Influence of Alginate Properties and Calcium Chloride Concentration on Alginate Bead Reticulation and Size: A Phenomenological Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alginate Characterization
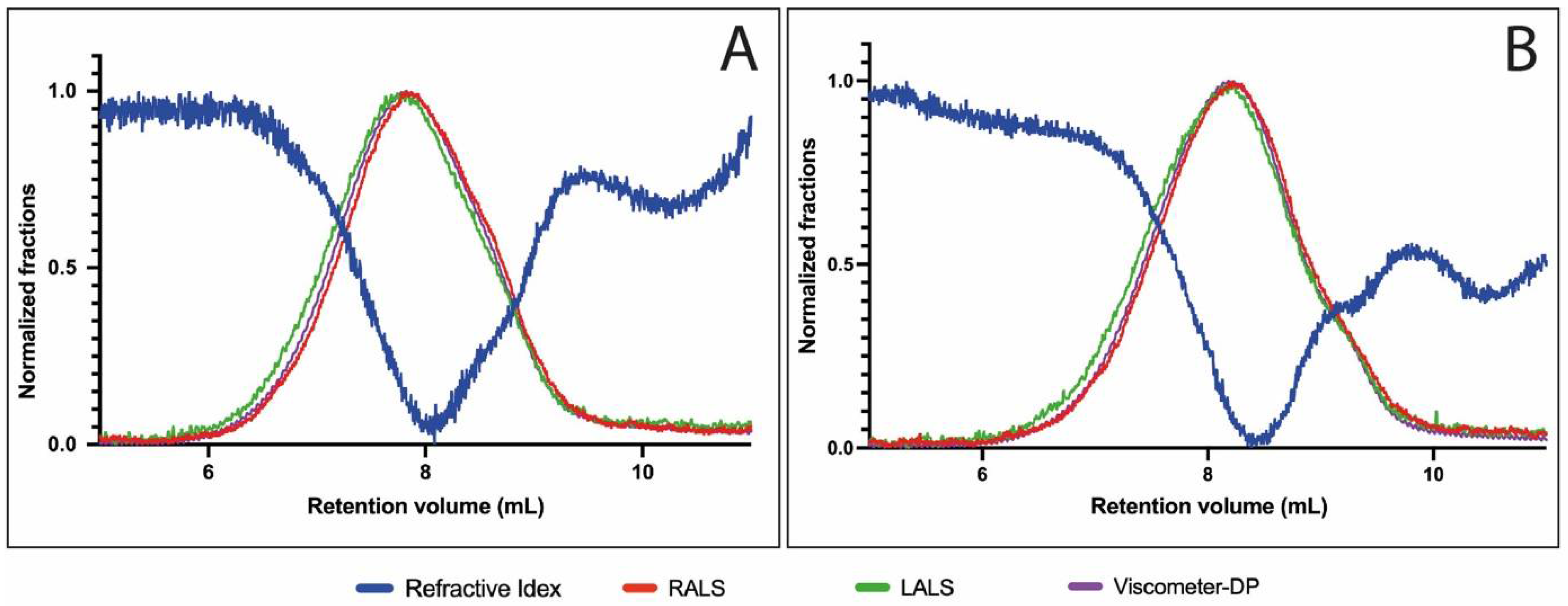
2.2.1. Molecular Weight with Size-Exclusion Chromatography (SEC) Coupled to Multi-Detectors
