Removal of Algae and Algal Toxins from a Drinking Water Source Using a Two-Stage Polymeric Ultrafiltration Membrane Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
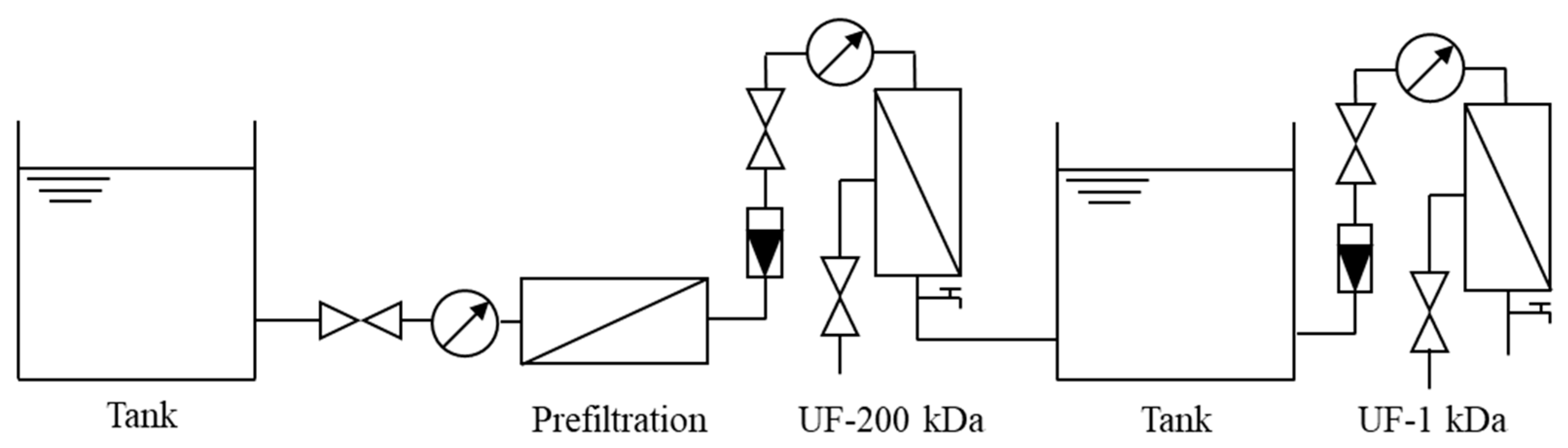
2.2. UF Process Setup
2.3. Analysis Methods
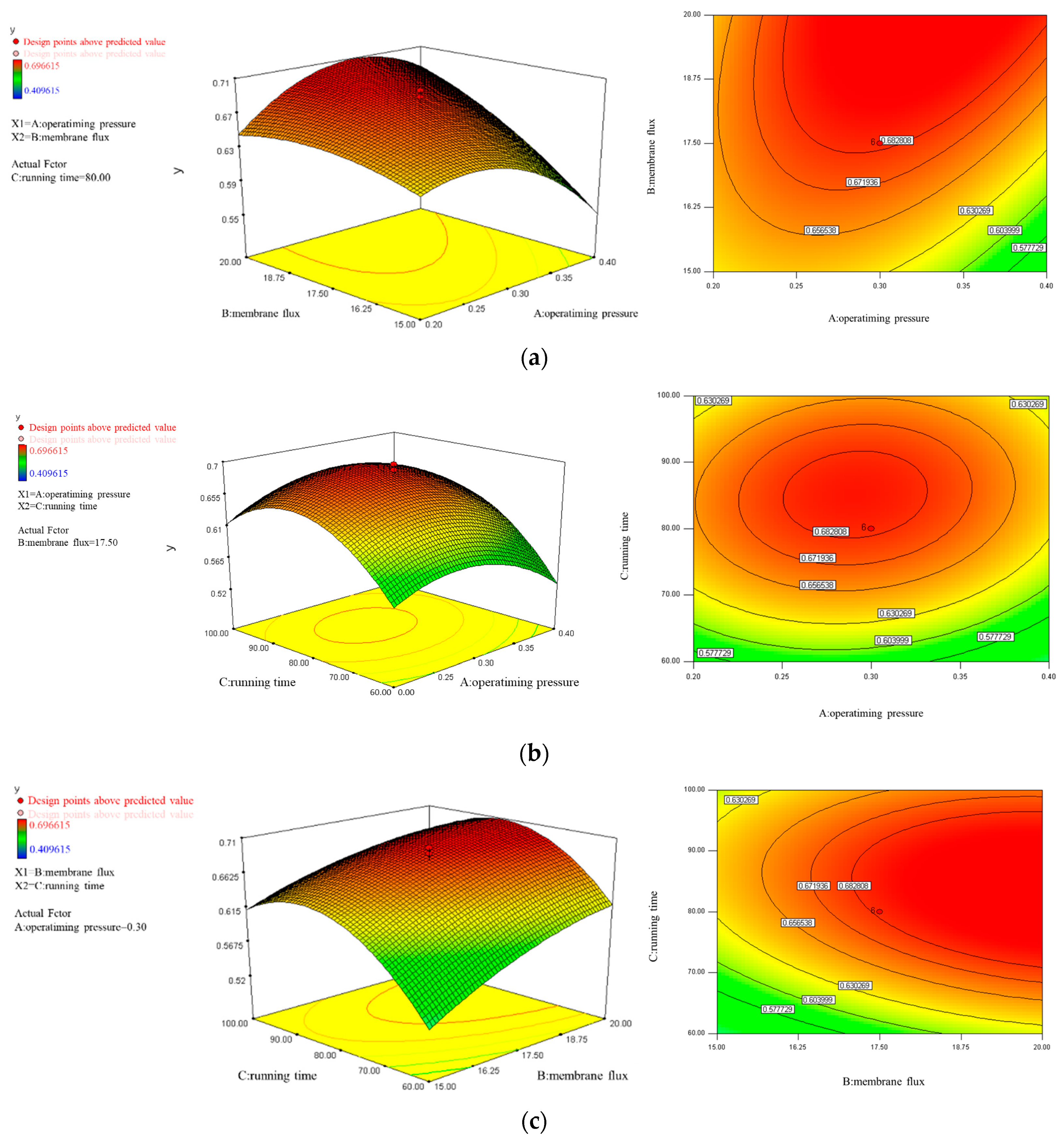
2.4. Response Surface Method Design
3. Results and Discussion
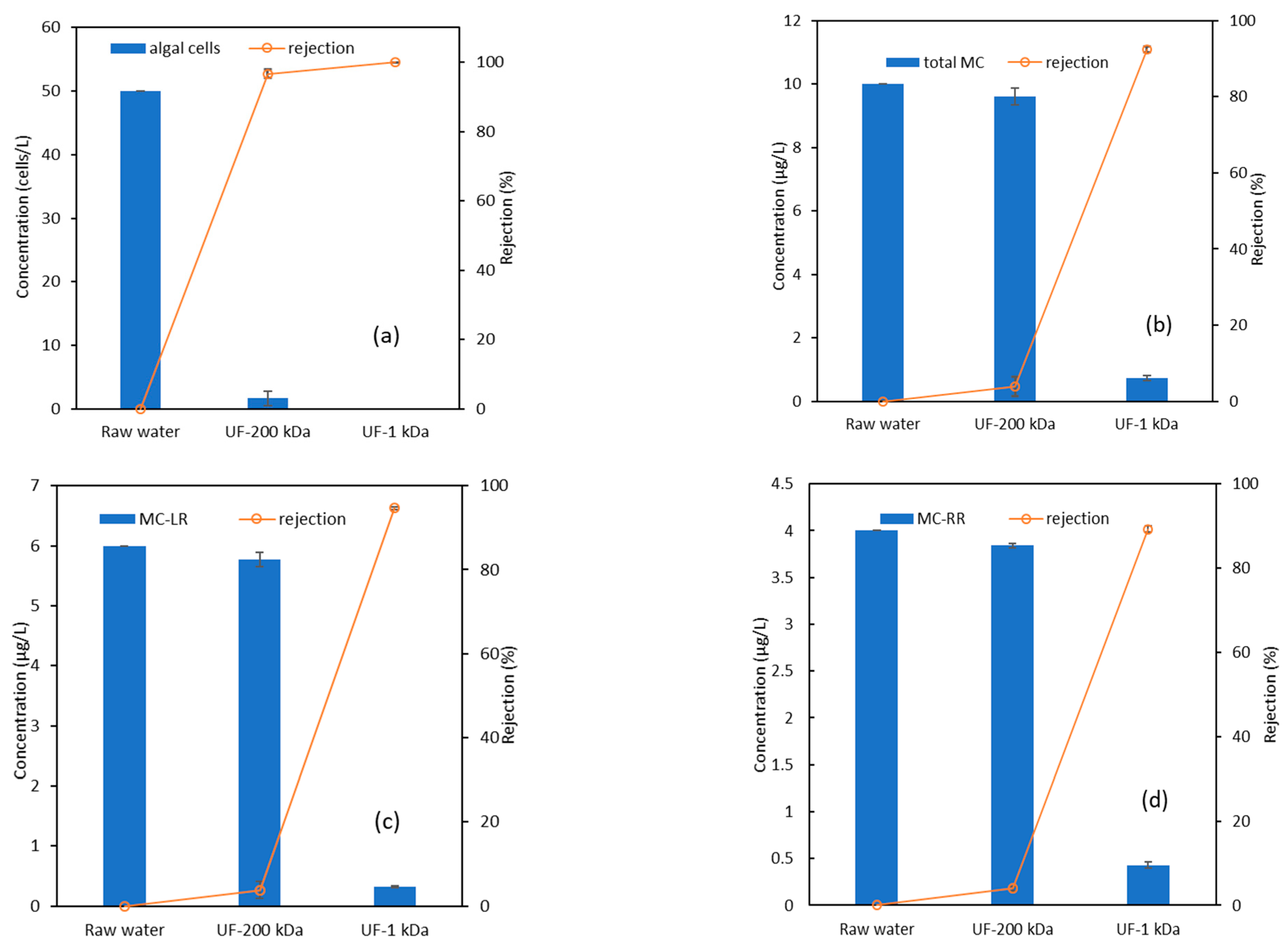
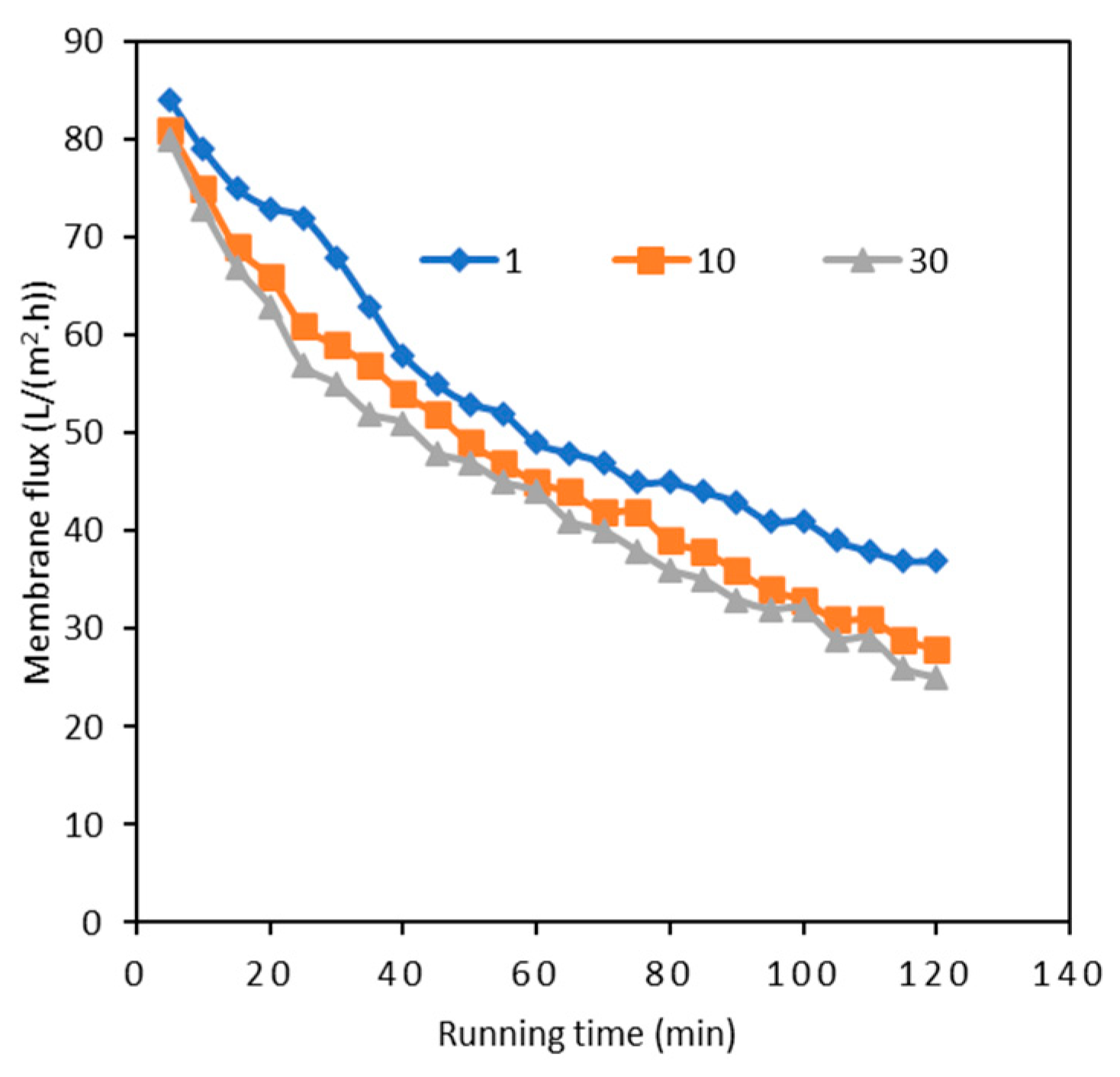
3.1. Overall Algal Pollutant Removal by the Two-Stage UF Process
3.2. Removal of Algal Toxins Using the UF-1 kDa Membrane
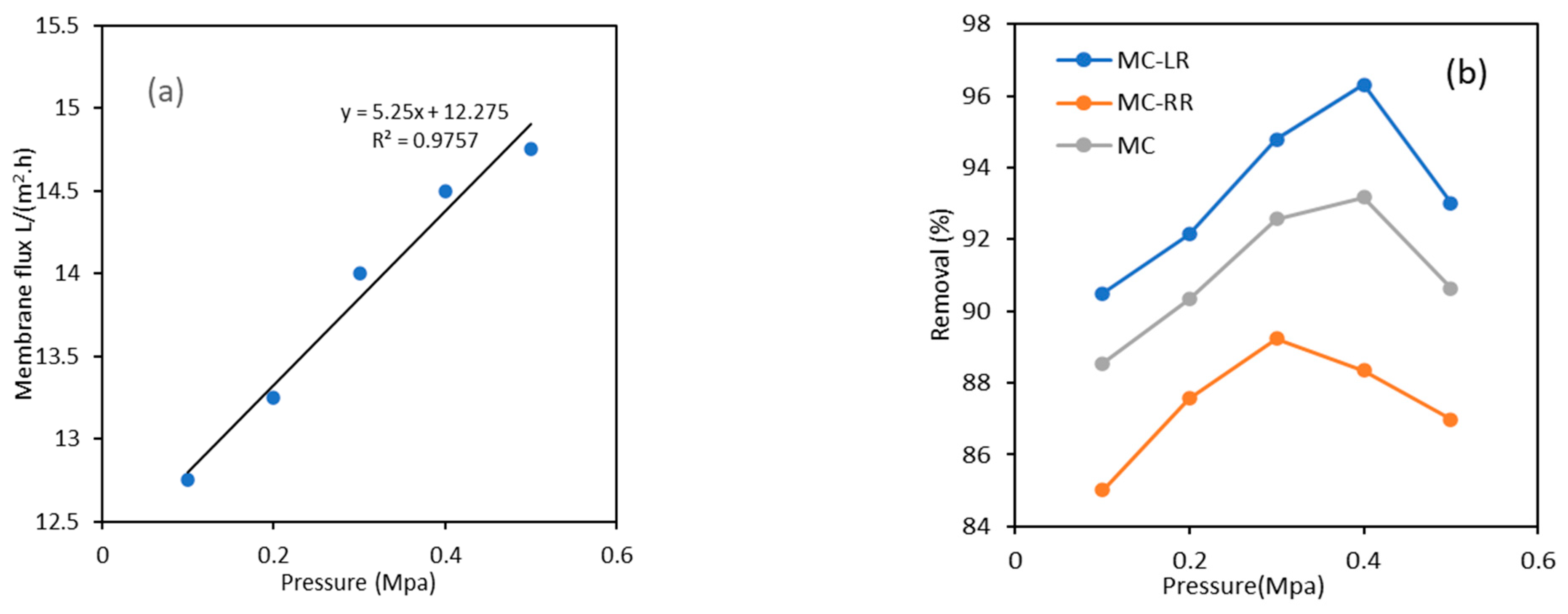
3.3. Analysis of MC Removal by UF Using RSM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Qi, F.; Liu, C.; Zhang, Y.; Wang, Y.; Song, Z.; Kumirska, J.; Sun, D. Cyanobacteria derived taste and odor characteristics in various lakes in China: Songhua Lake, Chaohu Lake and Taihu Lake. Ecotoxicol. Environ. Saf. 2019, 181, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, S.; Mu, X.; Hu, X.; Ma, Y. Research Characteristics on Cyanotoxins in Inland Water: Insights from Bibliometrics. Water 2022, 14, 667. [Google Scholar] [CrossRef]
- Seubert, E.L.; Trussell, S.; Eagleton, J.; Schnetzer, A.; Cetinic, I.; Lauri, P.; Jones, B.H.; Caron, D.A. Algal toxins and reverse osmosis desalination operations: Laboratory bench testing and field monitoring of domoic acid, saxitoxin, brevetoxin and okadaic acid. Water Res. 2012, 46, 6563–6573. