Iodine and Nickel Ions Adsorption by Conjugated Copolymers Bearing Repeating Units of Dicyclopentapyrenyl and Various Thiophene Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
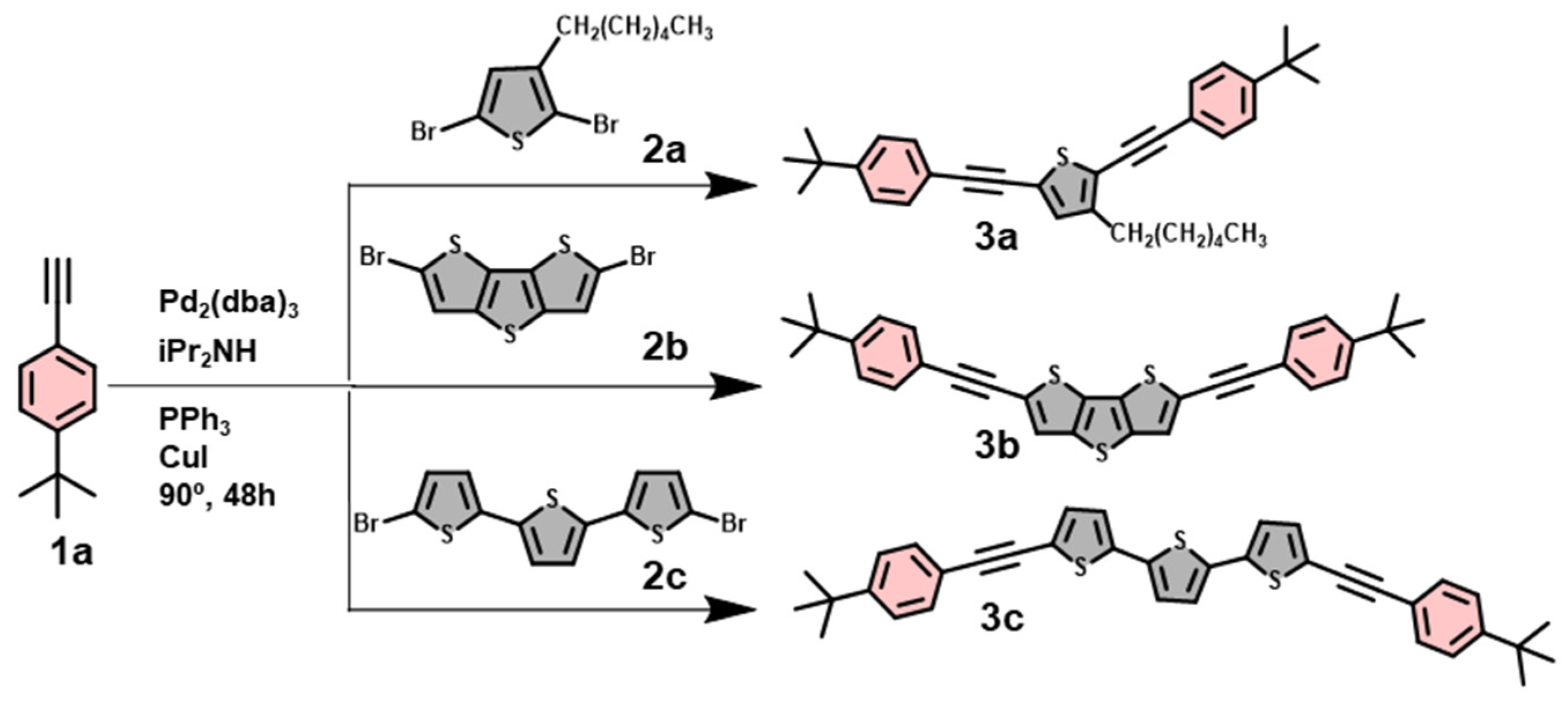
2.1.1. Synthesis of 3a (Procedure A)
2.1.2. Synthesis of 3b
2.1.3. Synthesis of 3c
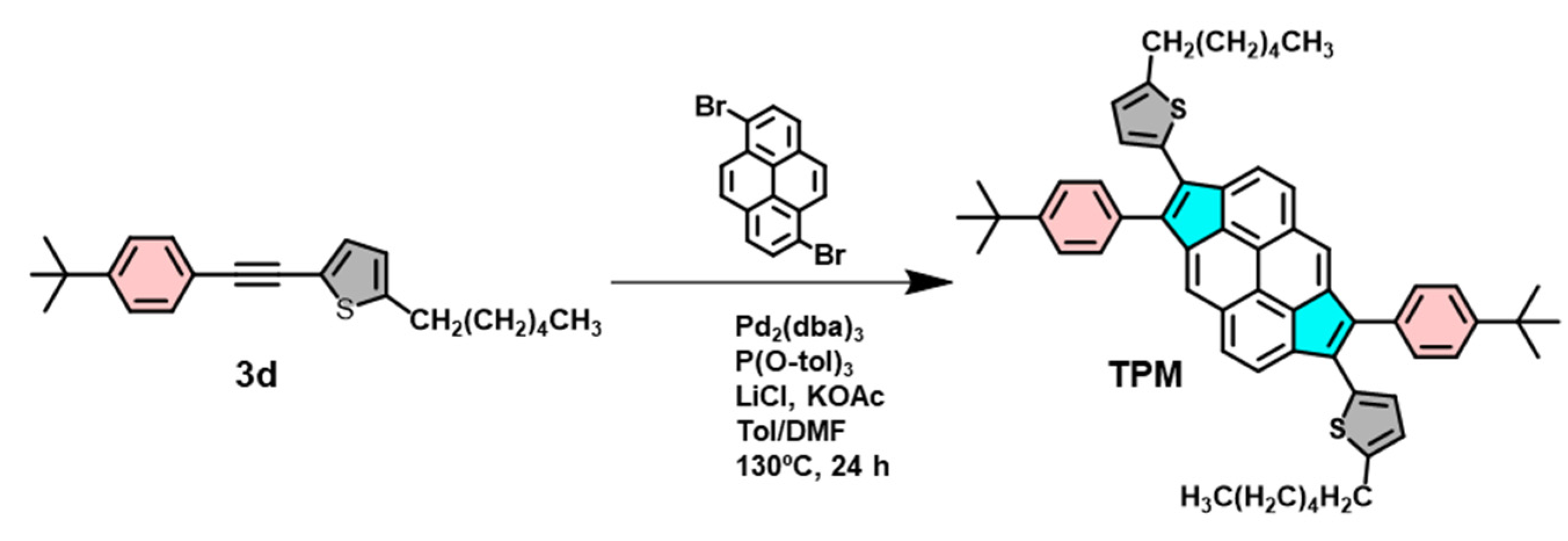
2.1.4. Synthesis of 3d
2.1.5. Synthesis of TPM
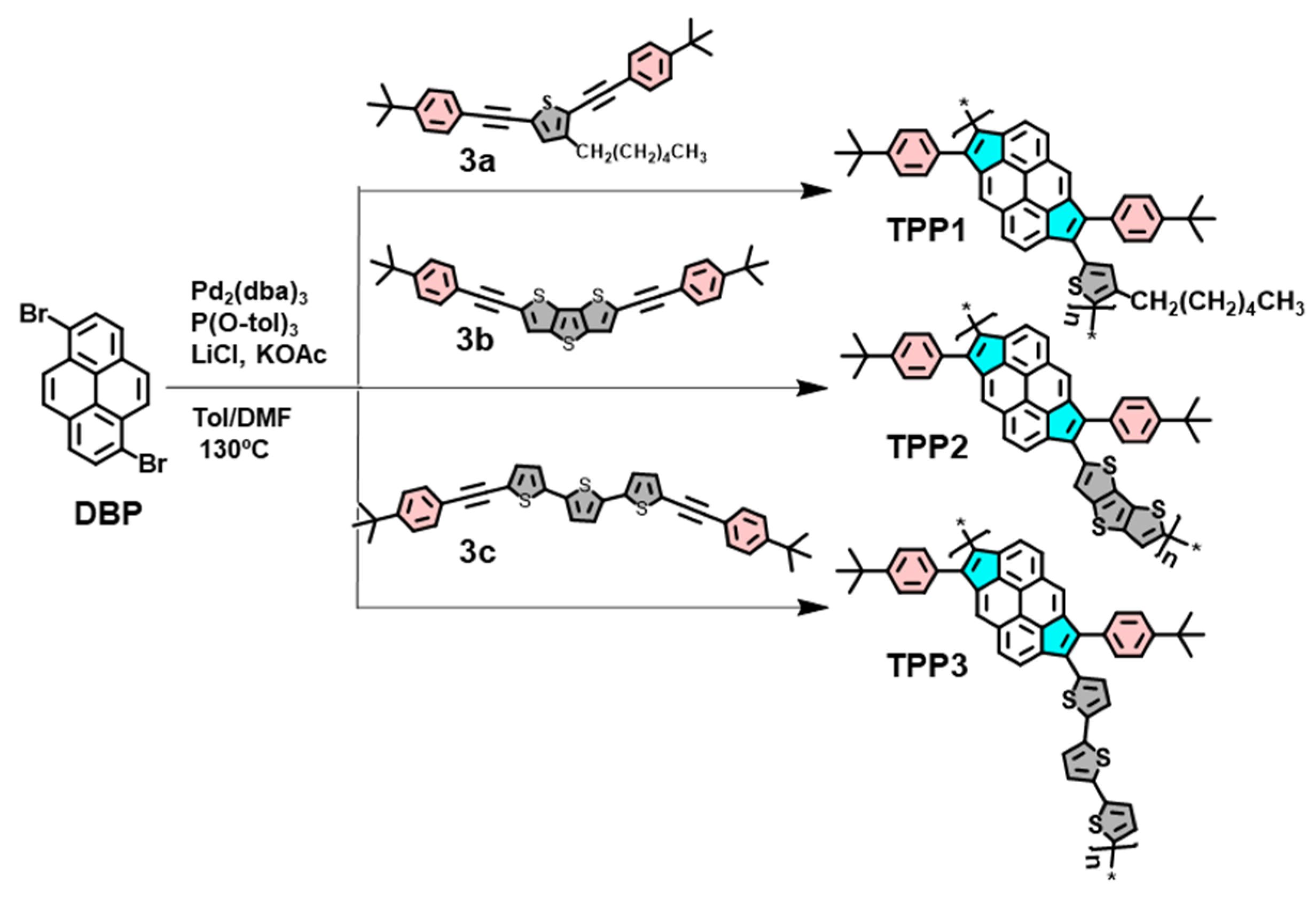
2.1.6. Synthesis of TPP1 (Procedure B)
2.1.7. Synthesis of TPP2
2.1.8. Synthesis of TPP3
2.2. Iodine Uptake Studies of TPP1–3
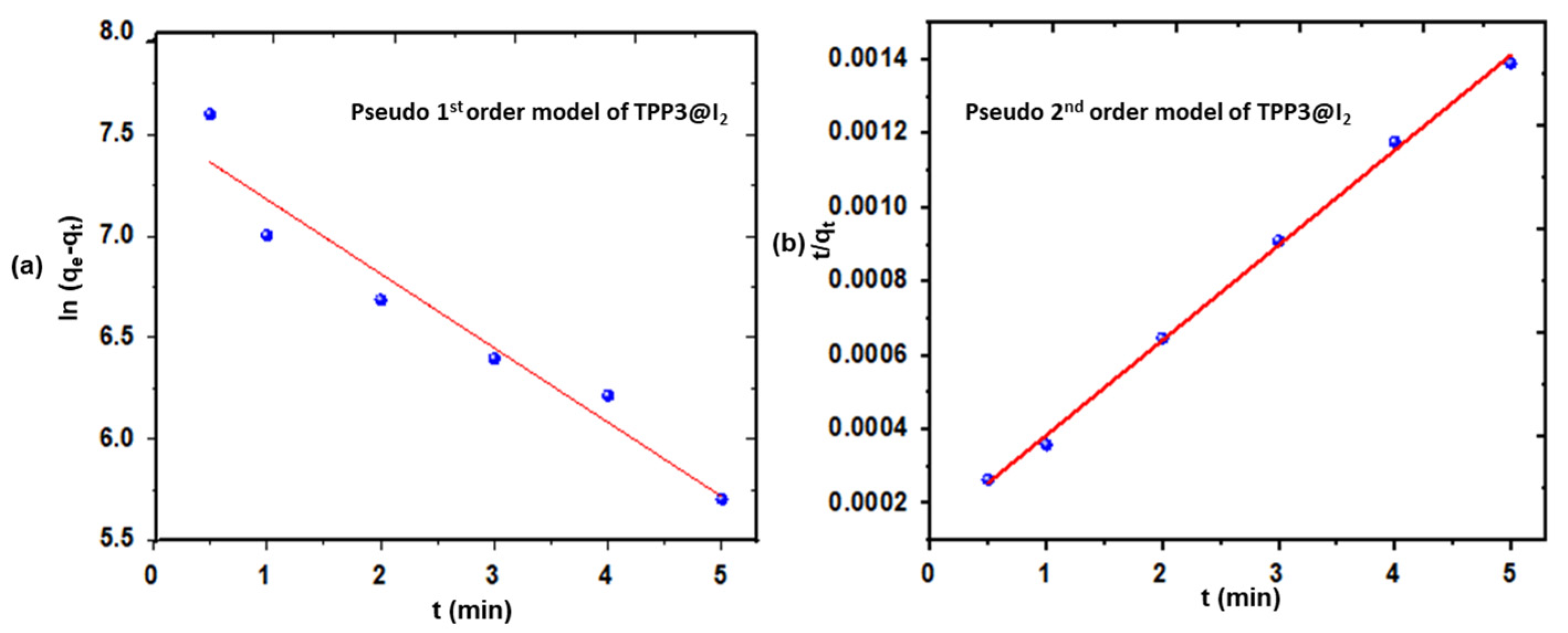
Adsorption Equilibrium and Kinetics
