Properties of Eco-Friendly Composites Based on Post-Consumer Recycled Resin Filled with Walnut Shell Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Filler Preparation
2.3. Walnut Shell Powder Composites
2.4. Composite Specimens
3. Research Methods and Characterizations
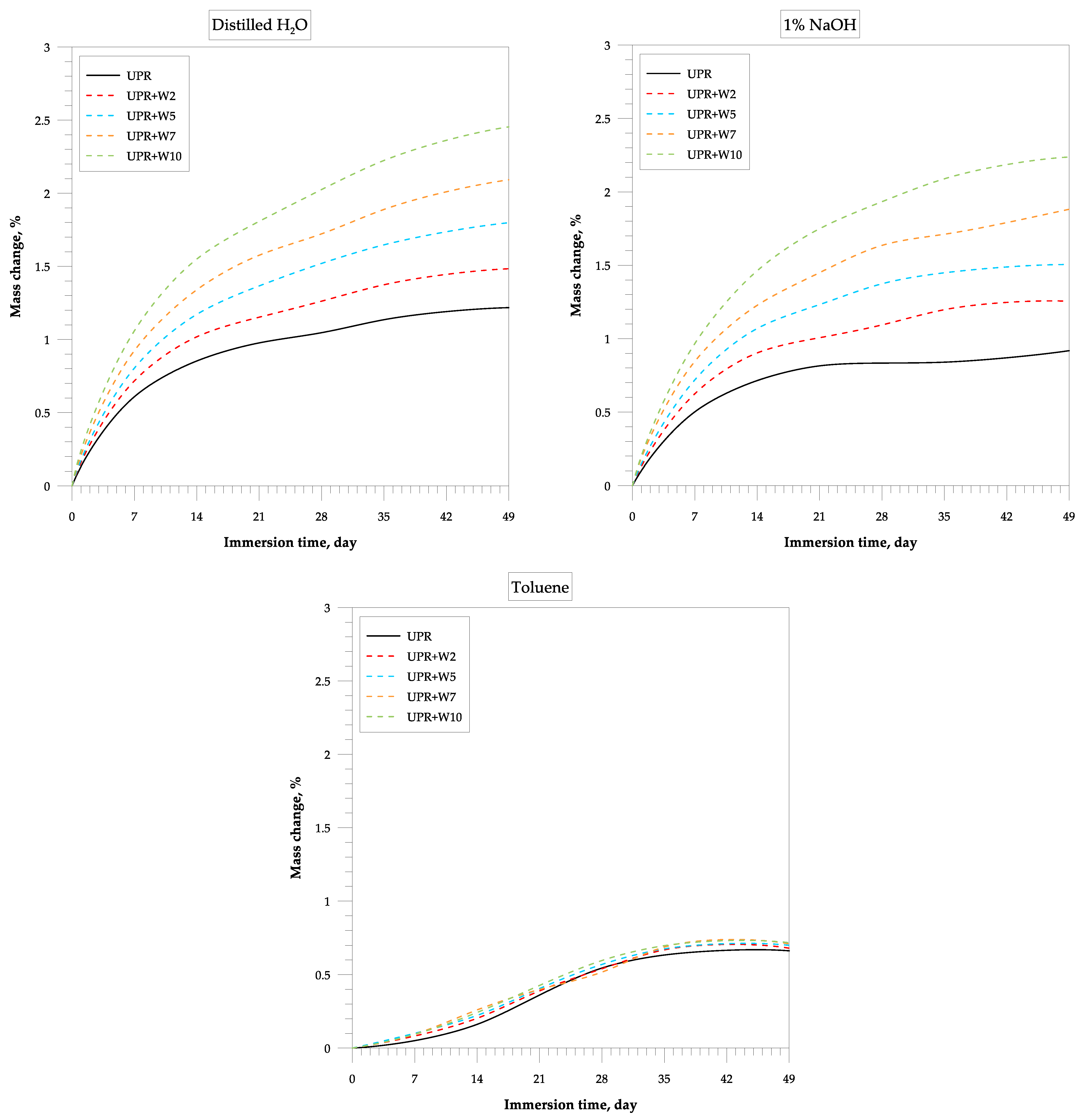
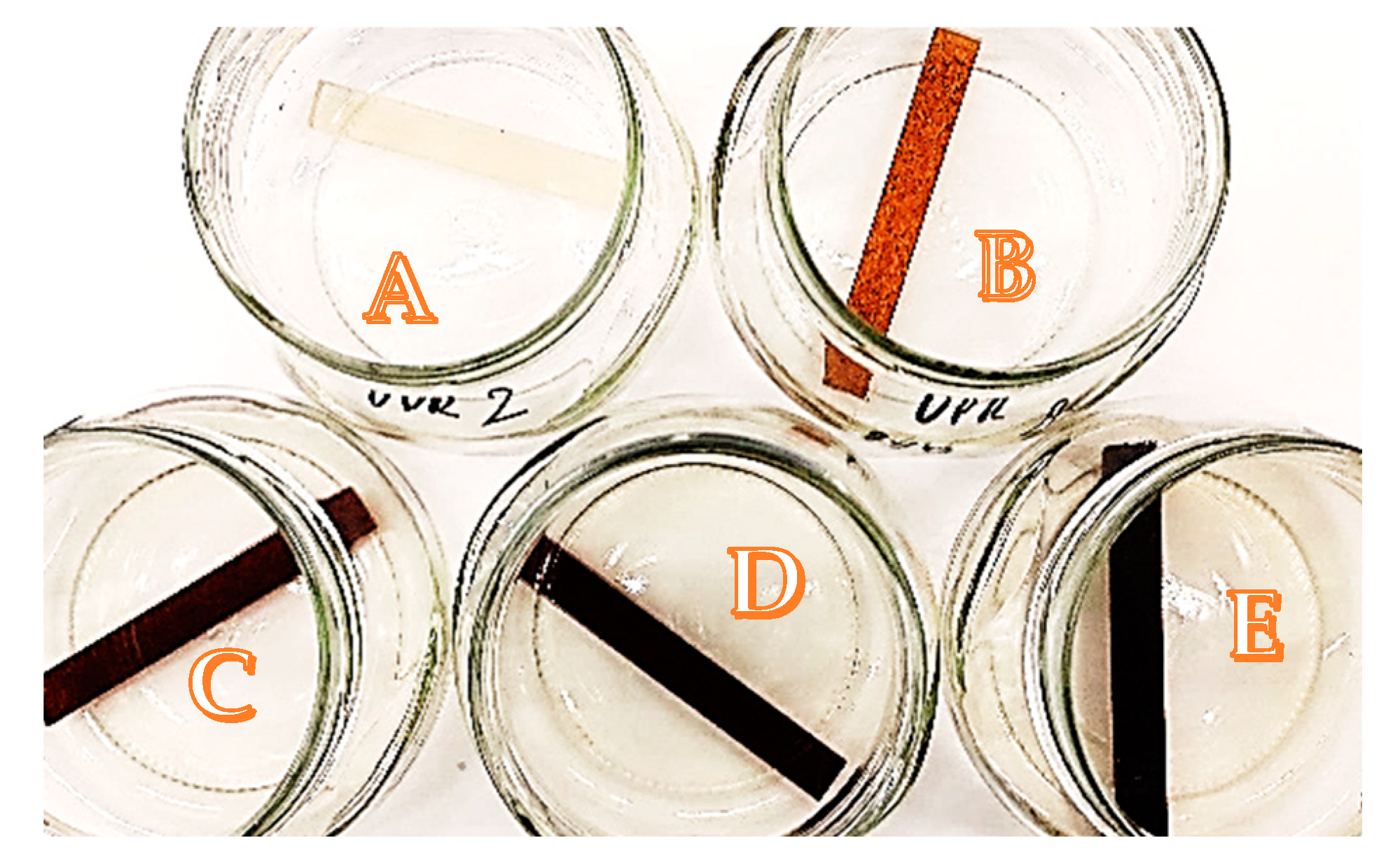
3.1. Chemical Resistance
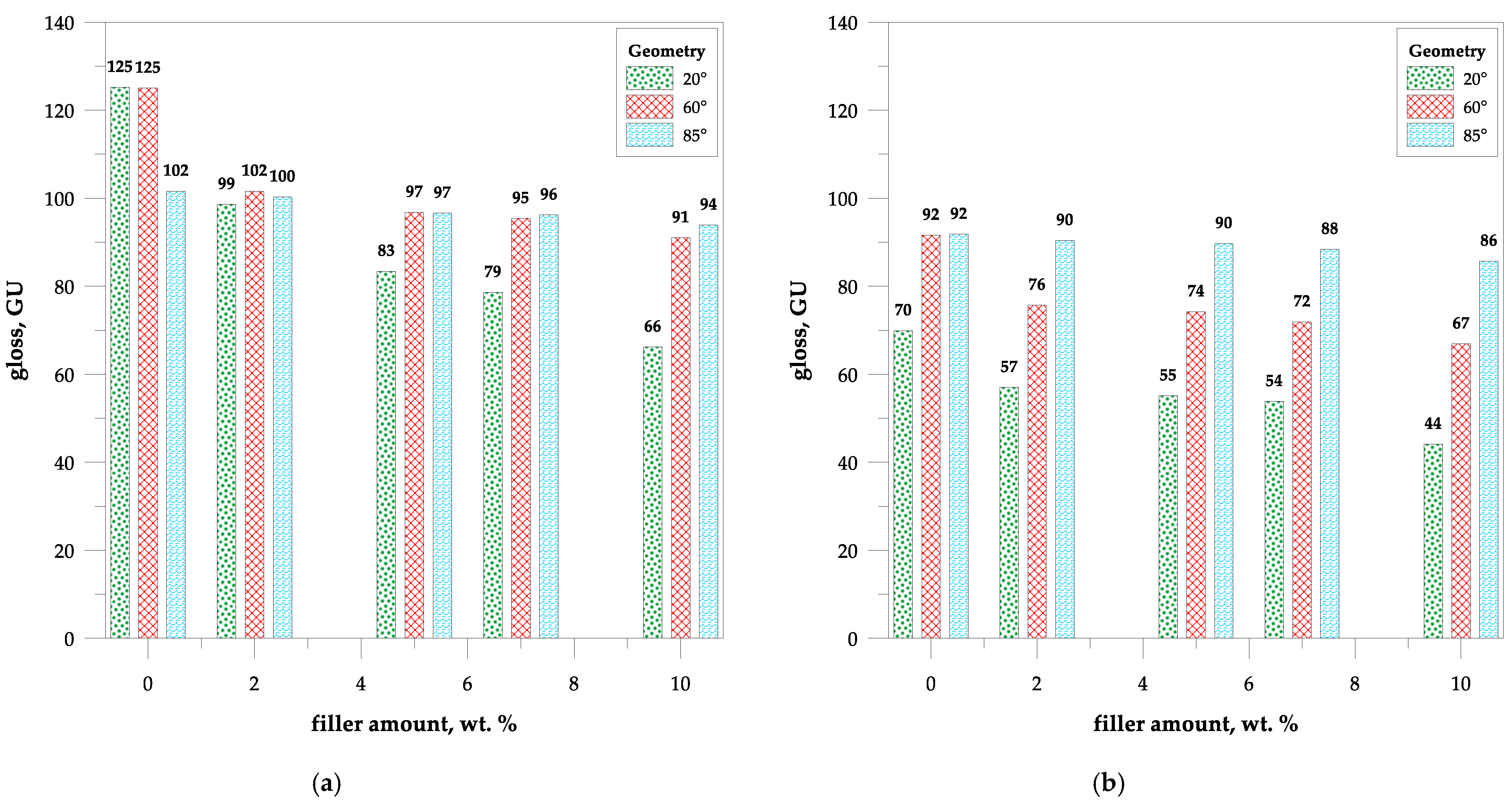
3.2. Gloss Measurement and Optical Properties
3.3. Thermal Stabilities
3.4. Hardness and Mechanical Properties
3.5. Accelerated Aging
3.6. Microwave Treatment
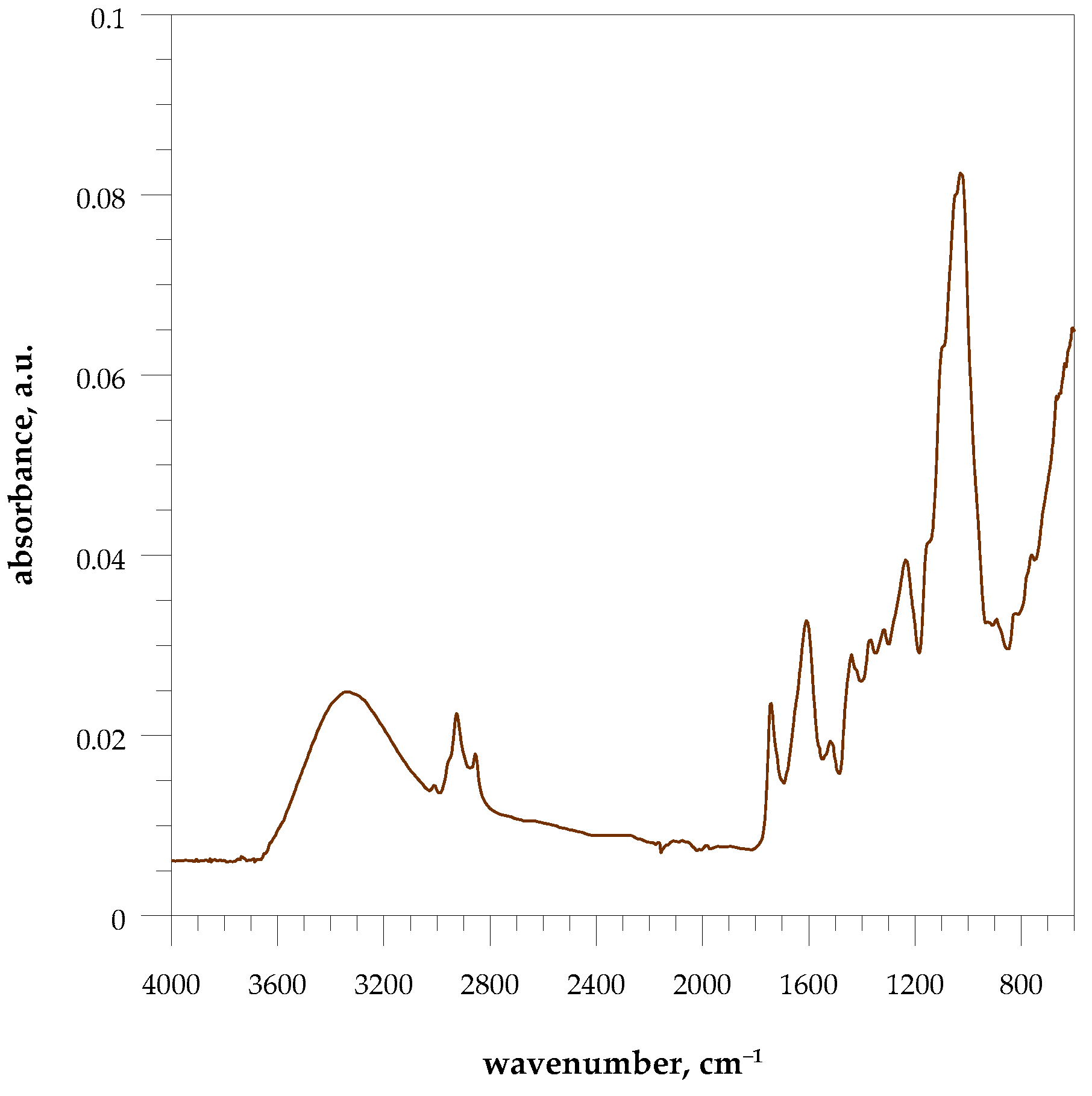
3.7. Elemental Analysis and FT-IR Spectroscopy
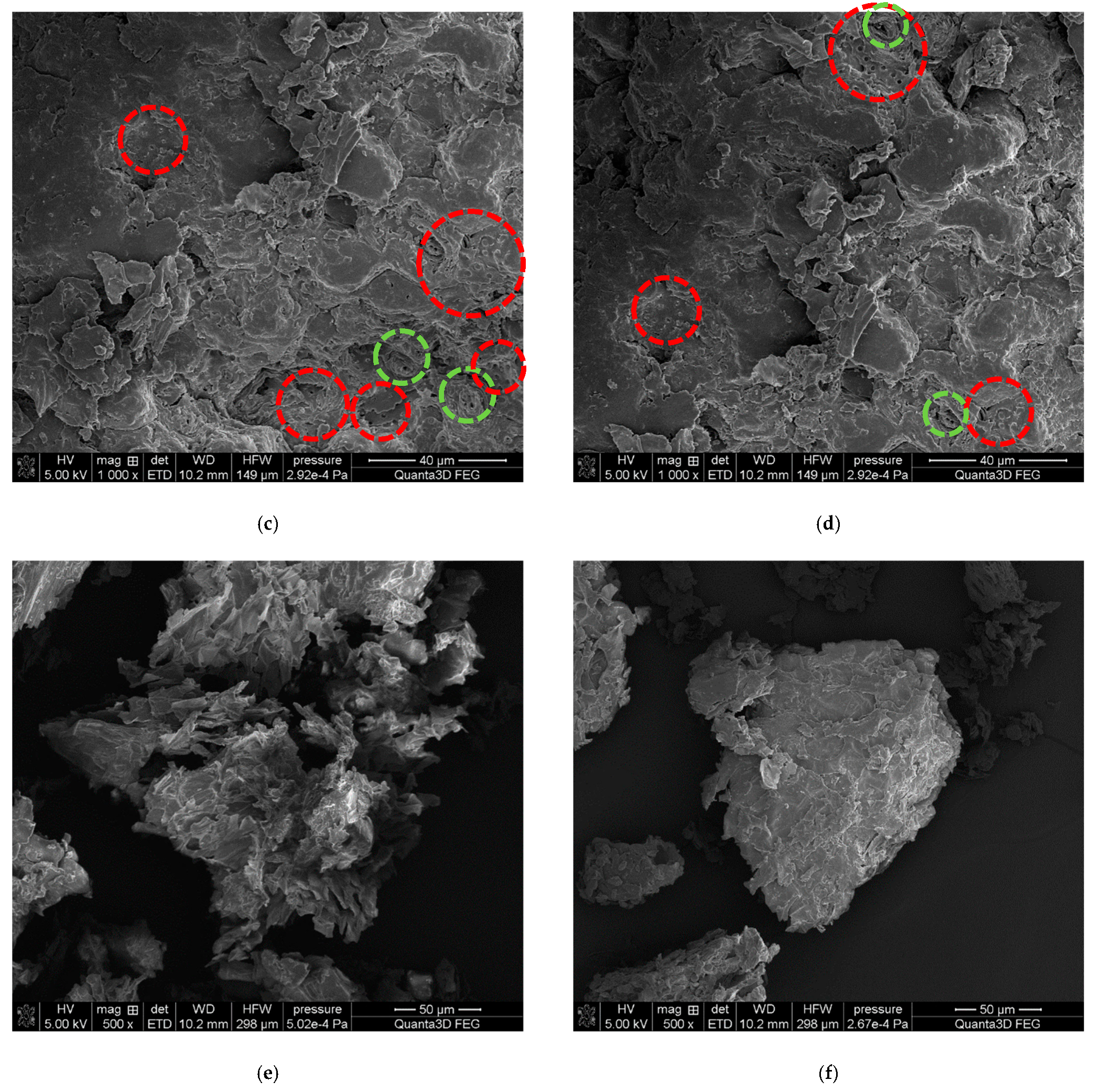
3.8. Morphology and Microstructure
4. Results and Discussion
4.1. Characterization of Eco Filler and Post-Consumer Resin