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Salim, E.H.; Azab, Y.A.; Ismail, A.H.M. Monitoring and removal of cyanobacterial toxins from drinking water by algal-activated carbon. Toxicol. Ind. Health 2016, 32, 1752–1762. [Google Scholar] [CrossRef]
- Gregor, J.E.; Fenton, E.; Brokenshire, G.; Van Den Brink, P.; O’Sullivan, B. Interactions of calcium and aluminium ions with alginate. Water Res. 1996, 30, 1319–1324. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Shao, Y.S.; Gao, N.Y.; Li, L.; Deng, J.; Zhu, M.Q.; Zhu, S.M. Effect of chlorine dioxide on cyanobacterial cell integrity, toxin degradation and disinfection by-product formation. Sci. Total Environ. 2014, 482, 208–213. [Google Scholar] [CrossRef]
- Gonsior, M.; Powers, L.C.; Williams, E.; Place, A.; Chen, F.; Ruf, A.; Hertkorn, N.; Schmitt-Kopplin, P. The chemodiversity of algal dissolved organic matter from lysed Microcystis aeruginosa cells and its ability to form disinfection by-products during chlorination. Water Res. 2019, 155, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. Co-Occurrence of Cyanobacteria and Cyanotoxins with Other Environmental Health Hazards: Impacts and Implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Lambert, T.W.; Holmes, C.F.B.; Hrudey, S.E. Microcystin class of toxins: Health effects and safety of drinking water supplies. Environ. Rev. 1994, 2, 167–186. [Google Scholar] [CrossRef]
