Synthesis, Thermal and Mechanical Properties of Fully Biobased Poly (hexamethylene succinate-co-2,5-furandicarboxylate) Copolyesters
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Chemical Structure and Molecular Weight Studies
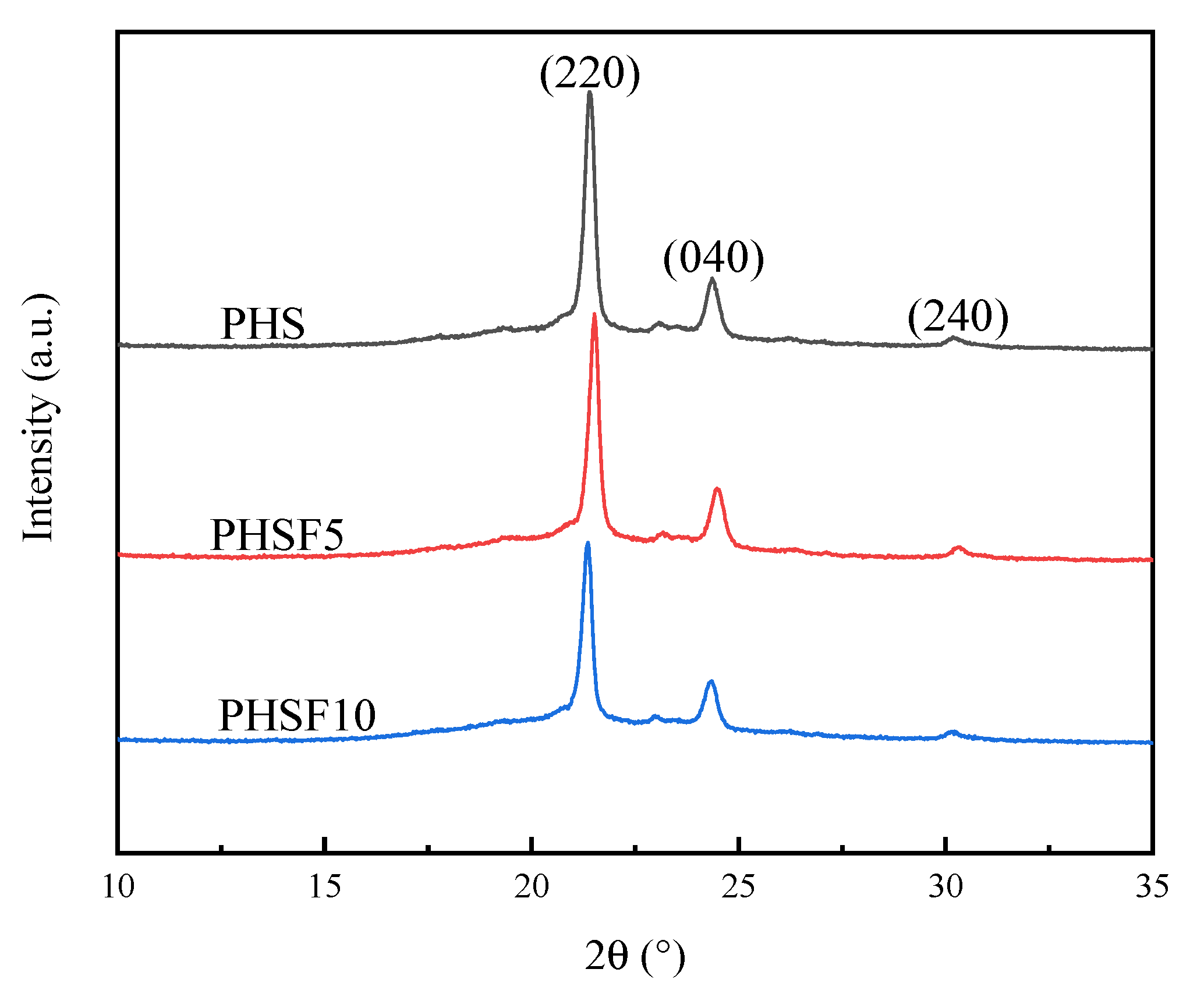
3.2. Crystal Structure Study
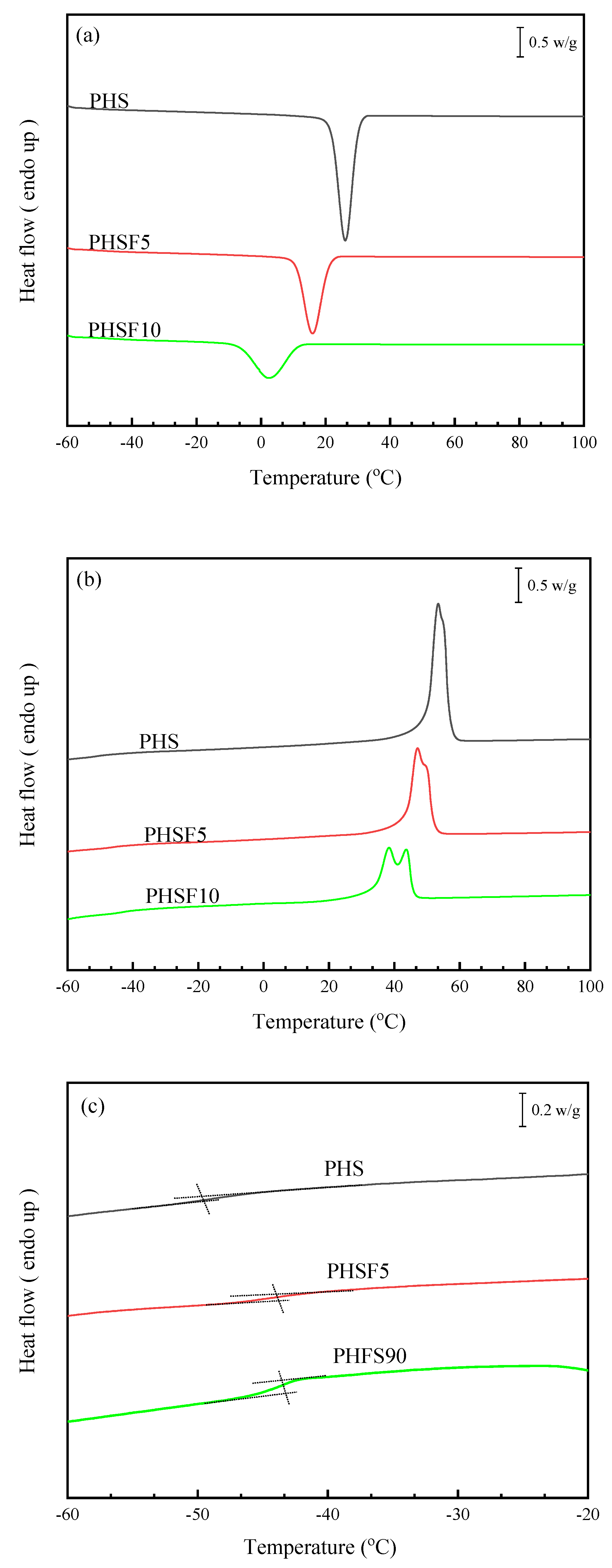
3.3. Basic Thermal Parameters Study
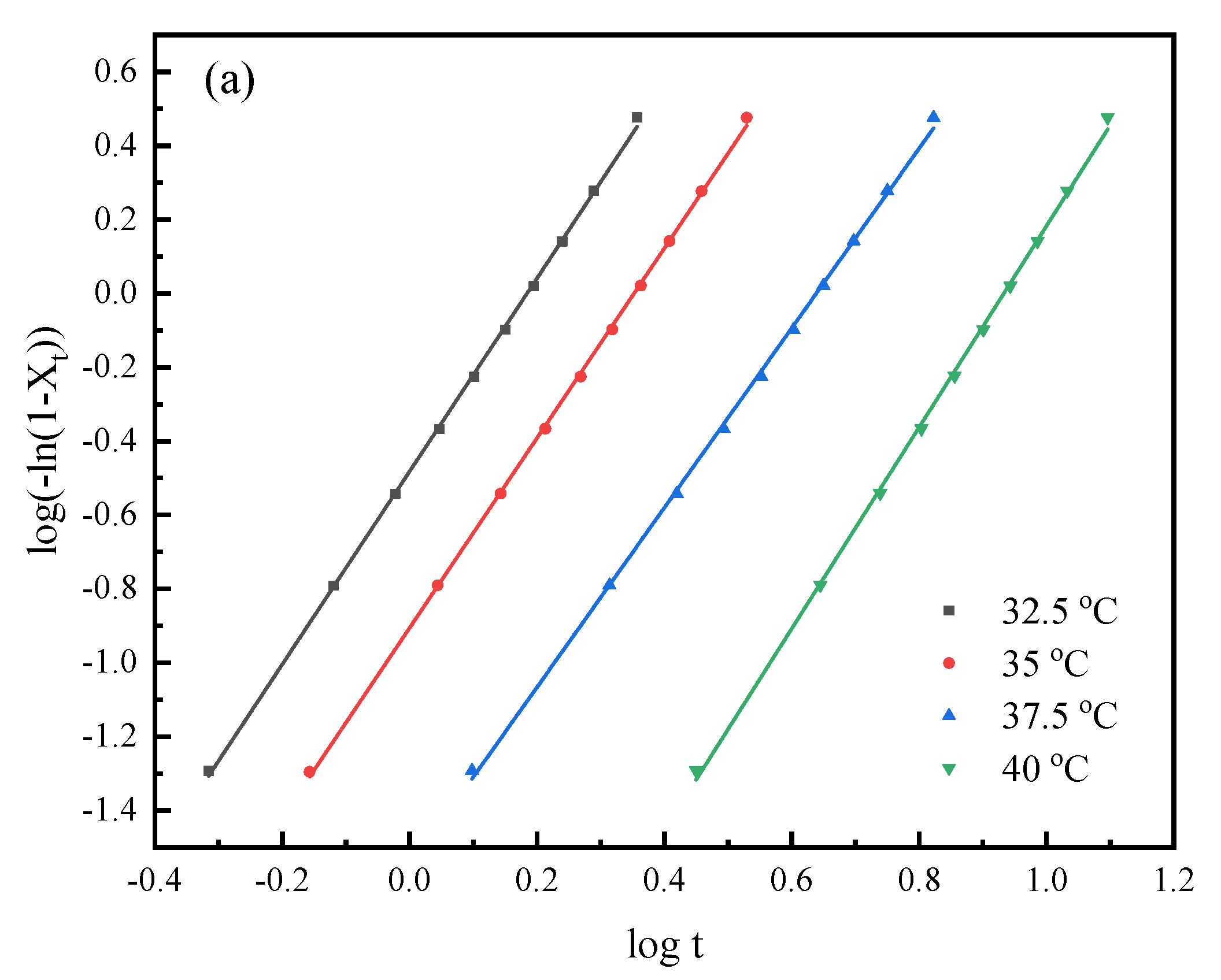
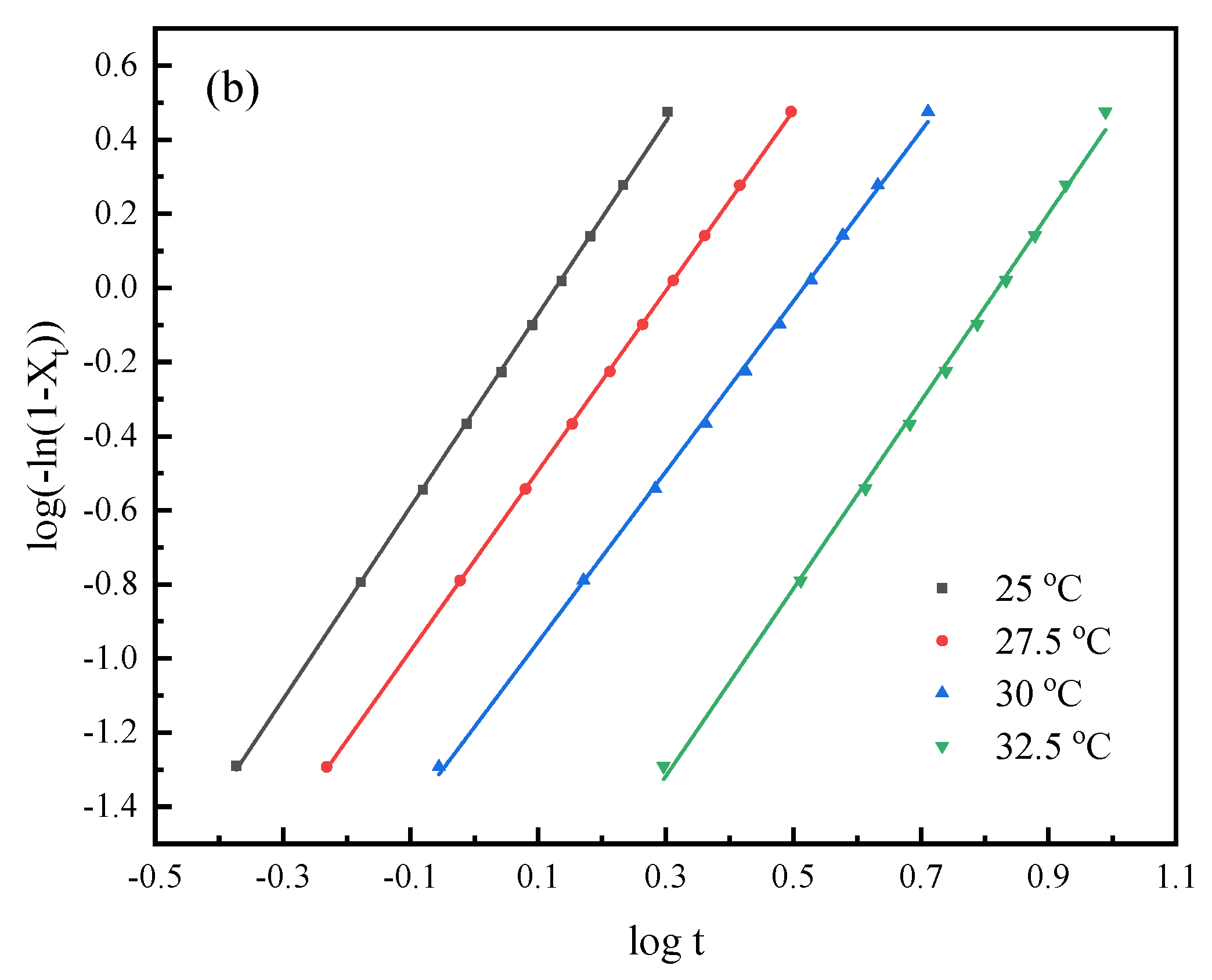
3.4. Isothermal Crystallization Kinetics Study
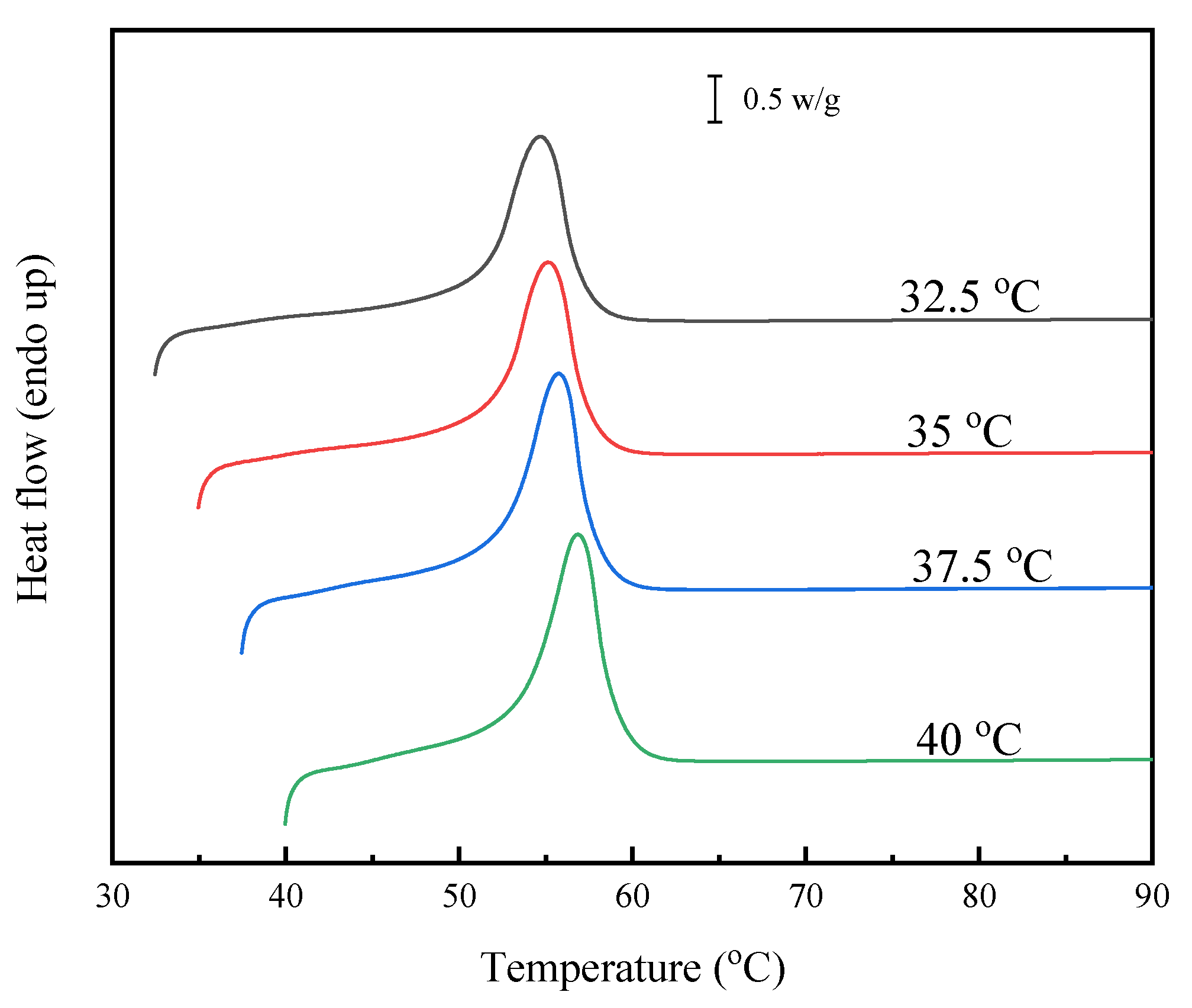
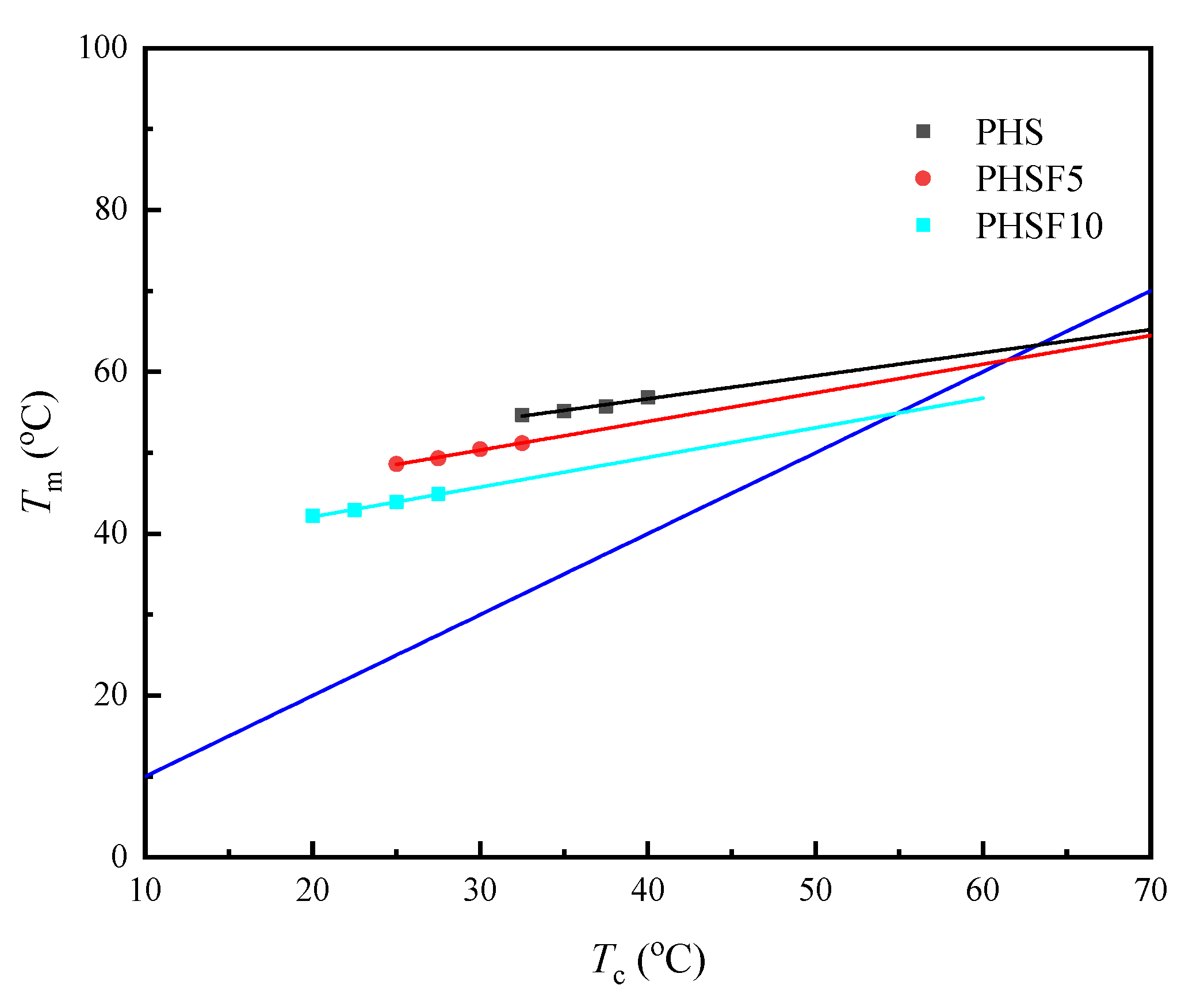
3.5. Melting Behavior and Equilibrium Melting-Point Study
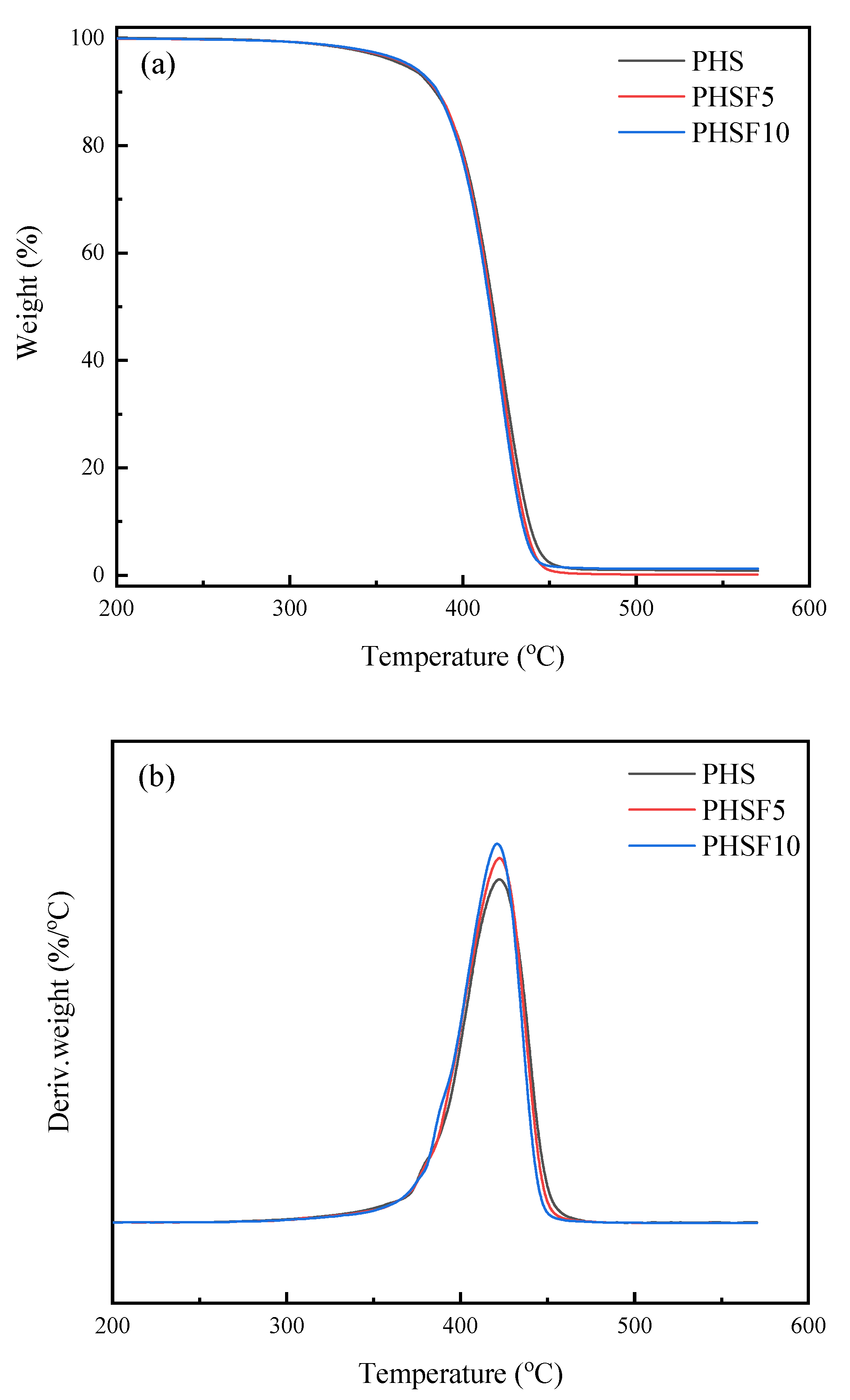
3.6. Thermal Stability Study
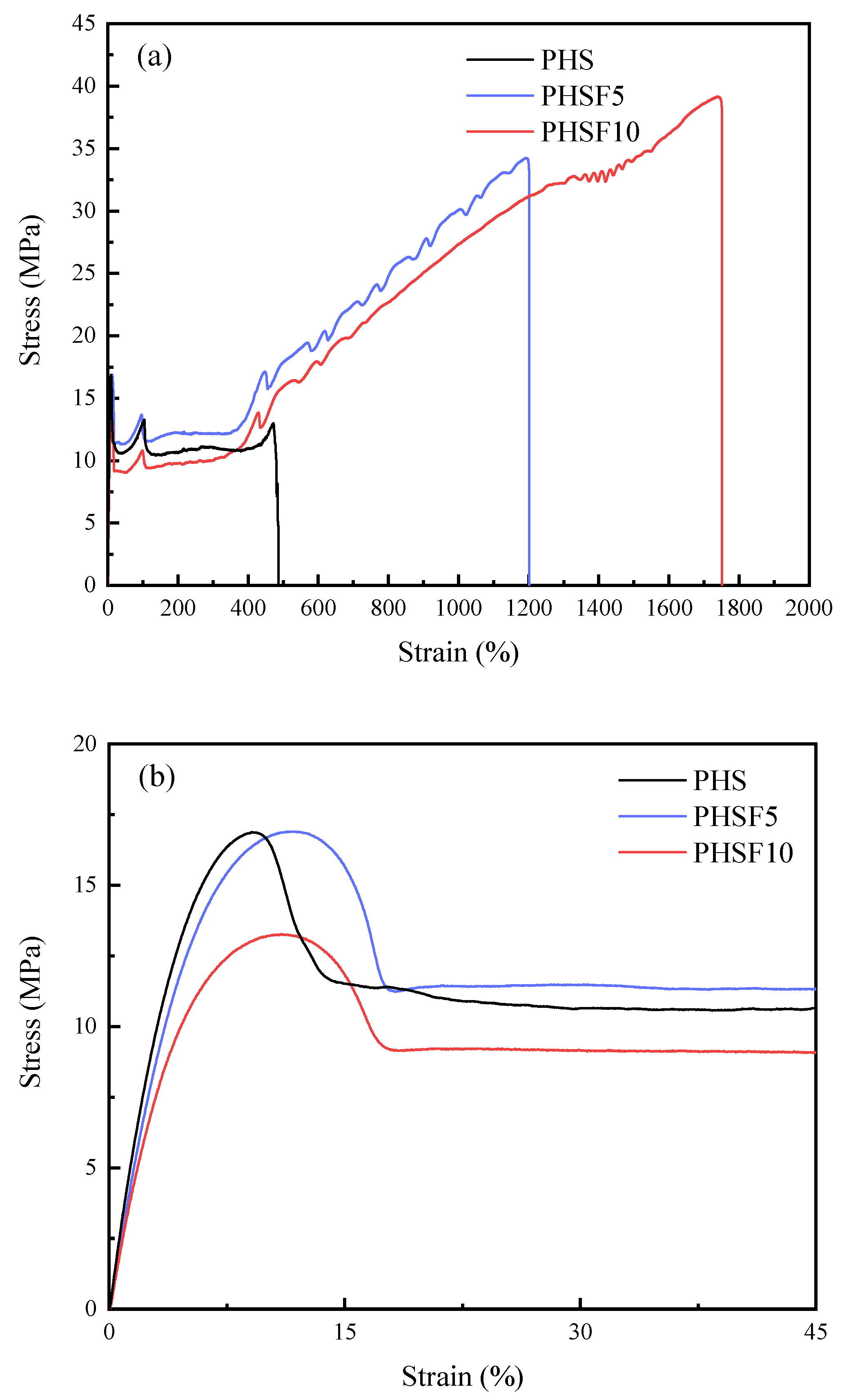
3.7. Mechanical Property Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study. Polymer 2005, 46, 12081–12092. [Google Scholar] [CrossRef]
- Bai, Z.; Su, T.; Wang, Z. Study on the synthesis and enzymatic hydrolysis of biodegradable aliphatic polyester. New Chem. Mater. 2019, 47, 152–156. [Google Scholar]