2.3. Nickel Ion Adsorption Studies of TPP1–3
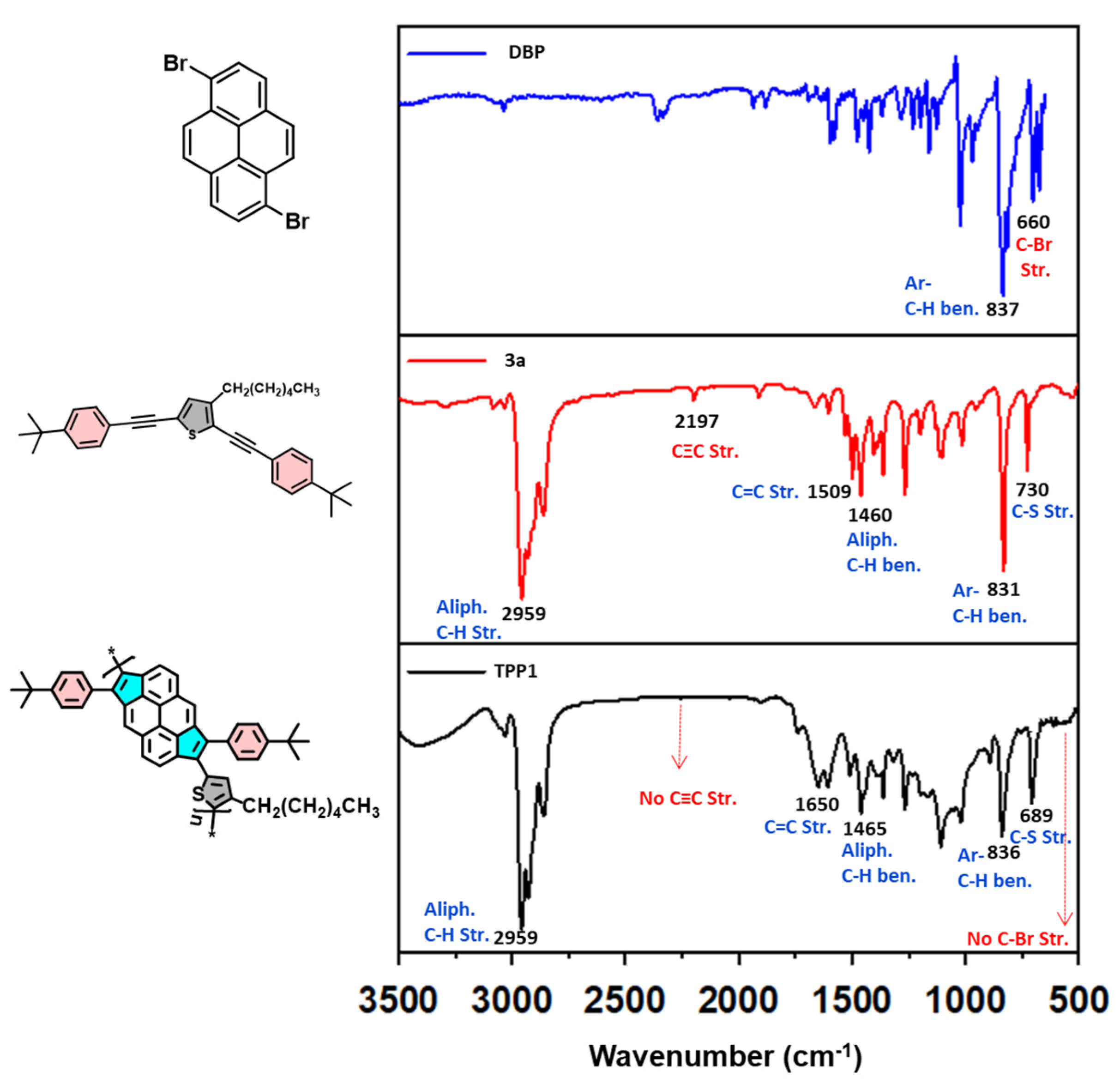
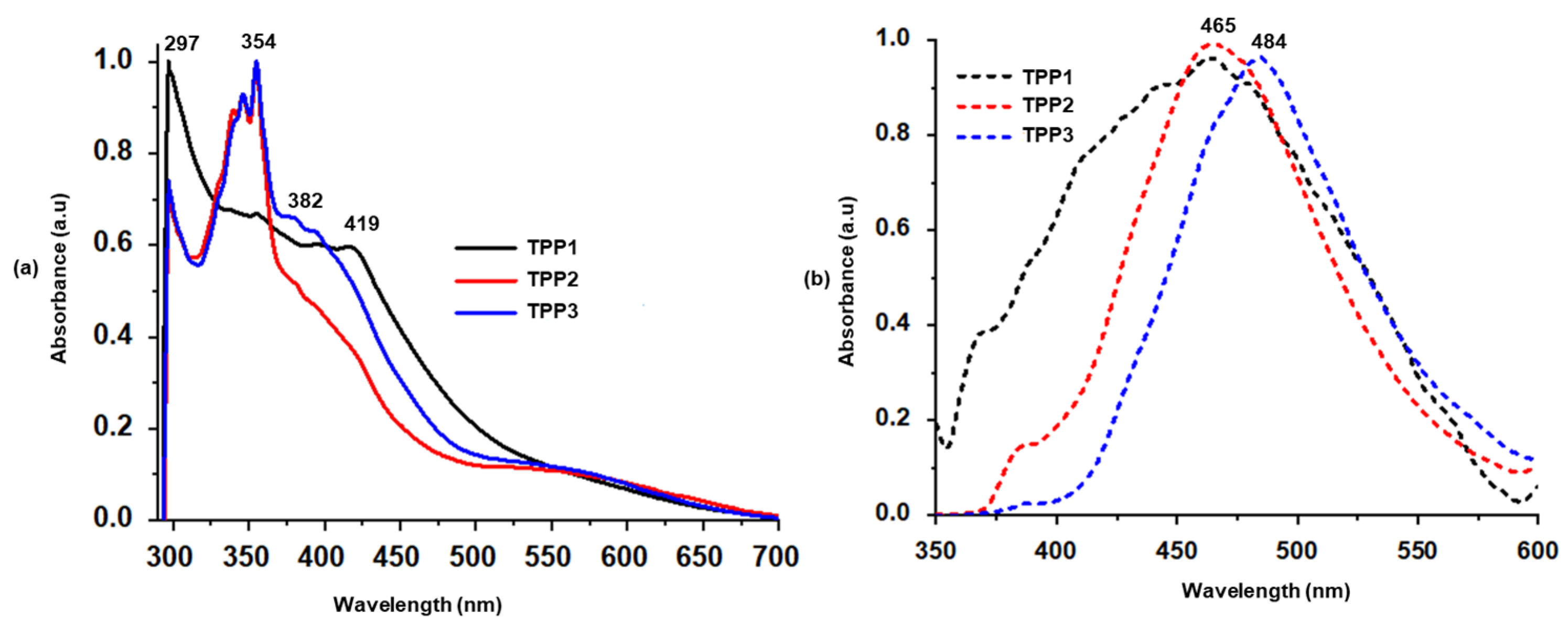
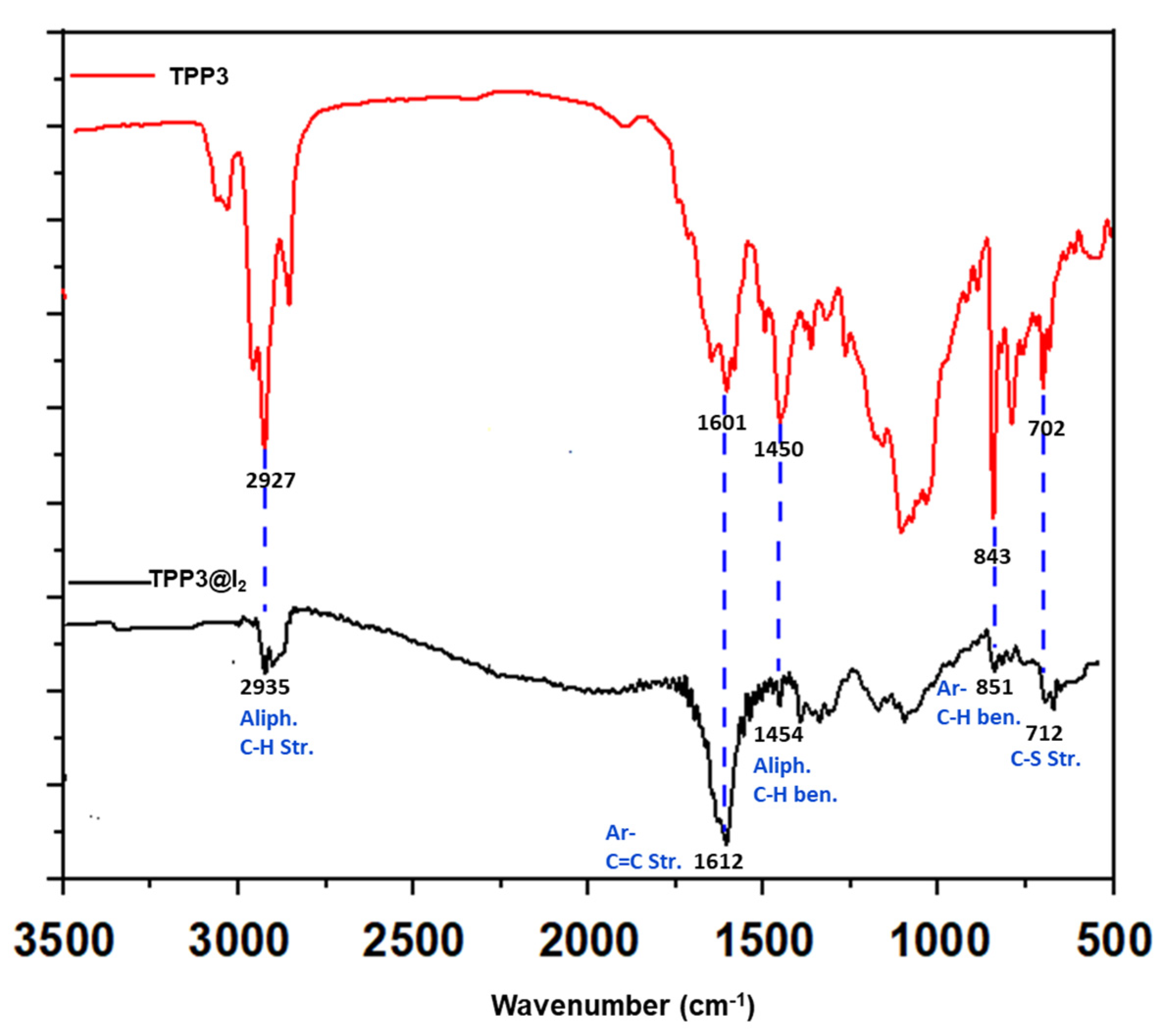
3. Results and Discussion
4. Surface Area and Porosity Analysis
5. Iodine Uptake
6. Nickel Ion Adsorption
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bheemireddy, S.R.; Hautzinger, M.P.; Li, T.; Lee, B.; Plunkett, K.N. Conjugated Ladder Polymers by a Cyclopentannulation Polymerization. J. Am. Chem. Soc. 2017, 139, 5801–5807. [Google Scholar] [CrossRef] [PubMed]
- Kukhta, N.A.; Luscombe, C.K. Gaining control over conjugated polymer morphology to improve the performance of organic electronics. Chem. Commun. 