- Zhang, S.; Du, X.; Liu, H.; Losiewic, M.D.; Chen, X.; Ma, Y.; Wang, R.; Tian, Z.; Shi, L.; Guo, H.; et al. The latest advances in the reproductive toxicity of microcystin-LR. Environ. Res. 2021, 192, 110254. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Puerto, M.; Gutiérrez-Praena, D.; Llana-Ruiz-Cabello, M.; Jos, A.; Cameán, A.M. Microcystin-RR: Occurrence, content in water and food and toxicological studies. A review. Environ. Res. 2019, 168, 467–489. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kwon, J.H.; Jo, G.J. Removal of algae and turbidity by floating-media and sand filtration. Desalination Water Treat. 2014, 52, 1007–1013. [Google Scholar] [CrossRef]
- Rapala, J.; Niemela, M.; Berg, K.A.; Lepisto, L.; Lahti, K. Removal of cyanobacteria, cyanotoxins, heterotrophic bacteria and endotoxins at an operating surface water treatment plant. Water Sci. Technol. 2006, 54, 23–28. [Google Scholar] [CrossRef]
- Xu, D.L.; Bai, L.M.; Tang, X.B.; Niu, D.Y.; Luo, X.S.; Zhu, X.W.; Li, G.B.; Liang, H. A comparison study of sand filtration and ultrafiltration in drinking water treatment: Removal of organic foulants and disinfection by-product formation. Sci. Total Environ. 