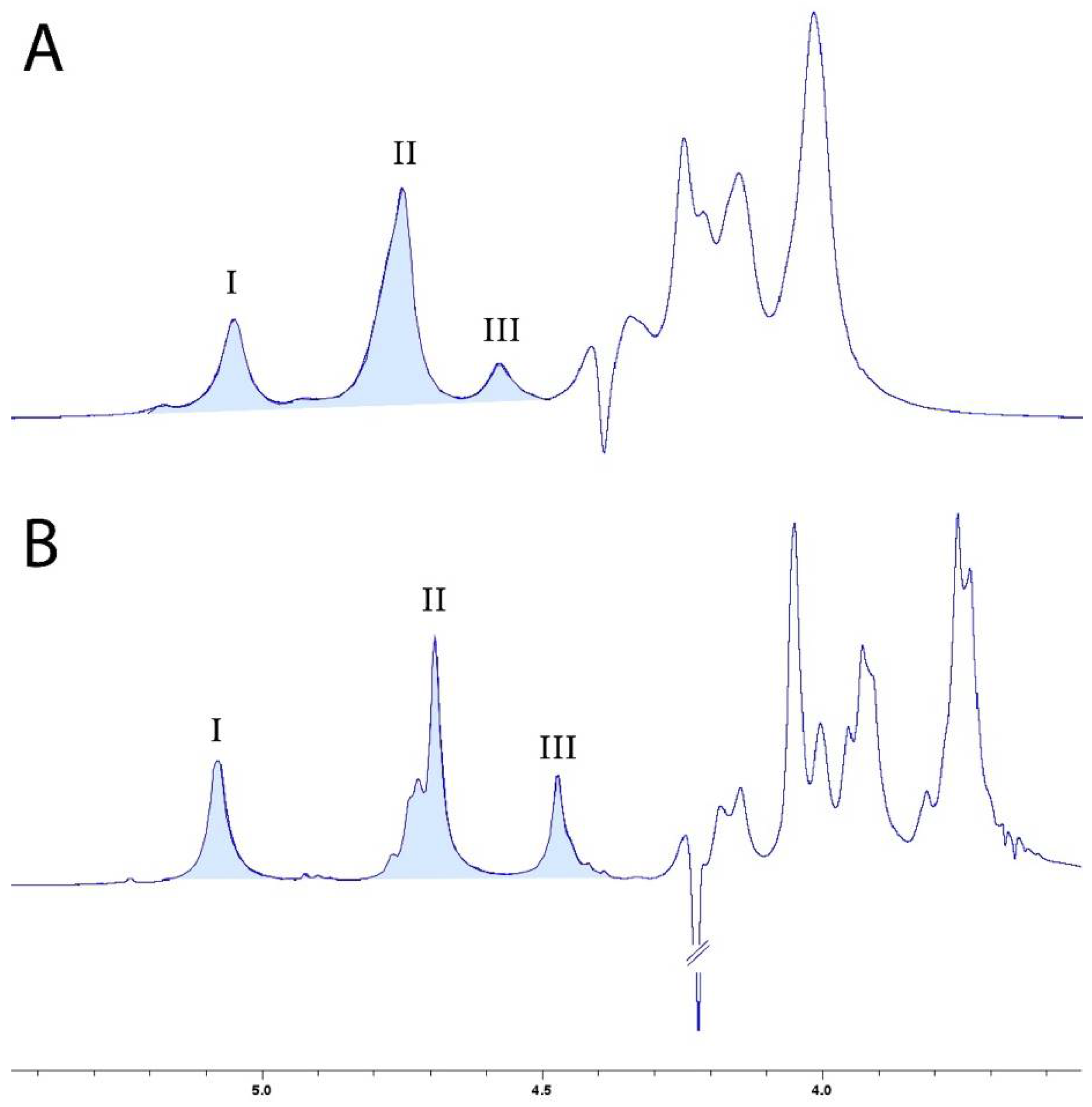
2.2.2. M/G Ratio with Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3. Preparation of Alginate Solution
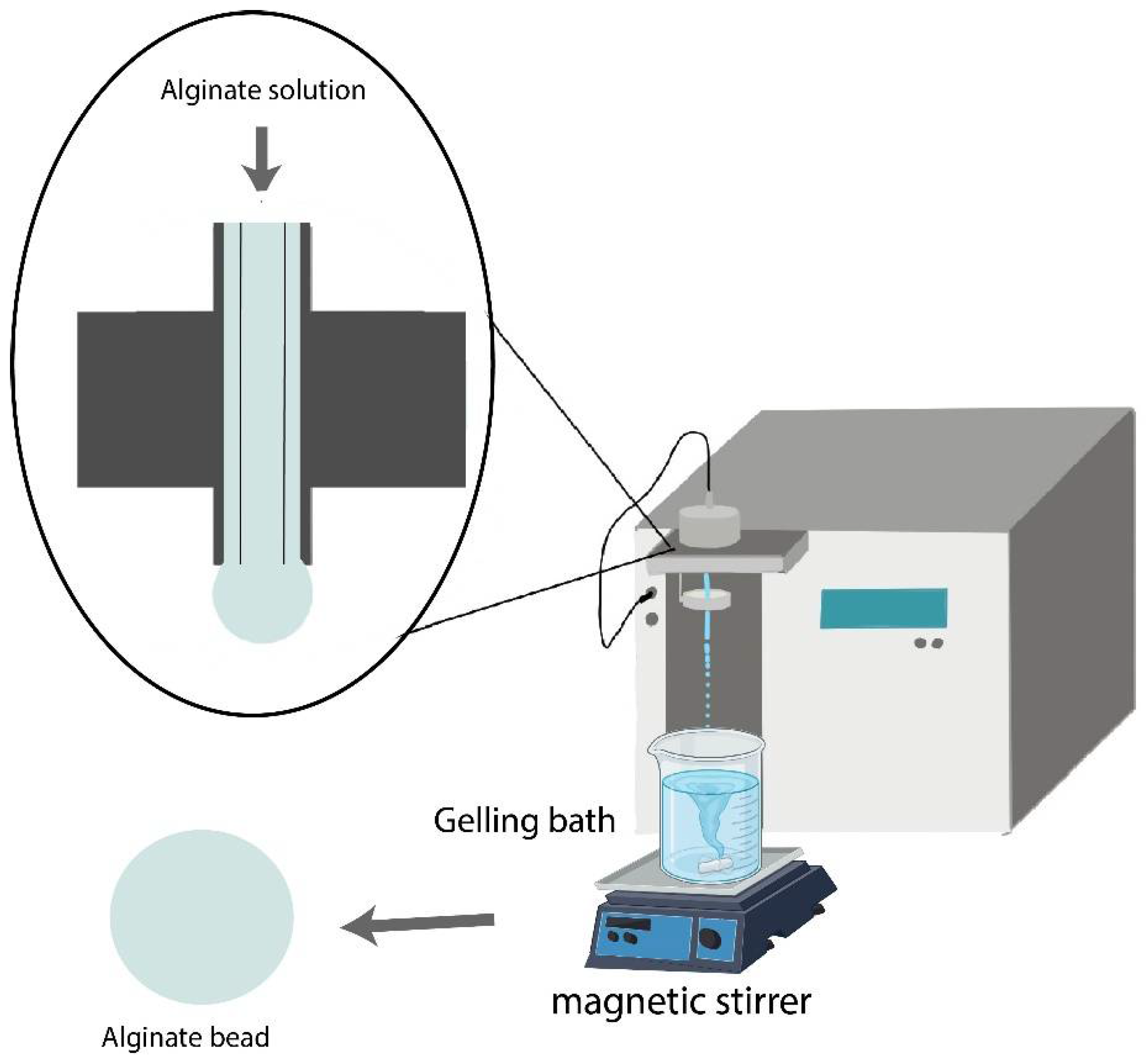
2.4. Preparation of Alginate Beads
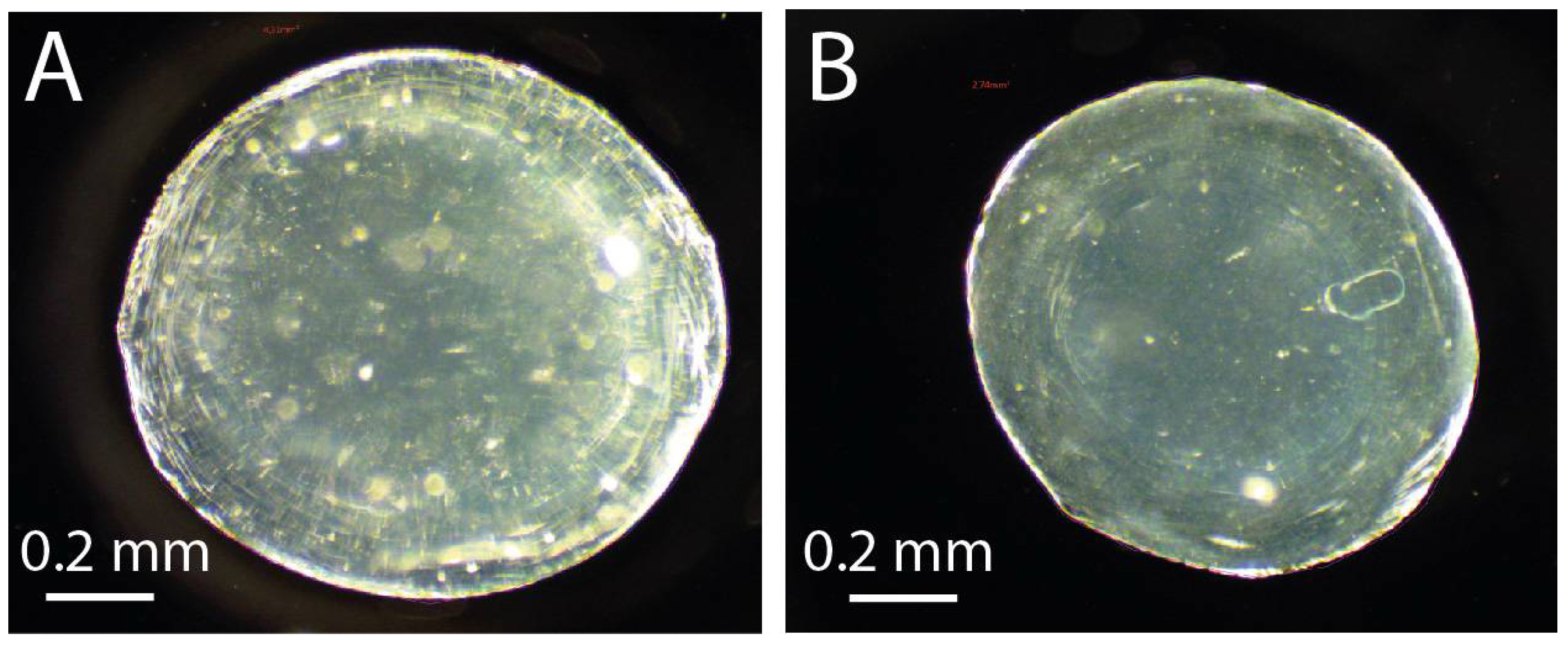
2.5. Bead Size Measurement
2.6. Experimental Design
2.7. Statistics