4.2. Thermal Properties of Composites
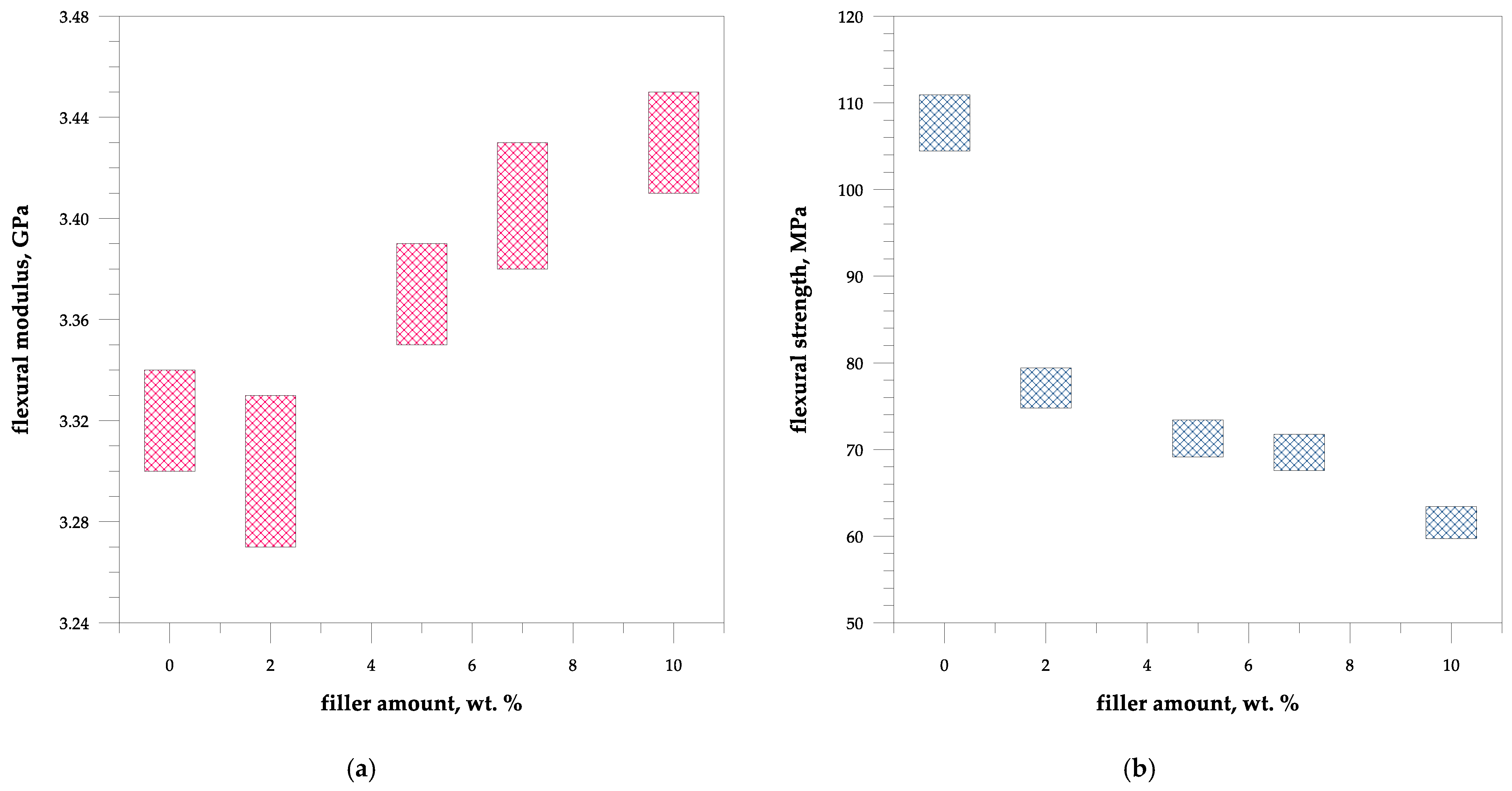
4.3. Mechanical Properties of Composites
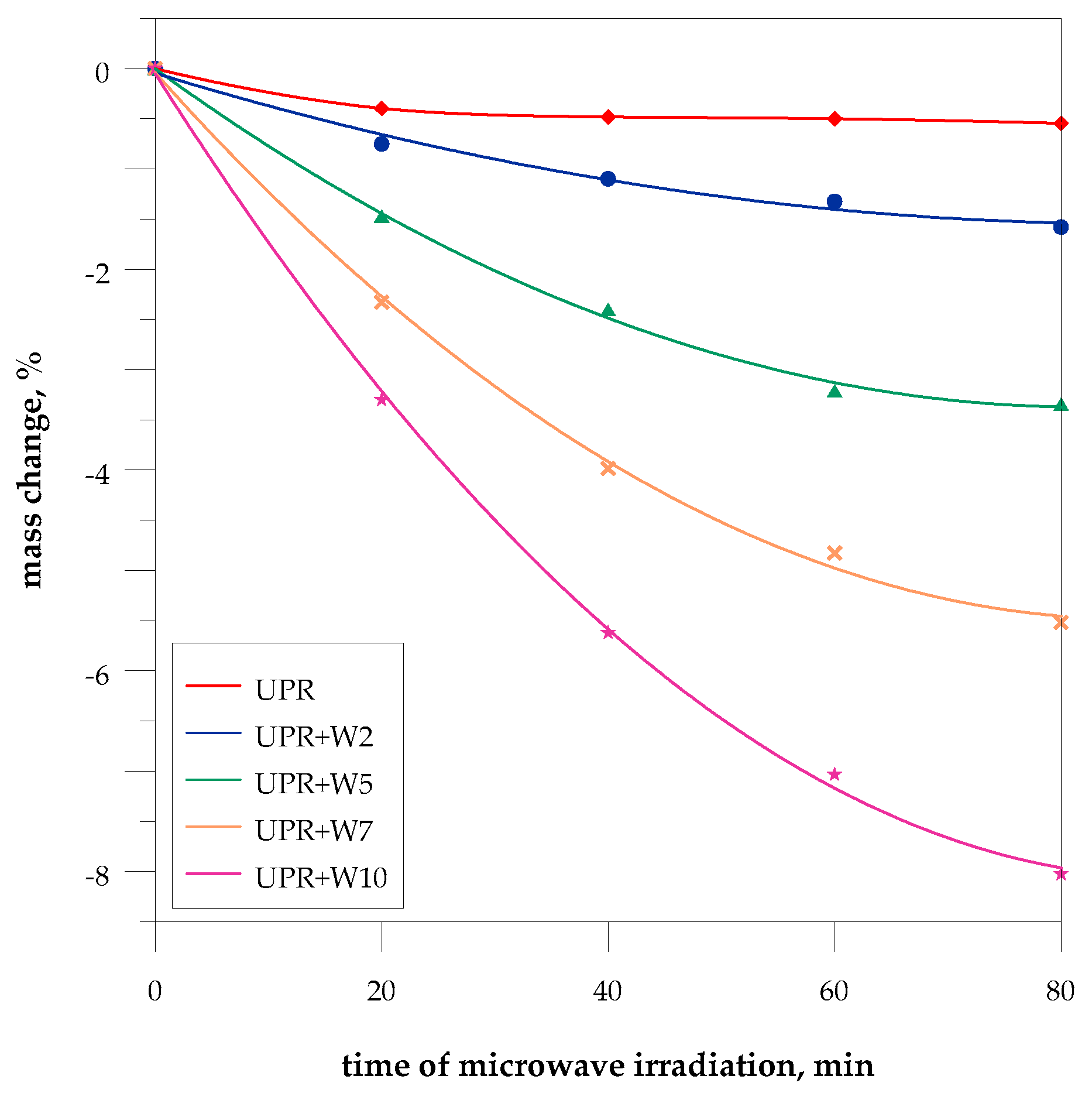
4.4. Influence of Microwaves on Composites
4.5. Influence of Solvents on Composites
4.6. Surface Gloss of Composites
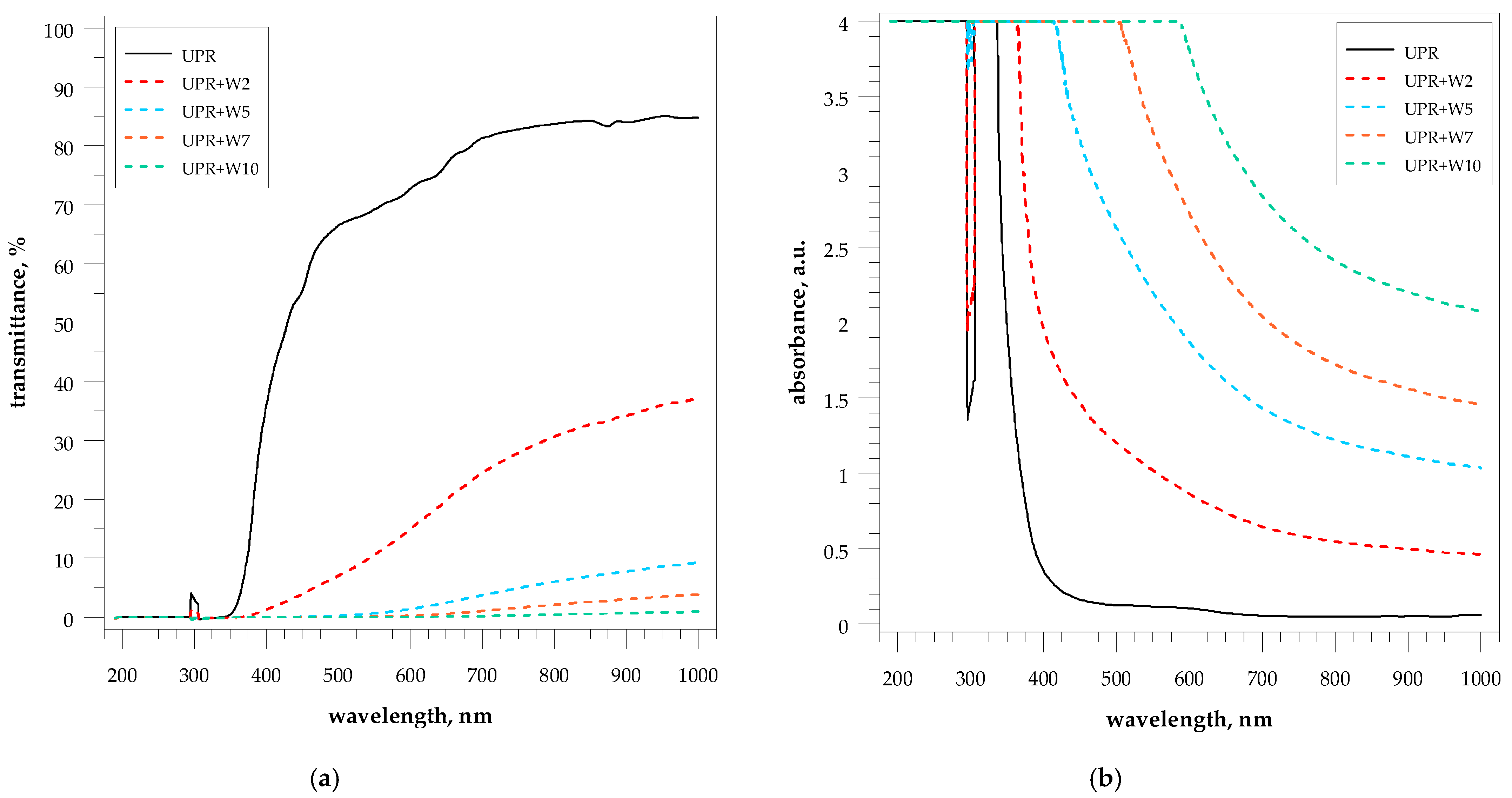
4.7. Optical Properties of Composites
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khantwal, R.; Gupta, G.; Negi, R.S. Walnut shell reinforced composite: A review. Int. J. Sci. Eng. Res. 2016, 7, 179–189. [Google Scholar]
- Food and Agriculture Organization Corporate Statistical Database (FAOSTAT); Food and Agriculture Organization of the United Nations: Rome, Italy, 2023.
- Han, H.; Wang, S.; Rakita, M.; Wang, Y.; Han, Q.; Xu, Q. Effect of ultrasound-assisted extraction of phenolic compounds on the characteristics of walnut shells. Food Nutr. Sci. 2018, 9, 1034–1045. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, H.; Khazaeian, A.; Tabarsa, T. The potential for using walnut (Juglans regia L.) shell as a raw material for wood-based particle board manufacturing. Compos. B Eng. 2012, 43, 3276–3280. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khanjanzadeh, H.; Salari, A. Effect of using walnut/almond shells on the physical, mechanical properties, and formaldehyde emission of particleboard. Compos. B Eng. 2013, 45, 858–863. [Google Scholar] [CrossRef]
- Rao, D.K.; Gope, P.C. Fracture toughness of walnut particles (Juglans regia L.) and coconut fibre reinforced hybrid biocomposite. Polym. Compos. 2015, 36, 167–173. [Google Scholar]
- Gope, P.C.; Rao, D.K. Fracture behaviour of epoxy biocomposite reinforced with short coconut fibres (Cocos nucifera) and walnut particles (Juglans regia L.). J. Thermoplast. Compos. Mater. 2016, 29, 1098–1117. [Google Scholar] [CrossRef]
- Ogah, A.O.; Afiukwa, J.N. Characterization and comparison of mechanical behavior of agrofiber-filled high-density polyethylene bio-composites. J. Reinf. Plast. Compos. 2014, 33, 37–46. [Google Scholar] [CrossRef]
- Mohammed, A.J. Study the effect of adding powder walnut shells on the mechanical properties and the flame resistance for low density polyethylene (LDPE). Int. J. Sci. Technol. 2014, 3, 18–22. [Google Scholar]
- Ayrilmis, N.; Kaymakci, A.; Ozdemir, F. Physical, mechanical, and thermal properties of polypropylene composites filled with walnut shell flour. J. Ind. Eng. Chem. 2013, 19, 908–914. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Quiles-Carrillo, L.; Richart, N.; Torres-Giner, S.; Montanes, N. Enhanced interfacial adhesion of polylactide/poly (ε-caprolactone)/walnut shell flour composites by reactive extrusion with maleinized linseed oil. Polymers 2019, 11, 758. [Google Scholar] [CrossRef]
- Sarsari, N.A.; Pourmousa, S.; Tajdini, A. Physical and mechanical properties of walnut shell flour-filled thermoplastic starch composites. Bioresources 2016, 11, 6968–6983. [Google Scholar]
- Sowińska-Baranowska, A.; Maciejewska, M.; Duda, P. The Potential Application of Starch and Walnut Shells as Biofillers for Natural Rubber (NR) Composites. Int. J. Mol. Sci. 2022, 23, 7968. [Google Scholar] [CrossRef]
- Güngör, A.; Akbay, I.K.; Özdemir, T. Waste walnut shell as an alternative bio-based filler for the EPDM: Mechanical, thermal, and kinetic studies. J. Mater. Cycles Waste Manag. 2019, 21, 145–155. [Google Scholar] [CrossRef]
- Gañán, P.; Barajas, J.; Zuluaga, R.; Castro, C.; Marín, D.; Tercjak, A.; Builes, D.H. The Evolution and Future Trends of Unsaturated Polyester Biocomposites: A Bibliometric Analysis. Polymers 2023, 15, 2970. [Google Scholar] [CrossRef] [PubMed]
- Uma Devi, L.; Bhagawan, S.S.; Thomas, S. Mechanical properties of pineapple leaf fiber-reinforced polyester composites. J. Appl. Polym. Sci. 1997, 64, 1739–1748. [Google Scholar] [CrossRef]
- Market Analysis Report. Unsaturated Polyester Resin Market Size, Share & Trends Analysis Report by Product (DCPD, Orthophthalic, Isophthalic), by End-Use, by Form (Liquid Form, Powder Form), by Region, and Segment Forecasts. Available online: https://www.grandviewresearch.com/industry-analysis/unsaturated-polyester-resin-upr-market (accessed on 10 September 2023).
