, A.V.; Uryupina, O.Y.; Roldughin, V.I. Modification of Epoxy and Amine Oligomers with Oligohexamethyleneguanidine Fatty-Acid Salts. Colloid J. 2018, 80, 306–311. [Google Scholar] [CrossRef]
- Shatalov, D.O.; Kedik, S.A.; Ivanov, I.S.; Aydakova, A.V.; Akhmedova, D.A.; Minenkov, D.S.; Beliakov, S.V.; Herbst, A.; Greiner, L.; Kozlovskaya, L.I.; et al. Development of a Promising Method for Producing Oligomeric Mixture of Branched Alkylene Guanidines to Improve Substance Quality and Evaluate Their Antiviral Activity against SARS-CoV-2. Molecules 2021, 26, 3472. [Google Scholar] [CrossRef] [PubMed]
- Shatalov, D.O.; Kedik, S.A.; Panov, A.V.; Zhavoronok, E.S.; Aydakova, A.V.; Kovalenko, A.V.; Morozova, O.A.; Makeeva, I.M.; Dezhurko-Korol, V.A. Antimicrobial activity of branched oligo(hexamethyleneguanidine) hydrochloride on oral pathogens. Russ. Open Med. J. 2018, 7, e0309. [Google Scholar] [CrossRef]
- Brown, D.F.; Kothari, D. Comparison of antibiotic discs from different sources. J. Clin. Pathol. 1975, 28, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Buttke, T.M.; McCubrey, J.A.; Owen, T.C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immunol. Methods 1993, 157, 233–240. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Larimer, C.; Winder, E.; Jeters, R.; Prowant, M.; Nettleship, I.; Addleman, R.S.; Bonheyo, G.T. A method for rapid quantitative assessment of biofilms with biomolecular staining and image analysis. Anal. Bioanal. Chem. 2016, 408, 999–1008. [Google Scholar] [CrossRef]
- Akkaya, Y.; Akyuz, S. Infrared and Raman spectra, ab initio calculations vibrational assignment of 4-aminosalicylic acid. Vib. Spectrosc. 2006, 42, 292–301. [Google Scholar] [CrossRef]
- Cao, C.; Wu, K.; Yuan, W.; Zhang, Y.; Wang, H. Synthesis of non-water soluble polymeric guanidine derivatives and application in preparation of antimicrobial regenerated cellulose. Fibers Polym. 2017, 18, 1040–1047. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; John, A.; Philip, D.; Istvan, K.; Keresztury, G. FT-IR, FT-Raman and FT-SERS spectra of 4-aminosalicylic acid sodium salt dihydrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Chen, S.; Wang, X.; Wang, J. Effect of PPEGMA content on the structure and hydrophilicity of PVDF/PPEGMA blends prepared by in situ polymerization. Colloid Polym. Sci. 2013, 291, 1573–1580. [Google Scholar] [CrossRef]
- Moradi, R.; Karimi-Sabet, J.; Shariaty-Niassar, M.; Koochaki, M.A. Preparation and characterization of polyvinylidene fluoride/graphene superhydrophobic fibrous films. Polymers 2015, 7, 1444–1463. [Google Scholar] [CrossRef]
- Lencar, C.C.; Ramakrishnan, S.; Sundararaj, U. Carbon Nanotube Migration in Melt-Compounded PEO/PE Blends and Its Impact on Electrical and Rheological Properties. Nanomaterials 2022, 12, 3772. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Ha, C.S.; Cho, Y.; Yoo, S.; Lee, W.K. Solvent-induced surface structure of poly(vinylidene fluoride)/biodegradable polyester blend films. Mol. Cryst. Liq. Cryst. 2014, 598, 23–27. [Google Scholar] [CrossRef]




| OHMG-PAS Content in the Polymer, wt.% | Polymer Solution, mL | OHMG-PAS Solution, mL | Methanol + DMSO (5:1 Ratio), mL |
|---|---|---|---|
| 0.00% | 15 | 0 | 6 |
| 1.39% | 15 | 1 | 5 |
| 2.78% | 15 | 2 | 4 |
| 4.17% | 15 | 3 | 3 |
| 5.56% | 15 | 4 | 2 |
| 6.94% | 15 | 5 | 1 |
| 8.33% | 15 | 6 | 0 |
| OHMG-PAS Content in the Polymer, wt.% | Diameter of M. smegmatis Inhibition Zone, mm | Diameter of the P. chlororaphis Inhibition Zone, mm |
|---|---|---|
| 0.00% | 0.0 | 0.0 |
| 1.39% | 0.8 | 1.0 |
| 2.78% | 1.4 | 2.0 |
| 4.17% | 4.2 | 6.7 |
| 5.56% | 6.3 | 10.7 |
| 6.94% | 7.3 | 13.3 |
| 8.33% | 10.6 | 15.7 |
| OHMG-PAS Content in the Polymer, wt.% | γtot, mN/m | γd, mN/m | γp, mN/m |
|---|---|---|---|
| 0.00% | 18.08 | 17.80 | 0.28 |
| 1.39% | 17.46 | 17.21 | 0.25 |
| 2.78% | 14.54 | 14.45 | 0.09 |
| 4.17% | 15.18 | 15.06 | 0.12 |
| 5.56% | 15.34 | 15.21 | 0.13 |
| 6.94% | 16.19 | 16.01 | 0.18 |
| 8.33% | 17.76 | 17.50 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowski, M.D.; Korobova, E.V.; Naumova, A.O.; Sedishev, I.P.; Markova, A.A.; Nguyen, M.T.; Kuzmin, V.A.; Nichugovskiy, A.I.; Arlyapov, V.A.; Yashtulov, N.A.; et al. Oligohexamethylene Guanidine Derivative as a Means to Prevent Biological Fouling of a Polymer-Based Composite Optical Oxygen Sensor. Polymers 2023, 15, 4508. https://doi.org/10.3390/polym15234508
Lisowski MD, Korobova EV, Naumova AO, Sedishev IP, Markova AA, Nguyen MT, Kuzmin VA, Nichugovskiy AI, Arlyapov VA, Yashtulov NA, et al. Oligohexamethylene Guanidine Derivative as a Means to Prevent Biological Fouling of a Polymer-Based Composite Optical Oxygen Sensor. Polymers. 2023; 15(23):4508. https://doi.org/10.3390/polym15234508
Chicago/Turabian StyleLisowski, Maxim D., Elizaveta V. Korobova, Alina O. Naumova, Igor P. Sedishev, Alina A. Markova, Minh Tuan Nguyen, Vladimir A. Kuzmin, Artemiy I. Nichugovskiy, Vyacheslav A. Arlyapov, Nikolay A. Yashtulov, and et al. 2023. "Oligohexamethylene Guanidine Derivative as a Means to Prevent Biological Fouling of a Polymer-Based Composite Optical Oxygen Sensor" Polymers 15, no. 23: 4508. https://doi.org/10.3390/polym15234508
APA StyleLisowski, M. D., Korobova, E. V., Naumova, A. O., Sedishev, I. P., Markova, A. A., Nguyen, M. T., Kuzmin, V. A., Nichugovskiy, A. I., Arlyapov, V. A., Yashtulov, N. A., & Melnikov, P. V. (2023). Oligohexamethylene Guanidine Derivative as a Means to Prevent Biological Fouling of a Polymer-Based Composite Optical Oxygen Sensor. Polymers, 15(23), 4508. https://doi.org/10.3390/polym15234508