3. Results and Discussion
3.1. Alginate Characterization
3.2. Influence of Process Variables on AlgLF Beads
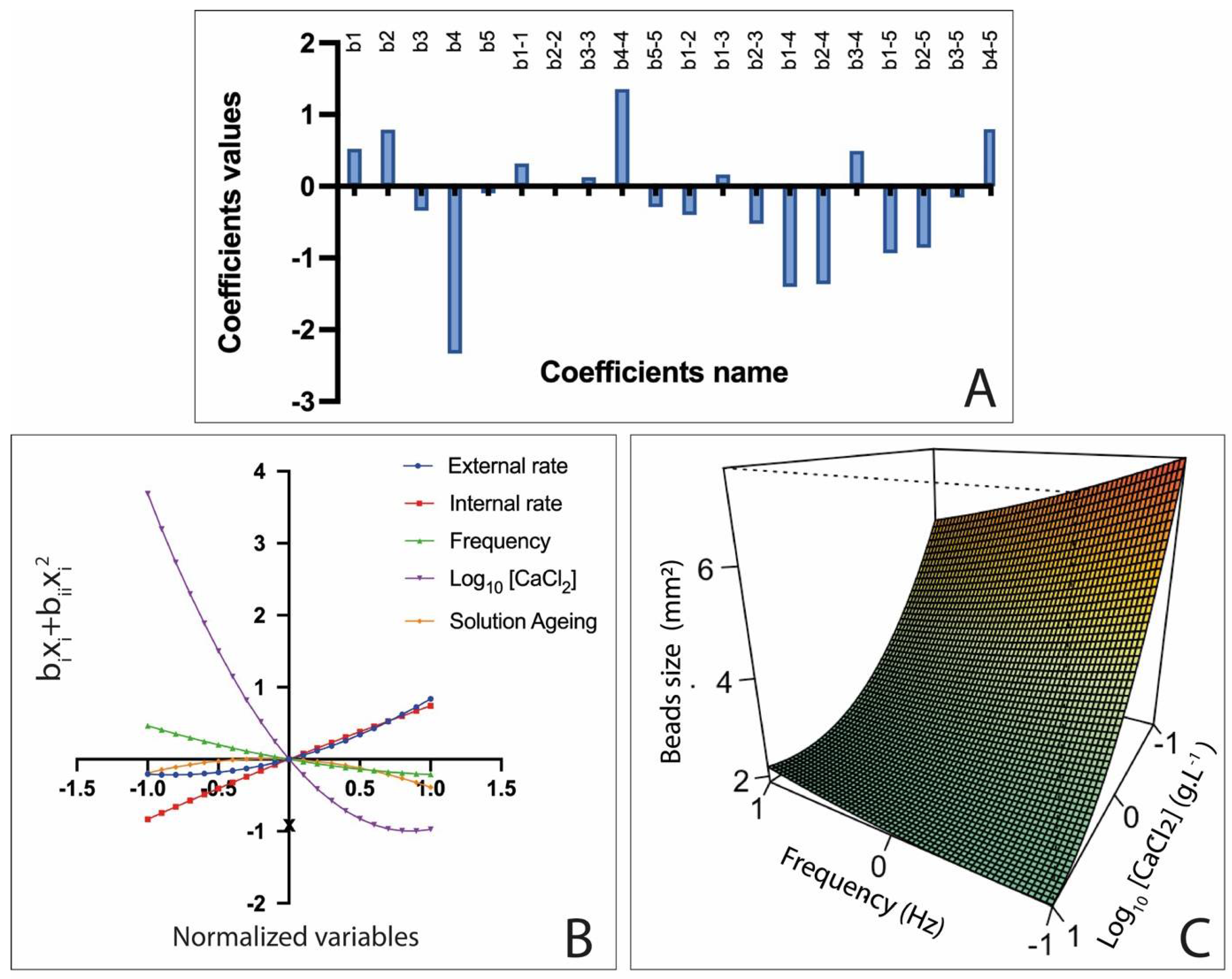
3.3. Influence of Variables on AlgP Beads
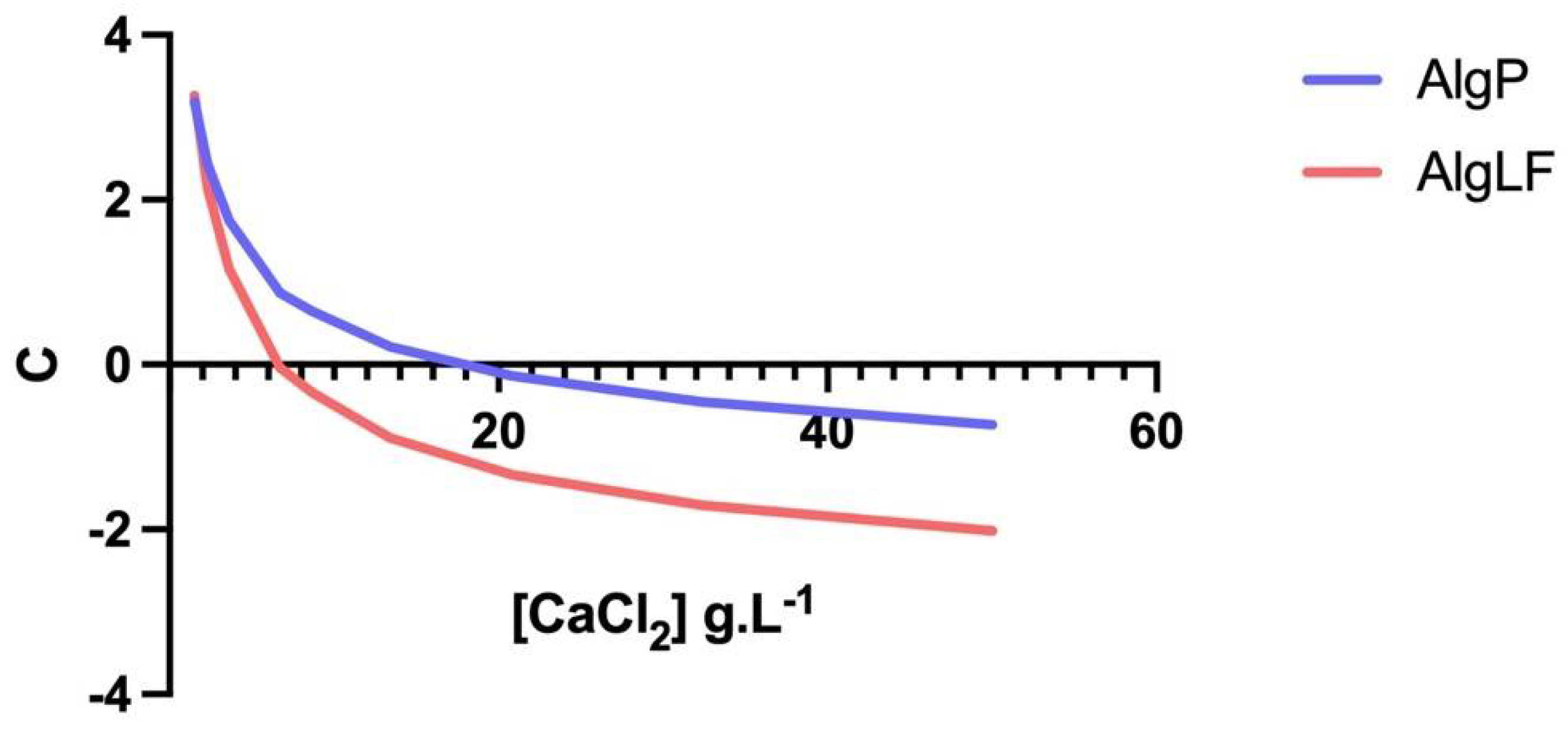
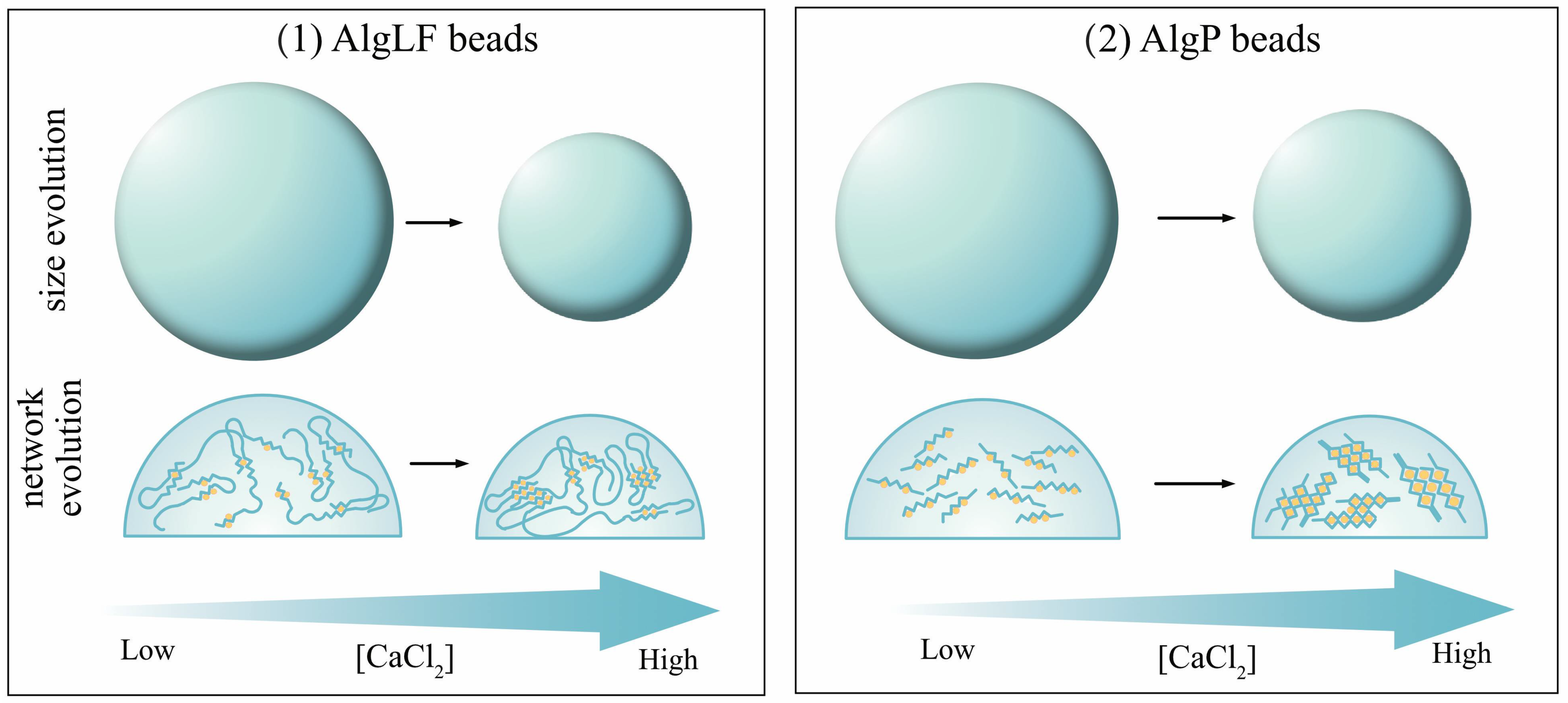
3.4. Alginate Characteristics’ Impact on Gelation
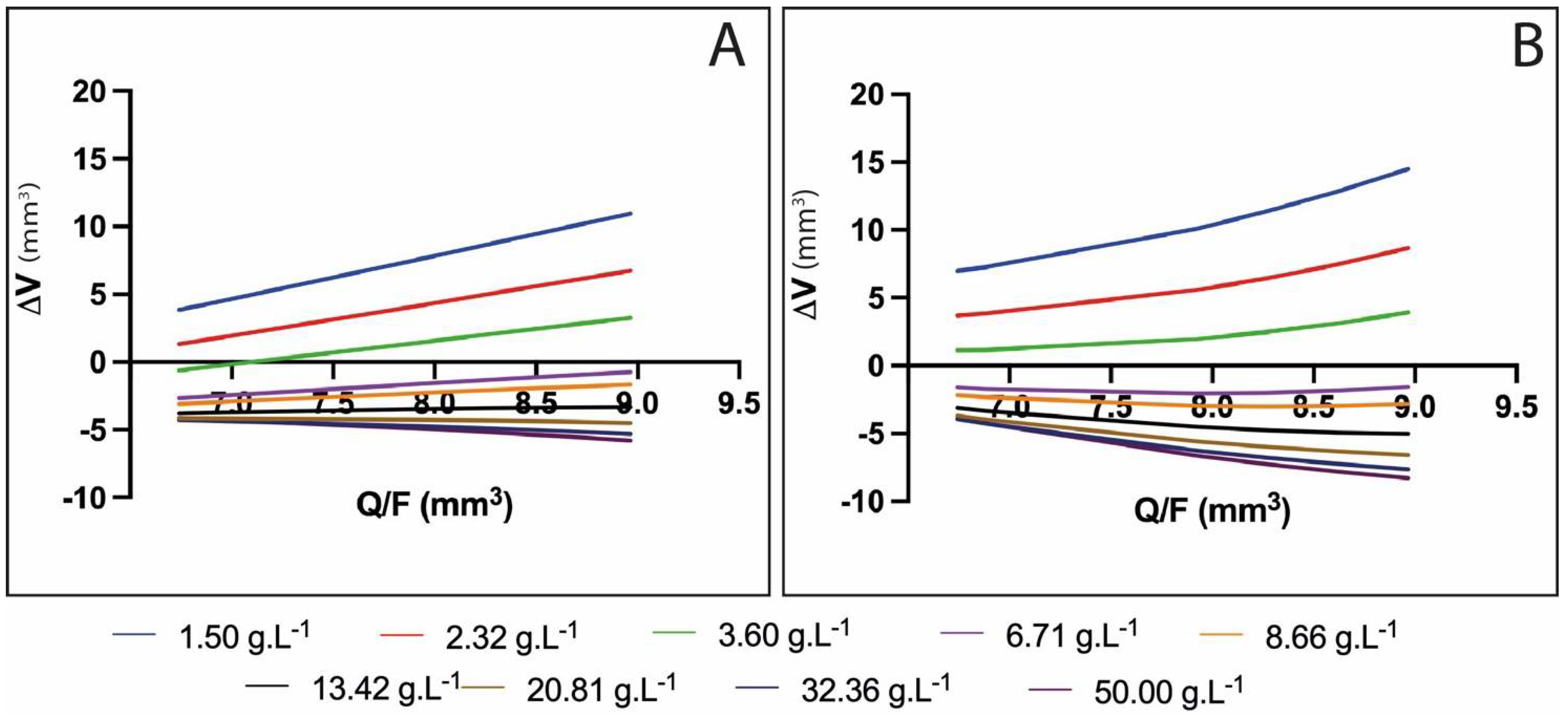
- For the lowest concentrations (below 3.6 g/m2), ΔV ≥ 0 was measured for all flow rates. This means that no significant shrinking occurred when the drop penetrated into the calcium solution. Due to the low ionic gradient between the internal water and calcium solution, no significant water release was observed and low and light reticulation occurred. In all cases, the slopes of the draws were positive (C > 0).
- For CaCl2 concentrations between 6.71 and 13.42 g/m2, significant water release and shrinking was observed, leading to V < 0 for all Q/F values. Nevertheless, the bead volume increased with (Q/F), due to the lower relative shrinkage for larger beads. The slopes of the draws were still positive (C > 0) but, as the CaCl2 concentration rose, the slope was reduced, corresponding to fewer beads growing with (Q/F). This means that a denser network was formed as more Ca2+ ions penetrated the alginate (enhancing reticulation, contracting the alginate network, and reducing the bead size).