- Gestí, S.; Casas, M.T.; Puiggalí, J. Crystalline structure of poly(hexamethylene succinate) and single crystal degradation studies. Polymer 2007, 48, 5088–5097. [Google Scholar] [CrossRef]
- Franco, L.; Puiggalí, J. Crystallization kinetics of poly(hexamethylene succinate). Eur. Polym. J. 2003, 39, 1575–1583. [Google Scholar] [CrossRef]
- Yang, H.; Qiu, Z. Crystallization kinetics and morphology of novel biodegradable poly(hexamethylene succinate-co-3 mol % ethylene succinate) with low and high molecular weights. Ind. Eng. Chem. Res. 2013, 52, 3537–3542. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Extension of theory of growth of chain-folded polymer crystals to large undercoolings. J. Appl. Phys. 1973, 44, 4340–4352. [Google Scholar] [CrossRef]
- Pan, S.; Qiu, Z. Fully biodegradable poly(hexamethylene succinate)/cellulose nanocrystals composites with enhanced crystallization rate and mechanical property. Polymers 2021, 13, 3667. [Google Scholar] [CrossRef]
- Tan, B.; Bi, S.; Emery, K.; Sobkowicz, M.J. Bio-based poly(butylene succinate-co-hexamethylene succinate) copolyesters with tunable thermal and mechanical properties. Eur. Polym. J. 2017, 86, 162–172. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, C.; Yu, Y.; Li, Y. Poly(hexamethylene succinate) copolyesters containing phosphorus pendent group: Retarded crystallization and solid-state microstructure. Polymer 2015, 71, 31–42. [Google Scholar] [CrossRef]
- Ye, H.M.; Liu, P.; Wang, C.X.; Meng, X.; Zhou, Q. Polymorphism regulation in poly(hexamethylene succinate-co-hexamethylene fumarate): Altering the hydrogen bonds in crystalline lattice. Polymer 2017, 108, 272–280. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, H.; Qiu, Z. Thermal properties and crystallization behavior of novel biodegradable poly(hexamethylene succinate-co-6mol% butylene succinate) and poly(hexamethylene succinate). J. Polym. Environ. 2018, 26, 1320–1327. [Google Scholar] [CrossRef]
- Li, X.; Hong, Z.; Sun, J.; Geng, Y.; Huang, Y.; An, H.; Ma, Z.; Zhao, B.; Shao, C.; Fang, Y.; et al. Identifying the phase behavior of biodegradable poly(hexamethylene succinate-co-hexamethylene adipate) copolymers with FTIR. J. Phys. Chem. B 2009, 113, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, Z.; Qiu, Z. Synthesis and properties of poly(hexamethylene 2,5-furandicarboxylate-co-adipate) copolyesters. Eur. Polym. J. 2021, 161, 110860. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.A.; Lotti, N. Fully biobased superpolymers of 2,5-furandicarboxylic acid with different functional properties: From rigid to flexible, high performant packaging materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Qu, X.; Zhou, G. Poly(hexamethylene 2,5-furandicarboxylate) copolyesters containing phosphorus: Synthesis, crystallization behavior, thermal, mechanical and flame retardant properties. Polym. Degrad. Stabil. 2018, 153, 272–280. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. Biobased poly(ethylene-co-hexamethylene 2,5-furandicarboxylate) (PEHF) copolyesters with superior tensile properties. Ind. Eng. Chem. Res. 2018, 57, 13094–13102. [Google Scholar] [CrossRef]
- Bozell, J.; Petersen, G. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- van Putten, R.J.; van der Waal, J.C.; Putten; Jan, C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-based polyesters from renewable resources: Crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Bourdet, A.; Esposito, A.; Thiyagarajan, S.; Delbreilh, L.; Affouard, F.; Knoop, R.; Dargent, E. Molecular mobility in amorphous biobased poly(ethylene 2,5-furandicarboxylate) and poly(ethylene 2,4-furandicarboxylate). Macromolecules 2018, 51, 1937–1945. [Google Scholar] [CrossRef]
- Fei, X.; Wang, J.; Zhu, J.; Wang, X.; Liu, X. Biobased poly(ethylene 2,5-furancoate): No longer an alternative, but an irreplaceable polyester in the polymer industry. ACS Sustain. Chem. Eng. 2020, 8, 8471–8485. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Shi, L.; Ying, W.B.; Wang, J.; Zhu, J. Modification of poly(butylene 2,5-furandicarboxylate) with lactic acid for biodegradable copolyesters with good mechanical and barrier properties. Ind. Eng. Chem. Res. 2018, 57, 11020–11030. [Google Scholar] [CrossRef]
- Papamokos, G.; Dimitriadis, T.; Bikiaris, D.N.; Papageorgiou, G.Z.; Floudas, G. Chain conformation, molecular dynamics, and thermal properties of poly(n-methylene 2,5-furanoates) as a function of methylene unit sequence length. Macromolecules 2019, 52, 6533–6546. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.