- Penczek, P.; Czub, P.; Pielichowski, J. Unsaturated Polyester Resins: Chemistry and Technology. In Crosslinking in Materials Science. Technical Applications; Abe, A., Dušek, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–95. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Duque-Ingunza, I.; López-Fonseca, R.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Synthesis of unsaturated polyester resin from glycolysed postconsumer PET wastes. J. Mater. Cycles Waste Manag. 2013, 15, 256–263. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Unsaturated Polyester Resin Nanocomposites Based on Post-Consumer Polyethylene Terephthalate. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef]
- Bórquez-Mendivil, A.; Hurtado-Macías, A.; Leal-Pérez, J.E.; Flores-Valenzuela, J.; Vargas-Ortíz, R.Á.; Cabrera-Covarrubias, F.G.; Almaral-Sánchez, J.L. Hybrid Coatings of SiO2–Recycled PET Unsaturated Polyester Resin by Sol-Gel Process. Polymers 2022, 14, 3280. [Google Scholar] [CrossRef]
- Wilson García, N.A.; Almaral Sánchez, J.L.; Vargas Ortiz, R.Á.; Hurtado Macías, A.; Flores Ramírez, N.; Aguilar Palazuelos, E.; Flores Valenzuela, J.; Castro Beltrán, A.; Alvarado Beltrán, C.G. Physical and mechanical properties of unsaturated polyester resin matrix from recycled PET (based PG) with corn straw fiber. J. Appl. Polym. Sci. 2021, 138, 51305. [Google Scholar] [CrossRef]
- EN ISO 175:2010; Plastics–Methods of Test for the Determination of the Effects of Immersion in Liquid Chemicals. International Organization of Standardization: Geneva, Switzerland, 2010.
- ASTM D2457; Standard Test Method for Specular Gloss of Plastic Films and Solid Plastics. ASTM International: West Conshohocken, PA, USA, 2013.
- EN ISO 11358-1:2014; Plastics—Thermogravimetry (TG) of Polymers—Part 1: General Principles. International Organization of Standardization: Geneva, Switzerland, 2014.
- ASTM D2583; Standard Test Method for Indentation Hardness of Rigid Plastics by Means of a Barcol Impressor. ASTM International: West Conshohocken, PA, USA, 2013.
- EN ISO 178:2019; Plastics—Determination of Flexural Properties. International Organization of Standardization: Geneva, Switzerland, 2019.
- EN ISO 4892-2:2013; Plastics—Methods of Exposure to Laboratory Light Sources—Part 2: Xenon-arc Lamps. International Organization of Standardization: Geneva, Switzerland, 2013.
- Pradhan, P.; Satapathy, A. Physico-mechanical characterization and thermal property evaluation of polyester composites filled with walnut shell powder. Polym. Polym. Compos. 2020, 30, 09673911221077808. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Guo, Y.; Yue, J. Isolation and Characterization of Nanocellulose with a Novel Shape from Walnut (Juglans regia L.) Shell Agricultural Waste. Polymers 2019, 11, 1130. [Google Scholar] [CrossRef]
- Enache, A.-C.; Samoila, P.; Cojocaru, C.; Apolzan, R.; Predeanu, G.; Harabagiu, V. An Eco-Friendly Modification of a Walnut Shell Biosorbent for Increased Efficiency in Wastewater Treatment. Sustainability 2023, 15, 2704. [Google Scholar] [CrossRef]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Green Composites Based on Unsaturated Polyester Resin from Recycled Poly(Ethylene Terephthalate) with Wood Flour as Filler—Synthesis, Characterization and Aging Effect. Polymers 2020, 12, 2966. [Google Scholar] [CrossRef] [PubMed]
- Bautista, Y.; Gozalbo, A.; Mestre, S.; Sanz, V. Thermal Degradation Mechanism of a Thermostable Polyester Stabilized with an Open-Cage Oligomeric Silsesquioxane. Materials 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Tibiletti, L.; Longuet, C.; Ferry, L.; Coutelen, P.; Mas, A.; Robin, J.J.; Lopez-Cuesta, J.M. Thermal degradation and fire behaviour of unsaturates polyesters filled with metallic oxides. Polym. Degrad. Stab. 2011, 96, 67–75. [Google Scholar] [CrossRef]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Effect of Eco-Friendly Peanut Shell Powder on the Chemical Resistance, Physical, Thermal, and Thermomechanical Properties of Unsaturated Polyester Resin Composites. Polymers 2021, 13, 3690. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef]
- Rayón, E.; Ferrandiz, S.; Rico, I.; López, J.; Arrieta, M.P. Microstructure, Mechanical, and Thermogravimetric Characterization of Cellulosic By-Products Obtained from Biomass Seeds. Int. J. Food Prop. 2015, 18, 1211–1222. [Google Scholar] [CrossRef]