2019, 691, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Aziz, M.T.; Richardson, S.D. Transformation of Algal Toxins during the Oxidation/Disinfection Processes of Drinking Water: From Structure to Toxicity. Environ. Sci. Technol. 2023, 57, 12944–12957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.X.; Lin, Y.L.; Fang, R.F.; Zhou, X.Y.; Liu, Z.; Dong, Z.Y.; Zhang, T.Y.; Xu, B. Removal of algae and algogenic odor compounds via combined pre-chlorination and powdered activated carbon adsorption for source water pretreatment. Sep. Purif. Technol. 2023, 304, 122365. [Google Scholar] [CrossRef]
- Roh, S.H.; Kwak, D.H.; Jung, H.J.; Hwang, K.J.; Baek, I.H.; Chun, Y.N.; Kim, S.I.; Lee, J.W. Simultaneous removal of algae and their secondary algal metabolites from water by hybrid system of DAF and PAC adsorption. Sep. Sci. Technol. 2008, 43, 113–131. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xue, J.; Sun, W.T.; Chen, W.J.; Liu, B.S.; Jin, L.; Li, J.N.; Li, J.J.; Tian, L.P.; Wang, X.B. Efficiency and mechanism of ozonated microbubbles for enhancing the removal of algae and algae-derived organic matter. Chemosphere 2023, 312, 137220. [Google Scholar] [CrossRef]
- Menezes, I.; Capelo-Neto, J.; Pestana, C.J.; Clemente, A.; Hui, J.N.; Irvine, J.T.S.; Gunaratne, H.Q.N.; Robertson, P.K.J.; Edwards, C.; Gillanders, R.N.; et al. Comparison of UV-A photolytic and UV/TiO2 photocatalytic effects on Microcystis aeruginosa PCC7813 and four microcystin analogues: A pilot scale study. J. Environ. Manag. 2021, 298, 113519. [Google Scholar] [CrossRef]