-H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furan dicarboxylate), a biobased alternative to PBT: Synthesis, physical properties, and crystal structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Liu, T.; Petermann, J. Multiple melting behavior in isothermally cold-crystallized isotactic polystyrene. Polymer 2001, 42, 6453–6461. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Panayiotou, C.G. Novel miscible poly(ethylene sebacate)/poly(4-vinyl phenol) blends: Miscibility, melting behavior and crystallization study. Polymer 2011, 52, 4553–4561. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Komura, M.; Ikehara, T.; Nishi, T. DSC and TMDSC study of melting behaviour of poly(butylene succinate) and poly(ethylene succinate). Polymer 2003, 44, 7781–7785. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Hoffman, J.D.; Weeks, J.J. X-ray study of isothermal thickening of lamellae in bulk polyethylene at the crystallization temperature. J. Chem. Phys. 1965, 42, 4301–4302. [Google Scholar] [CrossRef]
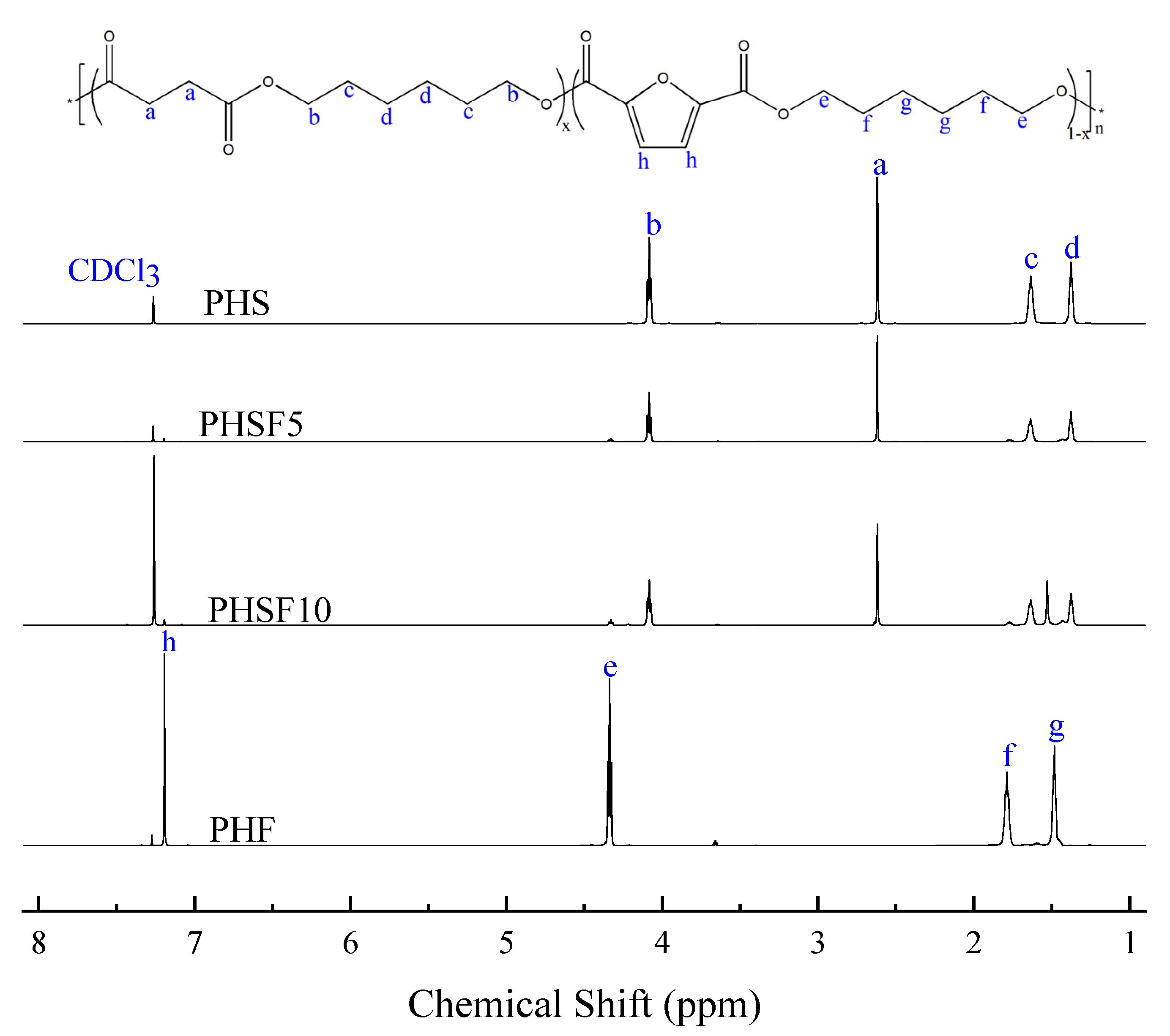

| Samples | SA/DMFD (mol%) | HS/HF (mol%) | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|---|---|