- For excessive CaCl2 concentrations (above 13.42 g/m2), Ca2+ supersaturation occurred, leading to repulsion between alginate chains, the large release of water from the capsule and free alginate dissolution into the surrounding solution (erosion).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Ding, M.; Zhang, T.; Wu, T.; Qiao, R.; Zhang, F.; Wang, X.; Zhong, J. Electrospraying Technique and Its Recent Application Advances for Biological Macromolecule Encapsulation of Food Bioactive Substances. Food Rev. Int. 2022, 38, 566–588. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.d.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic Emulsification: An Overview on the Preparation of Different Emulsifiers-Stabilized Emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of Encapsulates and Their Relations with Encapsulation Efficiency and Controlled Release of Bioactive Constituents: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef] [PubMed]
- Prabha, K.; Ghosh, P.; Abdullah, S.; Joseph, R.M.; Krishnan, R.; Rana, S.S.; Pradhan, R.C. Recent Development, Challenges, and Prospects of Extrusion Technology. Future Foods 2021, 3, 100019. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L. Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies. Mar. Drugs 2023, 21, 235. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.S.; Pushpamalar, J.; Choo, W.S. Advances in Extrusion-Dripping Encapsulation of Probiotics and Omega-3 Rich Oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and Delivery of Bioactive Citrus Pomace Polyphenols: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Grasdalen, H.; Larsen, B.; Smisrod, O. 13C-n.m.r. Studies of Monomeric Composition and Sequence in Alginate. Carbohydr. Res. 1981, 89, 179–191. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of Alginates from Ghanaian Brown Seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Jeoh, T.; Wong, D.E.; Strobel, S.A.; Hudnall, K.; Pereira, N.R.; Williams, K.A.; Arbaugh, B.M.; Cunniffe, J.C.; Scher, H.B. How Alginate Properties Influence in Situ Internal Gelation in Crosslinked Alginate Microcapsules (CLAMs) Formed by Spray Drying. PLoS ONE 2021, 16, e0247171. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and Microstructural Evolution of Alginate Beads in Simulated Gastrointestinal Fluids. Impact of M/G Ratio, Molecular Weight and PH. Carbohydr. Polym. 2019, 223, 115121. [Google Scholar] [CrossRef]
- Syanda, A.M.; Kringstad, V.I.; Blackford, S.J.I.; Kjesbu, J.S.; Ng, S.S.; Ma, L.; Xiao, F.; Coron, A.E.; Rokstad, A.M.A.; Modi, S.; et al. Sulfated Alginate Reduces Pericapsular Fibrotic Overgrowth on Encapsulated CGMP-Compliant HPSC-Hepatocytes in Mice. Front. Bioeng. Biotechnol. 2022, 9, 816542. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Larsen, F.H.; Steuernagel, S.; Engelsen, S.B. Direct Quantification of M/G Ratio from 13C CP-MAS NMR Spectra of Alginate Powders by Multivariate Curve Resolution. Carbohydr. Res. 2009, 344, 2014–2022. [Google Scholar] [CrossRef]
- Niizawa, I.; Espinaco, B.Y.; Zorrilla, S.E.; Sihufe, G.A. Natural Astaxanthin Encapsulation: Use of Response Surface Methodology for the Design of Alginate Beads. Int. J. Biol. Macromol. 2019, 121, 601–608. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Linder, M.; Khanji, A.N.; Probst, L.; Desobry, S. Study and Optimization of Core-Shell Capsules Produced by Annular Jet Breaking Coextrusion. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127475. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; Landín, M.; Altai, A.; Russo, P.; Aquino, R.P.; Del Gaudio, P. A Novel Method for the Production of Core-Shell Microparticles by Inverse Gelation Optimized with Artificial Intelligent Tools. Int. J. Pharm. 2018, 538, 97–104. [Google Scholar] [CrossRef]
- Solberg, A.; Draget, K.I.; Schatz, C.; Christensen, B.E. Alginate Blocks and Block Polysaccharides: A Review. Macromol. Symp. 2023, 408, 2200072. [Google Scholar] [CrossRef]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Vicente, A.A. Effect of Alginate Molecular Weight and M/G Ratio in Beads Properties Foreseeing the Protection of Probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef]
- Anani, J.; Noby, H.; Zkria, A.; Yoshitake, T.; Elkady, M. Monothetic Analysis and Response Surface Methodology Optimization of Calcium Alginate Microcapsules Characteristics. Polymers 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, A. Multiple Steps and Critical Behaviors of the Binding of Calcium to Alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef]