2022, 58, 6982–6997. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, W.; Szűcs, R.; Pascal, S.; Mocanu, A.; Bouit, P.A.; Nyulászi, L.; Hissler, M. Synthesis and electronic properties of polycyclic aromatic hydrocarbons doped with phosphorus and sulfur. Dalton Trans. 2016, 45, 1896–1903. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Qiao, Y.; Zhou, G. B2N2-Embedded Polycyclic Aromatic Hydrocarbons with Furan and Thiophene Derivatives Functionalized in Crossed Directions. Chem. Eur. J. 2019, 25, 9326–9338. [Google Scholar] [CrossRef]
- Ali, R.; Siddiqui, R. Dithieno[3,2-b:2′,3′-d]thiophene (DTT): An emerging heterocyclic building block for future organic electronic materials & functional supramolecular chemistry. RSC Adv. 2022, 12, 36073–36102. [Google Scholar] [CrossRef]
- Aumaitre, C.; Morin, J.-F. Polycyclic Aromatic Hydrocarbons as Potential Building Blocks for Organic Solar Cells. Chem. Rec. 2019, 19, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Ubaldo, P.; Colley, N.; Plunkett, K.; Wang, L. Electron Acceptability of Cyclopenta-Fused Polycyclic Aromatic Hydrocarbons: Effect of One Electron. 2022. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/63752a1556c6f44aab7059cb (accessed on 16 June 2023).