- Chen, M.; Jafvert, C.T. Anion Exchange on Cationic Surfactant Micelles, and a Speciation Model for Estimating Anion Removal on Micelles during Ultrafiltration of Water. Langmuir 2017, 33, 6540–6549. [Google Scholar] [CrossRef]
- Chen, M.; Shafer-Peltier, K.; Randtke, S.J.; Peltier, E. Competitive association of cations with poly(sodium 4-styrenesulfonate) (PSS) and heavy metal removal from water by PSS-assisted ultrafiltration. Chem. Eng. J. 2018, 344, 155–164. [Google Scholar] [CrossRef]
- Kammakakam, I.; Lai, Z. Next-generation ultrafiltration membranes: A review of material design, properties, recent progress, and challenges. Chemosphere 2023, 316, 137669. [Google Scholar] [CrossRef] [PubMed]
- Bhave, R.R. Inorganic Membranes Synthesis, Characteristics and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Chen, M.; Shen, S.; Zhang, F.; Zhang, C.; Xiong, J. Biodegradable Dissolved Organic Carbon (BDOC) Removal from Micro-Polluted Water Source Using Ultrafiltration: Comparison with Conventional Processes, Operation Conditions and Membrane Fouling Control. Polymers 2022, 14, 4689. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Q.; Chen, M.; Wang, Y.; Shen, S.; Zhang, Z.; Li, B.; Sun, B. Al30 polycation pillared montmorillonite preparation and phosphate adsorption removal from water. Surf. Interfaces 2022, 29, 101780. [Google Scholar] [CrossRef]