| Factor | Name | Unit | Low-Level Value | High-Level Value | Number of Levels |
|---|---|---|---|---|---|
| X1 | External flow rate | mL·min−1 | 30 | 50 | 5 |
| X2 | Internal flow rate | mL·min−1 | 4 | 18 | 7 |
| X3 | Frequency | Hz | 80 | 200 | 7 |
| X4 | Log10 [CaCl2] | g·L−1 | 0.176 | 1.699 | 7 |
| X5 | Solution aging | Hours | 24 | 72 | 3 |
| Log10 [CaCl2] | [CaCl2] g·L−1 |
|---|---|
| 0.176 | 1.50 |
| 0.328 | 2.12 |
| 0.785 | 6.09 |
| 0.937 | 8.66 |
| 1.089 | 12.29 |
| 1.546 | 35.21 |
| 1.699 | 50.00 |
| Sample | Composition, Fraction | Doublet Frequencies | M/G | ||||
|---|---|---|---|---|---|---|---|
| FG | FM | FGG | FGM | FMM | FMG | ||
| AlgLF | 0.30 | 0.70 | 0.11 | 0.18 | 0.52 | 0.18 | 2.35 |
| AlgP | 0.37 | 0.63 | 0.29 | 0.07 | 0.56 | 0.07 | 1.72 |
| Response | Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Significance |
|---|---|---|---|---|---|---|
| Bead size (Area) | Regression | 47.9642 | 20 | 2.3982 | 3.735 | 0.920 ** |
| Residual | 8.3476 | 13 | 0.6421 | |||
| Lack-of-fit | 6.2833 | 10 | 0.6283 | 0.913 | 6.04 | |
| Pure error | 2.0643 | 3 | 0.6881 | |||
| Total | 56.3118 | 33 |
| Coefficient | Standard Error | t-Value | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| AlgLF | AlgP | AlgLF | AlgP | AlgLF | AlgP | AlgLF | AlgP | |
| b0 | 2.893 | 3.133 | 0.401 | 0.227 | 7.22 | 13.81 | <0.01 *** | <0.01 *** |
| b1 | 0.523 | 0.848 | 0.327 | 0.185 | 1.60 | 4.58 | 13.4 | 0.0514 *** |
| b2 | 0.788 | 0.758 | 0.327 | 0.185 | 2.41 | 4.10 | 3.16 * | 0.126 ** |
| b3 | −0.338 | −0.480 | 0.327 | 0.185 | −1.03 | −2.59 | 32.1 | 2.22 * |
| b4 | −2.330 | −1.914 | 0.327 | 0.185 | −7.12 | −10.34 | <0.01 *** | <0.01 *** |
| b5 | −0.098 | 0.253 | 0.327 | 0.185 | −0.30 | 1.37 | 76.9 | 19.5 |
| b1-1 | 0.317 | −0.098 | 0.694 | 0.393 | 0.46 | −0.25 | 65.5 | 80.8 |
| b2-2 | −0.046 | 0.306 | 0.694 | 0.393 | −0.07 | 0.78 | 94.8 | 45.0 |
| b3-3 | 0.128 | −0.253 | 0.654 | 0.370 | 0.20 | −0.68 | 84.8 | 50.6 |
| b4-4 | 1.358 | 1.199 | 0.621 | 0.351 | 2.19 | 3.41 | 4.75 * | 0.462 ** |
| b5-5 | −0.290 | 0.557 | 0.594 | 0.336 | −0.49 | 1.66 | 63.3 | 12.2 |
| b1-2 | −0.398 | 0.306 | 0.925 | 0.524 | −0.43 | 0.58 | 67.4 | 56.9 |
| b1-3 | 0.165 | −0.451 | 1.035 | 0.585 | 0.16 | −0.77 | 87.5 | 45.5 |
| b2-3 | −0.524 | 0.638 | 1.034 | 0.585 | −0.51 | 1.09 | 62.1 | 29.6 |
| b1-4 | −1.402 | −0.855 | 1.075 | 0.608 | −1.30 | −1.41 | 21.5 | 18.3 |
| b2-4 | −1.363 | −0.375 | 1.075 | 0.608 | −1.27 | −0.62 | 22.7 | 54.8 |
| b3-4 | 0.494 | 0.747 | 1.075 | 0.608 | 0.46 | 1.23 | 65.3 | 24.1 |
| b1-5 | −0.932 | 0.522 | 1.095 | 0.620 | −0.85 | 0.84 | 41.0 | 41.5 |
| b2-5 | −0.856 | −1.018 | 1.095 | 0.620 | −0.78 | −1.64 | 44.8 | 12.4 |
| b3-5 | −0.158 | −1.261 | 1.095 | 0.620 | −0.14 | −2.03 | 88.8 | 6.3 |
| b4-5 | 0.799 | −0.100 | 1.095 | 0.620 | 0.73 | −0.16 | 47.8 | 87.5 |
| Response | Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Significance |
|---|---|---|---|---|---|---|
| Bead size (Area) | Regression | 38.2615 | 20 | 1.9131 | 9.301 | <0.01 *** |
| Residual | 2.6738 | 13 | 0.2057 | |||
| Lack-of-fit | 2.5397 | 10 | 0.2540 | 5.683 | 9.0 | |
| Pure error | 0.1341 | 3 | 0.0447 | |||
| Total | 40.9352 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennacef, C.; Desobry, S.; Jasniewski, J.; Leclerc, S.; Probst, L.; Desobry-Banon, S. Influence of Alginate Properties and Calcium Chloride Concentration on Alginate Bead Reticulation and Size: A Phenomenological Approach. Polymers 2023, 15, 4163. https://doi.org/10.3390/polym15204163
Bennacef C, Desobry S, Jasniewski J, Leclerc S, Probst L, Desobry-Banon S. Influence of Alginate Properties and Calcium Chloride Concentration on Alginate Bead Reticulation and Size: A Phenomenological Approach. Polymers. 2023; 15(20):4163. https://doi.org/10.3390/polym15204163
Chicago/Turabian StyleBennacef, Chanez, Stéphane Desobry, Jordane Jasniewski, Sébastien Leclerc, Laurent Probst, and Sylvie Desobry-Banon. 2023. "Influence of Alginate Properties and Calcium Chloride Concentration on Alginate Bead Reticulation and Size: A Phenomenological Approach" Polymers 15, no. 20: 4163. https://doi.org/10.3390/polym15204163
APA StyleBennacef, C., Desobry, S., Jasniewski, J., Leclerc, S., Probst, L., & Desobry-Banon, S. (2023). Influence of Alginate Properties and Calcium Chloride Concentration on Alginate Bead Reticulation and Size: A Phenomenological Approach. Polymers, 15(20), 4163. https://doi.org/10.3390/polym15204163