- Wagner, J.; Zimmermann Crocomo, P.; Kochman, M.A.; Kubas, A.; Data, P.; Lindner, M. Modular Nitrogen-Doped Concave Polycyclic Aromatic Hydrocarbons for High-Performance Organic Light-Emitting Diodes with Tunable Emission Mechanisms. Angew. Chem. Int. Ed. 2022, 61, e202202232. [Google Scholar] [CrossRef]
- Rescifina, A.; Chiacchio, U.; Corsaro, A.; Piperno, A.; Romeo, R. Isoxazolidinyl polycyclic aromatic hydrocarbons as DNA-intercalating antitumor agents. Eur. J. Med. Chem. 2011, 46, 129–136. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Bargakshatriya, R.; Pramanik, S.K.; Alameddine, B. High Uptake of the Carcinogenic Pararosaniline Hydrochloride Dye from Water Using Carbazole-Containing Conjugated Copolymers Synthesized from a One-Pot Cyclopentannulation Reaction. ACS Appl. Mater. Interfaces 2023, 15, 28149–28157. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Alameddine, B. Synthesis of conjugated polymers via cyclopentannulation reaction: Promising materials for iodine adsorption. Polym. Chem. 2020, 11, 3066–3074. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, P.; Williams, L.; Prakash, S.; Joseph, A. Design and synthesis of thiophene containing bis-chalcone-based mesoporous polymers for volatile iodine capture. J. Hazard. Mater. Adv. 2023, 10, 100272. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Moustafa, M.S.; Al-Mousawi, S.; Alameddine, B. Sizable iodine uptake of porous copolymer networks bearing Tröger’s base units. Polymer 2021, 229, 123996. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Habib, S.S.; Husain, A.A.; Al-Mousawi, S.; Alameddine, B. Synthesis of Iron(II) Clathrochelate-Based Poly(vinylene sulfide) with Tetraphenylbenzene Bridging Units and Their Selective Oxidation into Their Corresponding Poly(vinylene sulfone) Copolymers: Promising Materials for Iodine Capture. Polymers 2022, 14, 3727. [Google Scholar] [CrossRef]
- Baig, N.; Shetty, S.; Al-Mousawi, S.; Alameddine, B. Conjugated microporous polymers using a copper-catalyzed [4+2] cyclobenzannulation reaction: Promising materials for iodine and dye adsorption. Polym. Chem. 2021, 12, 2282–2292. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, D.W.; Hong, S.J.; Kim, J.; Kim, M.; Kim, J.; Lee, H.S.; Park, T.-H.; Kim, H.-K.; Park, J.I.; et al. Selective removal of radioactive iodine from water using reusable Fe@Pt adsorbents. Water Res. 2022, 222, 118864. [Google Scholar] [CrossRef]
- Yao, T.; Li, H.; Ren, Y.; Feng, M.; Hu, Y.; Yan, H.; Peng, L. Extraction and recovery of phenolic compounds from aqueous solution by thermo-separating magnetic ionic liquid aqueous two-phase system. Sep. Purif. Technol. 2022, 282, 120034. [Google Scholar] [CrossRef]
- Beghi, I.; Lind, T.; Prasser, H.-M. Experimental studies on retention of iodine in a wet scrubber. Nucl. Eng. Des. 2018, 326, 234–243. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, X.; Cheng, Z.; Liao, Y.; Duan, M. Porous ZIF-8@polyacrylonitrile composite beads for iodine capture. RSC Adv. 2021, 11, 30259–30269. [Google Scholar] [CrossRef]
- Emam, H.E.; El-Shahat, M.; Abdelhameed, R.M. Iodine removal efficiently from wastewater by magnetic Fe3O4 incorporated within activated porous cellulose. Ind. Crops Prod. 2023, 193, 116201. [Google Scholar] [CrossRef]
- Tian, S.; Yi, Z.; Chen, J.; Fu, S. In situ growth of UiO-66-NH2 in wood-derived cellulose for iodine adsorption. J. Hazard. Mater. 2023, 443, 130236. [Google Scholar] [CrossRef]