| PHS | 100/0 | 100/0 | 4.06 × 104 | 6.75 × 104 | 1.66 |
| PHSF5 | 95/5 | 94.9/5.1 | 4.88 × 104 | 9.30 × 104 | 1.91 |
| PHSF10 | 90/10 | 89.9/10.1 | 5.16 × 104 | 9.30 × 104 | 1.80 |
| Samples | Td | Tmax | Tg | Tm | ΔHm | Tcc | ΔHcc | Tmo |
|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | (J/g) | (°C) | (J/g) | (°C) | |
| PHS | 365.7 | 422.2 | −48.8 | 53.4 | 68.3 | 26.1 | 66.2 | 63.3 |
| PHSF5 | 368.5 | 422.4 | −43.9 | 47.1/50.1 | 50.9 | 15.9 | 49.4 | 61.4 |
| PHSF10 | 369.2 | 421.1 | −43.1 | 38.4/43.4 | 43.1 | 2.4 | 37.6 | 54.9 |
| Samples | Tc (°C) | n | k (min−n) | t0.5 (min) |
|---|---|---|---|---|
| PHS | 32.5 | 2.6 | 3.29 × 10−1 | 1.32 |
| 35 | 2.6 | 1.24 × 10−1 | 1.95 | |
| 37.5 | 2.4 | 2.81 × 10−2 | 3.74 | |
| 40 | 2.7 | 2.87 × 10−3 | 7.49 | |
| PHSF5 | 25 | 2.6 | 4.67 × 10−1 | 1.16 |
| 27.5 | 2.4 | 1.84 × 10−1 | 1.73 | |
| 30 | 2.3 | 6.53 × 10−2 | 2.79 | |
| 32.5 | 2.5 | 8.38 × 10−3 | 5.72 | |
| PHSF10 | 20 | 2.3 | 1.11 × 10−1 | 2.20 |
| 22.5 | 2.5 | 2.97 × 10−2 | 3.56 | |
| 25 | 2.5 | 1.12 × 10−2 | 5.06 | |
| 27.5 | 2.5 | 2.69 × 10−3 | 8.83 |