- Ding, J.; Wang, S.; Xie, P.; Zou, Y.; Wan, Y.; Chen, Y.; Wiesner, M.R. Chemical cleaning of algae-fouled ultrafiltration (UF) membrane by sodium hypochlorite (NaClO): Characterization of membrane and formation of halogenated by-products. J. Membr. Sci. 2020, 598, 117662. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, H.; Zhao, S.; Yang, H.; Wang, P.; Li, P.; Sun, H.; Jason Niu, Q. Semi-aromatic polyamide nanofiltration membranes with tuned surface charge and pore size distribution designed for the efficient removal of Ca2+ and Mg2+. Sep. Purif. Technol. 2019, 220, 162–175. [Google Scholar] [CrossRef]
- Novoa, A.F.; Vrouwenvelder, J.S.; Fortunato, L. Membrane Fouling in Algal Separation Processes: A Review of Influencing Factors and Mechanisms. Front. Chem. Eng. 2021, 3, 687422. [Google Scholar] [CrossRef]
- Rickman, M.; Pellegrino, J.; Davis, R. Fouling phenomena during membrane filtration of microalgae. J. Membr. Sci. 2012, 423–424, 33–42. [Google Scholar] [CrossRef]
- GB5749-2022; Standards for Drinking Water Quality. State Administration for Market Regulation and Standardization Administration of the P.R.C.: Beijing, China, 2022.