- Yao, T.; Feng, C.; Chen, W.; Chen, S. Selective separation and simultaneous recoveries of amino acids by temperature-sensitive magnetic ionic liquid aqueous biphasic system. J. Mol. Liq. 2023, 371, 121099. [Google Scholar] [CrossRef]
- Liu, R.; Tan, K.T.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent organic frameworks: An ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 2021, 50, 120–242. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Qin, Y.; Ni, C.; Dai, W.; Zou, J. Efficient Capture of Volatile Iodine by Thiophene-Containing Porous Organic Polymers. ACS Appl. Polym. Mater. 2020, 2, 5121–5128. [Google Scholar] [CrossRef]
- Janeta, M.; Bury, W.; Szafert, S. Porous Silsesquioxane–Imine Frameworks as Highly Efficient Adsorbents for Cooperative Iodine Capture. ACS Appl. Mater. Interfaces 2018, 10, 19964–19973. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.; Mohanty, P. Iodine sequestration using cyclophosphazene based inorganic-organic hybrid nanoporous materials: Role of surface functionality and pore size distribution. J. Mol. Liq. 2019, 283, 58–64. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous Organic Materials: Strategic Design and Structure–Function Correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Gupta, N.; Chattopadhyaya, M.C. Dynamics of adsorption of Ni(II), Co(II) and Cu(II) from aqueous solution onto newly synthesized poly[N-(4-[4-(aminophenyl)methylphenylmethacrylamide])]. Arab. J. Chem. 2017, 10, S1645–S1653. [Google Scholar] [CrossRef]
- Yao, T.; Song, J.; Yan, H.; Chen, S. Functionalized aqueous biphasic system coupled with HPLC for highly sensitive detection of quinolones in milk. LWT 2023, 173, 114398. [Google Scholar] [CrossRef]
- Türkmen, D.; Bakhshpour, M.; Akgönüllü, S.; Aşır, S.; Denizli, A. Heavy Metal Ions Removal From Wastewater Using Cryogels: A Review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Shaker, O.A.; Safwat, S.M.; Matta, M.E. Nickel removal from wastewater using electrocoagulation process with zinc electrodes under various operating conditions: Performance investigation, mechanism exploration, and cost analysis. Environ. Sci. Pollut. Res. 2023, 30, 26650–26662. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Yao, T.; Song, J.; Hong, Y.; Gan, Y.; Ren, X.; Du, K. Application of cellulose to chromatographic media: Cellulose dissolution, and media fabrication and derivatization. J. Chromatogr. A 2023, 1705, 464202. [Google Scholar] [CrossRef]
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res. 2022, 222, 118888. [Google Scholar] [CrossRef]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.; Matis, K.A. Hybrid flotation—Membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Wang, S.; Yang, C.; Zhao, P.; Huang, B.; Wang, Q.; Wang, H. Study on the Adsorption Mechanism of Cobalt and Nickel in Manganese Sulfate by δ-MnO2. ACS Omega 2022, 7, 37452–37464. [Google Scholar] [CrossRef]
- Muhammad Ekramul Mahmud, H.N.; Huq, A.K.O.; Yahya, R.b. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Z.; Feng, S.; Wang, D.; Liu, H. Engineering Functionality in Organic Porous Networks by Multicomponent Polymerization. Macromolecules 2021, 54, 7642–7652. [Google Scholar] [CrossRef]
- Guan, H.; Zou, D.; Yu, H.; Liu, M.; Liu, Z.; Sun, W.; Xu, F.; Li, Y. Adsorption Behavior of Iodine by Novel Covalent Organic Polymers Constructed Through Heterostructural Mixed Linkers. Front. Mater. 2019, 6, 12. [Google Scholar] [CrossRef]
- Yao, T.; Li, Q.; Li, H.; Peng, L.; Liu, Y.; Du, K. Extractive resolution of racemic phenylalanine and preparation of optically pure product by chiral magnetic ionic liquid aqueous two-phase system. Sep. Purif. Technol. 2021, 274, 119024. [Google Scholar] [CrossRef]