- Singh, P.; Khan, N.; Singh, M. Experimental investigation of mechanical properties of walnut shell particles (WNSP) reinforced polyester composites. Int. J. Emerg. Technol. Innov. Res. 2018, 5, 140–149. [Google Scholar]
- Jacob, J.; Chia, L.H.L.; Boey, F.Y.C. Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Naik, T.P.; Singh, I.; Sharma, A.K. Processing of polymer matrix composites using microwave energy: A review. Compos. A Appl. Sci. Manuf. 2022, 156, 106870. [Google Scholar] [CrossRef]
- Iglesias, J.G.; González-Benito, J.; Aznar, A.J.; Bravo, J.; Baselga, J. Effect of glass fiber surface treatments on mechanical strength of epoxy based composite materials. J. Colloid Interface Sci. 2002, 250, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Choqueuse, D. 12—Ageing of composites in marine vessels. In Ageing of Composites; Martin, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 326–353. [Google Scholar]
- Weitsman, Y.J. Effects of Fluids on Polymeric Composites—A Review. In Comprehensive Composite Materials; Kelly, A., Zweben, C., Eds.; Pergamon: Oxford, UK, 2000; pp. 369–401. [Google Scholar]
- Mouzakis, D.; Zoga, H.; Galiotis, C. Accelerated environmental ageing study of polyester/glass fiber reinforced composites (GFRPCs). Compos. B Eng. 2008, 39, 467–475. [Google Scholar] [CrossRef]
- Beg, M.D.H.; Pickering, K.L. Accelerated weathering of un-bleached and bleached Kraft wood fiber reinforced polypropylene composites. Polym. Degrad. 2008, 93, 1939–1946. [Google Scholar] [CrossRef]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Investigation of Degradation of Composites Based on Unsaturated Polyester Resin and Vinyl Ester Resin. Materials 2022, 15, 1286. [Google Scholar] [CrossRef]
- Nikafshar, S.; Mojgan Nejad, M. Evaluating efficiency of different UV-stabilizers/absorbers in reducing UV-degradation of lignin. Holzforschung 2022, 76, 235–244. [Google Scholar] [CrossRef]


| Sample | (°C) 1 | (°C) 2 | (°C) 3 | (°C) 4 | (%) 5 | (%) 6 |
|---|---|---|---|---|---|---|
| Walnut Shell Powder (WSP) | 75.9 | 197.5 | 290.8 | 66.7; 227.9 277.1; 404.0; 662.6 | −8.20; −10.48; −53.73; −22.10; −2.15 | 3.34 |
| pure UPR | 319.4 | 339.8 | 390.3 | 387.9; 520.4 | −83.30; −14.83 | --- |
| UPR + W2 | 312.5 | 340.3 | 388.2 | 388.1; 494.3 | −82.81; −15.48 | 0.25 |
| UPR + W5 | 308.3 | 337.6 | 387.7 | 390.1; 489.1 | −80.27; −16.57 | 1.36 |
| UPR + W7 | 300.7 | 334.3 | 386.6 | 390.6; 473.2 | −77.73; −18.15 | 1.81 |
| UPR + W10 | 292.2 | 329.8 | 385.4 | 391.1; 470.8 | −76.80; −19.54 | 2.27 |
| Sample | Optical Properties at Wavelength: | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 350 nm | 450 nm | 550 nm | 650 nm | 750 nm | 850 nm | 950 nm | ||||||||
| T 1 | A 2 | T | A | T | A | T | A | T | A | T | A | T | A | |
| pure UPR | 0.446 | 2.398 | 55.327 | 0.258 | 69.177 | 0.161 | 76.846 | 0.116 | 82.782 | 0.083 | 84.245 | 0.076 | 85.078 | 0.072 |
| UPR + W2 | --- | 4.000 | 3.828 | 1.468 | 10.521 | 1.019 | 19.865 | 0.738 | 27.855 | 0.588 | 32.706 | 0.517 | 36.015 | 0.475 |
| UPR + W5 | --- | 4.000 | 0.058 | 3.217 | 0.627 | 2.200 | 2.434 | 1.613 | 4.884 | 1.311 | 6.950 | 1.158 | 8.569 | 1.068 |
| UPR + W7 | --- | 4.000 | --- | 4.000 | 0.063 | 3.264 | 0.543 | 2.318 | 1.549 | 1.854 | 2.571 | 1.629 | 3.444 | 1.500 |
| UPR + W10 | --- | 4.000 | --- | 4.000 | --- | 4.000 | 0.064 | 3.211 | 0.273 | 2.587 | 0.545 | 2.289 | 0.790 | 2.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pączkowski, P. Properties of Eco-Friendly Composites Based on Post-Consumer Recycled Resin Filled with Walnut Shell Powder. Polymers 2023, 15, 4389. https://doi.org/10.3390/polym15224389
Pączkowski P. Properties of Eco-Friendly Composites Based on Post-Consumer Recycled Resin Filled with Walnut Shell Powder. Polymers. 2023; 15(22):4389. https://doi.org/10.3390/polym15224389
Chicago/Turabian StylePączkowski, Przemysław. 2023. "Properties of Eco-Friendly Composites Based on Post-Consumer Recycled Resin Filled with Walnut Shell Powder" Polymers 15, no. 22: 4389. https://doi.org/10.3390/polym15224389
APA StylePączkowski, P. (2023). Properties of Eco-Friendly Composites Based on Post-Consumer Recycled Resin Filled with Walnut Shell Powder. Polymers, 15(22), 4389. https://doi.org/10.3390/polym15224389