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Motsa, M.M.; Nkambule, T.I.; Mamba, B.B. Rejection of trace organic compounds by membrane processes: Mechanisms, challenges, and opportunities. Rev. Chem. Eng. 2023, 39, 875–910. [Google Scholar] [CrossRef]

| Factor | Code | Level | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | ||
| Operating pressure (MPa) | X1 | 0.15 | 0.2 | 0.3 | 0.4 | 0.45 |
| Flux (L/(m2·h)) | X2 | 13.75 | 15 | 17.5 | 20 | 21.25 |
| Operating time (min) | X3 | 45 | 60 | 80 | 100 | 110 |
| Run | Code | Response Value | ||
|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | |
| Operating Pressure (MPa) | Flux (L/(m2·h)) | Operating Time (min) | ||
| 1 | 0.2 | 15 | 100 | 0.579987 |
| 2 | 0.3 | 17.5 | 80 | 0.696281 |
| 3 | 0.4 | 20 | 100 | 0.64231 |
| 4 | 0.2 | 15 | 60 | 0.543629 |
| 5 | 0.2 | 20 | 100 | 0.591414 |
| 6 | 0.3 | 17.5 | 80 | 0.688964 |
| 7 | 0.3 | 21.25 | 80 | 0.695781 |
| 8 | 0.3 | 17.5 | 80 | 0.673976 |
| 9 | 0.3 | 17.5 | 45 | 0.409615 |
| 10 | 0.4 | 15 | 100 | 0.526855 |
| 11 | 0.3 | 17.5 | 80 | 0.672186 |
| 12 | 0.2 | 20 | 60 | 0.579512 |
| 13 | 0.3 | 17.5 | 80 | 0.668224 |
| 14 | 0.45 | 17.5 | 80 | 0.580424 |
| 15 | 0.4 | 15 | 60 | 0.436491 |
| 16 | 0.3 | 17.5 | 80 | 0.696615 |
| 17 | 0.15 | 17.5 | 80 | 0.609293 |
| 18 | 0.3 | 13.75 | 80 | 0.610765 |
| 19 | 0.3 | 17.5 | 110 | 0.6292 |
| 20 | 0.4 | 20 | 60 | 0.608986 |
| Quadratic Sum | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 0.12 | 9 | 0.013 | 29.56 | <0.0001 |
| R = 0.9638 | R2 = 0.9312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Xiong, J.; Zhang, C.; Wu, X.; Tian, Y. Removal of Algae and Algal Toxins from a Drinking Water Source Using a Two-Stage Polymeric Ultrafiltration Membrane Process. Polymers 2023, 15, 4495. https://doi.org/10.3390/polym15234495
Zhang F, Xiong J, Zhang C, Wu X, Tian Y. Removal of Algae and Algal Toxins from a Drinking Water Source Using a Two-Stage Polymeric Ultrafiltration Membrane Process. Polymers. 2023; 15(23):4495. https://doi.org/10.3390/polym15234495
Chicago/Turabian StyleZhang, Fan, Jianglei Xiong, Cong Zhang, Xue Wu, and Yuming Tian. 2023. "Removal of Algae and Algal Toxins from a Drinking Water Source Using a Two-Stage Polymeric Ultrafiltration Membrane Process" Polymers 15, no. 23: 4495. https://doi.org/10.3390/polym15234495
APA StyleZhang, F., Xiong, J., Zhang, C., Wu, X., & Tian, Y. (2023). Removal of Algae and Algal Toxins from a Drinking Water Source Using a Two-Stage Polymeric Ultrafiltration Membrane Process. Polymers, 15(23), 4495. https://doi.org/10.3390/polym15234495