- Fato, F.P.; Li, D.-W.; Zhao, L.-J.; Qiu, K.; Long, Y.-T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef]
- Yao, C.; Li, G.; Wang, J.; Xu, Y.; Chang, L. Template-free synthesis of porous carbon from triazine based polymers and their use in iodine adsorption and CO2 capture. Sci. Rep. 2018, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, B.; Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F. Tuning the optical properties of ethynylene triptycene-based copolymers via oxidation of their alkyne groups into α-diketones. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 931–937. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, L.; Madejová, J. Near-IR study of the impact of alkyl-ammonium and -phosphonium cations on the hydration of montmorillonite. J. Mol. Struct. 2022, 1256, 132568. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Alameddine, B. Synthesis and Iodine Adsorption Properties of Organometallic Copolymers with Propeller-Shaped Fe(II) Clathrochelates Bridged by Different Diaryl Thioether and Their Oxidized Sulfone Derivatives. Polymers 2022, 14, 4818. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Luo, J.; Kong, L.; Zhao, J.; Zhang, Y.; Du, H.; Chen, S.; Xie, Y. The synthesis of triazine-thiophene-thiophene conjugated porous polymers and their composites with carbon as anode materials in lithium-ion batteries. RSC Adv. 2021, 11, 10688–10698. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-J.; Zhao, X.-Q.; Wang, P.-F.; Wang, H.; Han, B.-H. Thiophene-based conjugated microporous polymers: Synthesis, characterization and efficient gas storage. Sci. China Chem. 2017, 60, 1067–1074. [Google Scholar] [CrossRef]
- Mokhtari, N.; Dinari, M. Developing novel amine-linked covalent organic frameworks towards reversible iodine capture. Sep. Purif. Technol. 2022, 301, 121948. [Google Scholar] [CrossRef]
- Yuan, G.; Lu, Y.; Yang, C. Effect of different synthesis methodologies on the adsorption of iodine. Heliyon 2023, 9, e16975. [Google Scholar] [CrossRef]
- Qiao, Y.; Lv, N.; Xue, X.; Zhou, T.; Che, G.; Xu, G.; Wang, F.; Wu, Y.; Xu, Z. Highly Efficient Iodine Capture and CO2 Adsorption using a Triazine-Based Conjugated Microporous Polymers. ChemistrySelect 2022, 7, e202200234. [Google Scholar] [CrossRef]
- Hastings, A.M.; Ray, D.; Hanna, S.L.; Jeong, W.; Chen, Z.; Oliver, A.G.; Gagliardi, L.; Farha, O.K.; Hixon, A.E. Leveraging Nitrogen Linkages in the Formation of a Porous Thorium–Organic Nanotube Suitable for Iodine Capture. Inorg. Chem. 2022, 61, 9480–9492. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Hamadi, H.; Nobakht, V. Capture of iodine in solution and vapor phases by newly synthesized and characterized encapsulated Cu2O nanoparticles into the TMU-17-NH2 MOF. J. Hazard. Mater. 2020, 399, 122872. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-X.; Li, X.-J.; Zong, J.-S.; You, D.-J.; Liang, A.-P.; Zhou, Y.-L.; Li, X.-Q.; Liu, L.-L. Fabrication of Protonated Two-Dimensional Metal–Organic Framework Nanosheets for Highly Efficient Iodine Capture from Water. Inorg. Chem. 2022, 61, 13883–13892. [Google Scholar] [CrossRef]
- Hassan, A.; Alam, A.; Chandra, S.; Prince; Das, N. Triptycene-based and imine linked porous uniform microspheres for efficient and reversible scavenging of iodine from various media: A systematic study. Environ. Sci. Adv. 2022, 1, 320–330. [Google Scholar] [CrossRef]
- Xu, J.; Xie, W.; Yao, C.; Xu, G.; Zhang, S.; Xu, Y. Preparation of sulfur-containing conjugated microporous polymer for adsorbing iodine and Fe3+ sensing. J. Environ. Chem. Eng. 2021, 9, 106399. [Google Scholar] [CrossRef]
- Shetty, S.; Baig, N.; Hassan, A.; Al-Mousawi, S.; Das, N.; Alameddine, B. Fluorinated Iron(ii) clathrochelate units in metalorganic based copolymers: Improved porosity, iodine uptake, and dye adsorption properties. RSC Adv. 2021, 11, 14986–14995. [Google Scholar] [CrossRef]
- Baseri, H.; Tizro, S. Treatment of nickel ions from contaminated water by magnetite-based nanocomposite adsorbents: Effects of thermodynamic and kinetic parameters and modeling with Langmuir and Freundlich isotherms. Process Saf. Environ. Prot. 2017, 109, 465–477. [Google Scholar] [CrossRef]
- Coşkun, R.; Soykan, C.; Saçak, M. Adsorption of copper(II), nickel(II) and cobalt(II) ions from aqueous solution by methacrylic acid/acrylamide monomer mixture grafted poly(ethylene terephthalate) fiber. Sep. Purif. Technol. 2006, 49, 107–114. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A. Removal of nickel (II) from aqueous solution by biosorption on A. barbadensis Miller waste leaves powder. Appl. Water Sci. 2019, 9, 96. [Google Scholar] [CrossRef]
- Onursal, N.; Altunkaynak, Y.; Baran, A.; Dal, M.C. Adsorption of nickel (II) ions from aqueous solutions using Malatya clay: Equilibrium, kinetic, and thermodynamic studies. Environ. Prog. Sustain. Energy 2023, 42, e14150. [Google Scholar] [CrossRef]
- Antić, K.; Onjia, A.; Vasiljević-Radović, D.; Veličković, Z.; Tomić, S.L. Removal of Nickel Ions from Aqueous Solutions by 2-Hydroxyethyl Acrylate/Itaconic Acid Hydrogels Optimized with Response Surface Methodology. Gels 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]

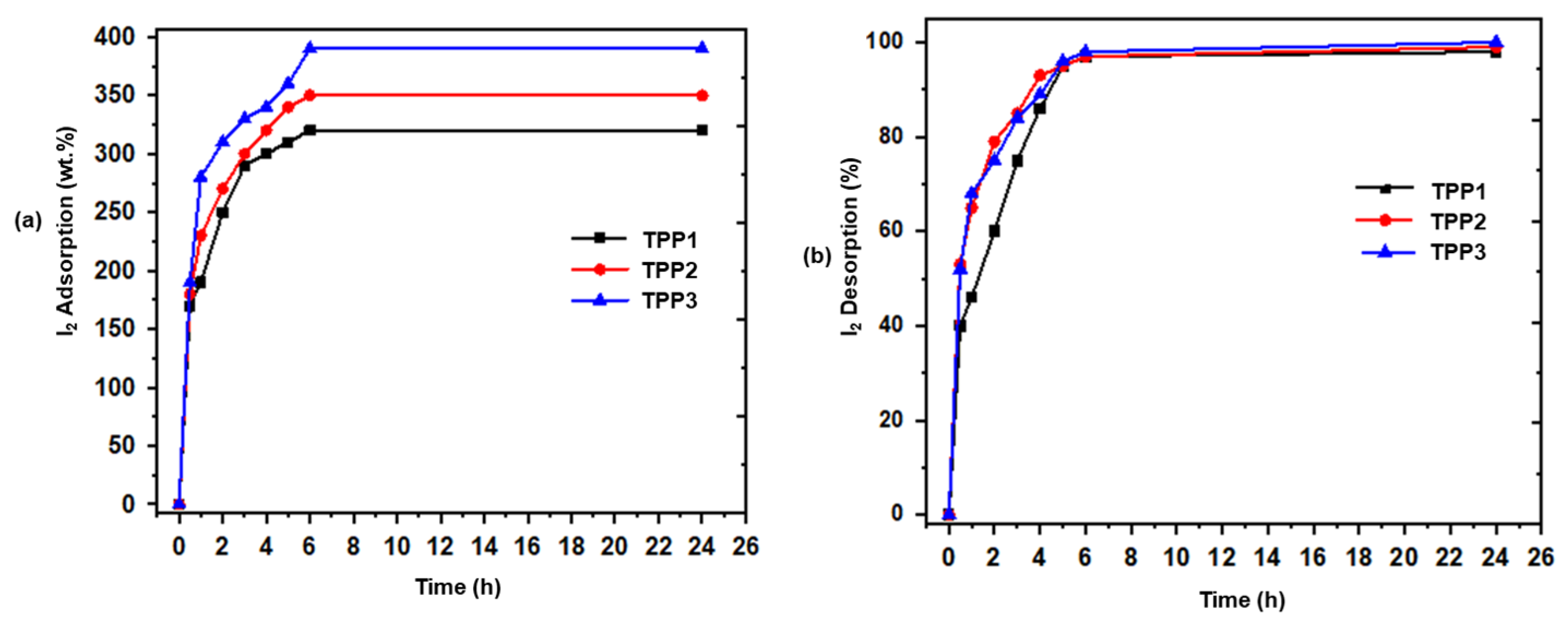
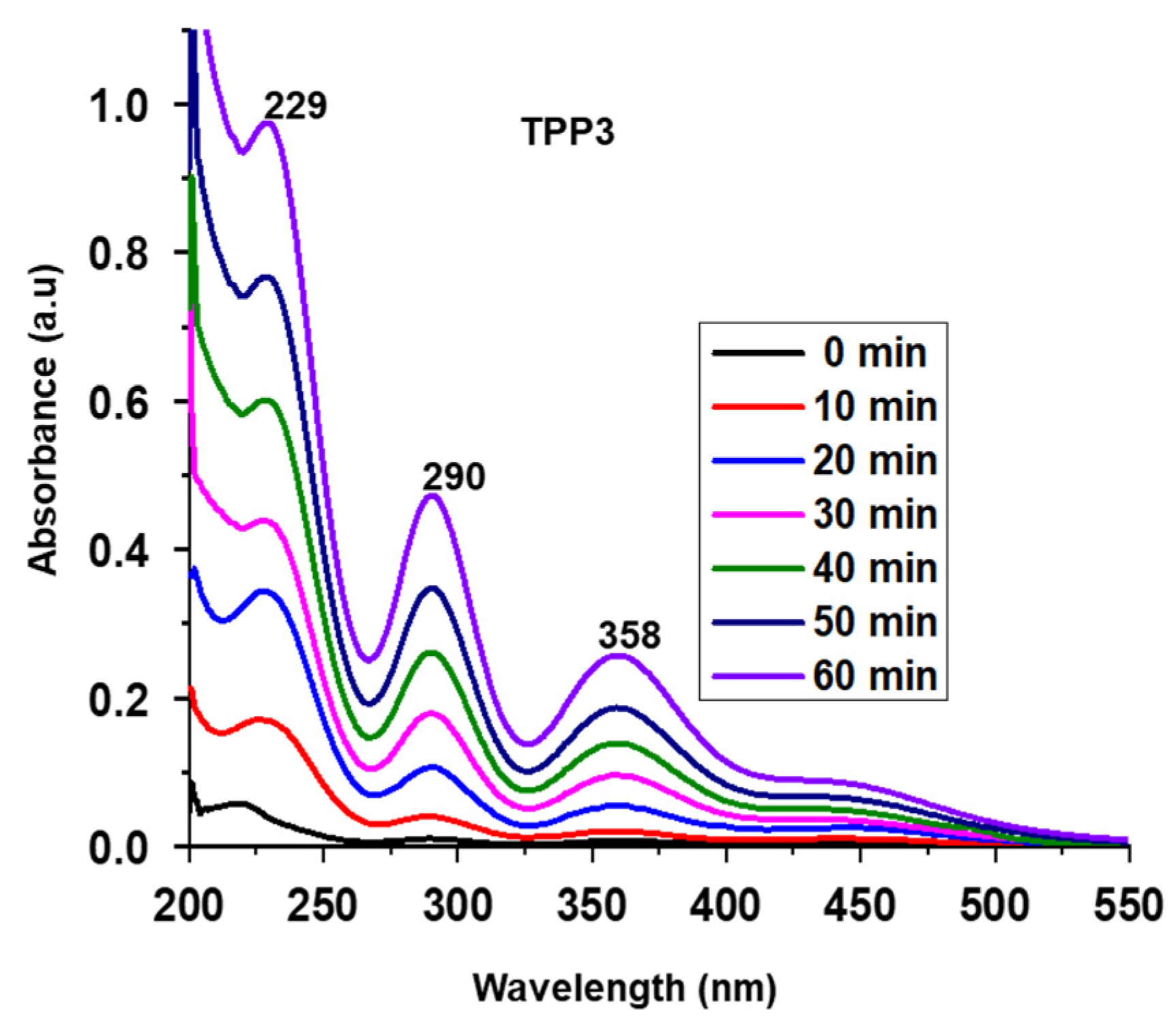
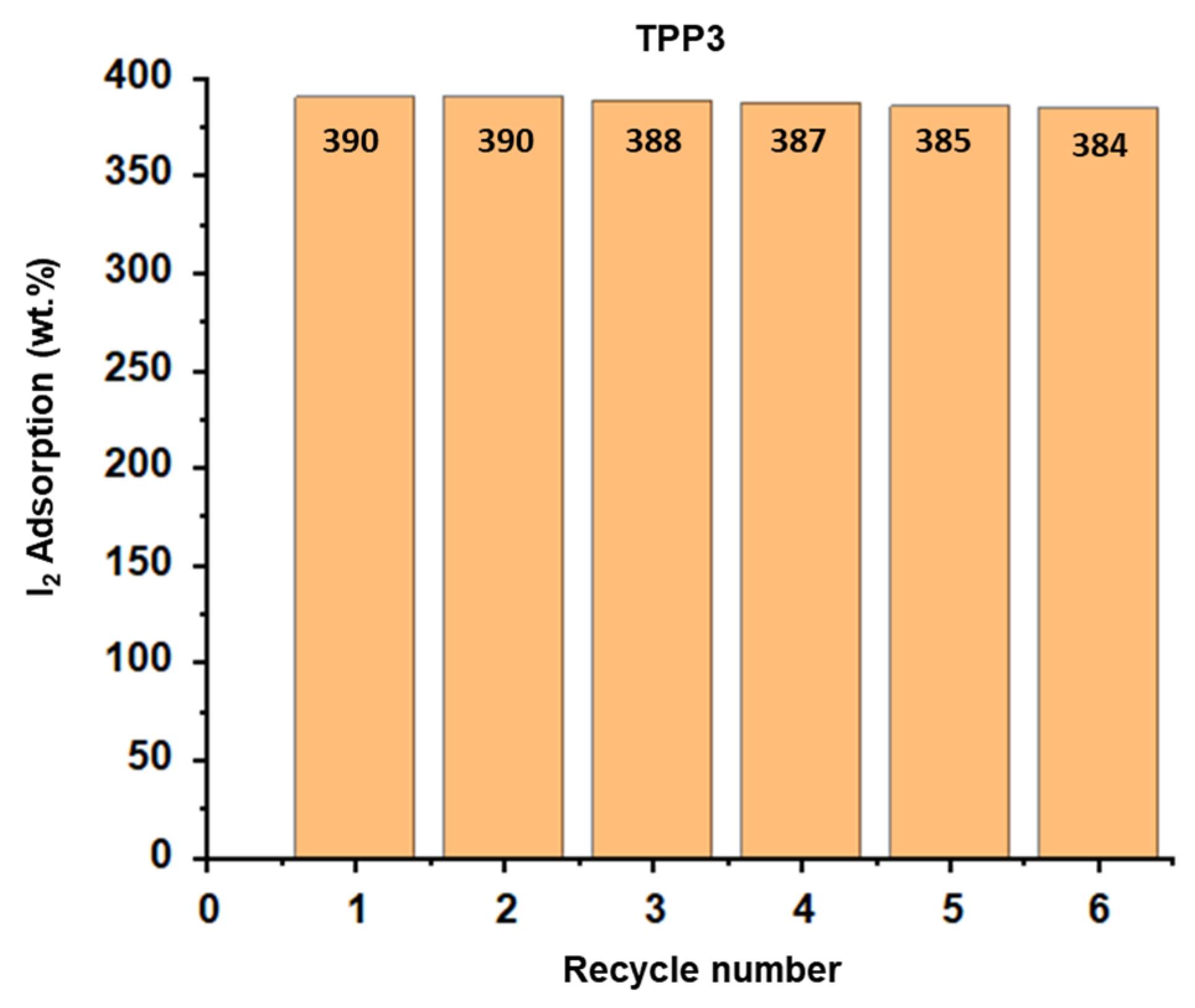

| Entry | Polymer | Wt.% I2 Adsorption after 24 h | % I2 Desorption after 24 h |
|---|---|---|---|
| 1 | TPP1 | 320 | 98 |
| 2 | TPP2 | 350 | 99 |
| 3 | TPP3 | 390 | 100 |
| Polymer | Pseudo 1st-Order Model | Pseudo 2nd-Order Model | |||||
|---|---|---|---|---|---|---|---|
| qe,exp (mg g−1) | qe,cal (mg g−1) | k1 (min−1) | R2 | qe,cal (mg g−1) | k2 (min−1) | R2 | |
| TPP3@I2 | 3900 | 1894 | −0.01526 | 0.9278 | 3887 | 0.000528 | 0.9976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shetty, S.; Baig, N.; Wahed, S.A.; Hassan, A.; Das, N.; Alameddine, B. Iodine and Nickel Ions Adsorption by Conjugated Copolymers Bearing Repeating Units of Dicyclopentapyrenyl and Various Thiophene Derivatives. Polymers 2023, 15, 4153. https://doi.org/10.3390/polym15204153
Shetty S, Baig N, Wahed SA, Hassan A, Das N, Alameddine B. Iodine and Nickel Ions Adsorption by Conjugated Copolymers Bearing Repeating Units of Dicyclopentapyrenyl and Various Thiophene Derivatives. Polymers. 2023; 15(20):4153. https://doi.org/10.3390/polym15204153
Chicago/Turabian StyleShetty, Suchetha, Noorullah Baig, Sk Abdul Wahed, Atikur Hassan, Neeladri Das, and Bassam Alameddine. 2023. "Iodine and Nickel Ions Adsorption by Conjugated Copolymers Bearing Repeating Units of Dicyclopentapyrenyl and Various Thiophene Derivatives" Polymers 15, no. 20: 4153. https://doi.org/10.3390/polym15204153
APA StyleShetty, S., Baig, N., Wahed, S. A., Hassan, A., Das, N., & Alameddine, B. (2023). Iodine and Nickel Ions Adsorption by Conjugated Copolymers Bearing Repeating Units of Dicyclopentapyrenyl and Various Thiophene Derivatives. Polymers, 15(20), 4153. https://doi.org/10.3390/polym15204153