| Samples | Et | σy | σb | εb |
|---|---|---|---|---|
| (MPa) | (MPa) | (MPa) | (%) | |
| PHS | 394.7 ± 7.6 | 16.9 ± 1.1 | 12.9 ± 0.9 | 498.5 ± 4.78 |
| PHSF5 | 378.2 ± 24.2 | 16.9 ± 1.5 | 33.4 ± 1.4 | 1228.4 ± 94.3 |
| PHSF10 | 305.0 ± 6.5 | 11.0 ± 0.1 | 39.2 ± 0.8 | 1757.6 ± 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Chen, M.; Jiang, Z.; Qiu, Z. Synthesis, Thermal and Mechanical Properties of Fully Biobased Poly (hexamethylene succinate-co-2,5-furandicarboxylate) Copolyesters. Polymers 2023, 15, 427. https://doi.org/10.3390/polym15020427
Wang C, Chen M, Jiang Z, Qiu Z. Synthesis, Thermal and Mechanical Properties of Fully Biobased Poly (hexamethylene succinate-co-2,5-furandicarboxylate) Copolyesters. Polymers. 2023; 15(2):427. https://doi.org/10.3390/polym15020427
Chicago/Turabian StyleWang, Chengqian, Mingkun Chen, Zhiguo Jiang, and Zhaobin Qiu. 2023. "Synthesis, Thermal and Mechanical Properties of Fully Biobased Poly (hexamethylene succinate-co-2,5-furandicarboxylate) Copolyesters" Polymers 15, no. 2: 427. https://doi.org/10.3390/polym15020427
APA StyleWang, C., Chen, M., Jiang, Z., & Qiu, Z. (2023). Synthesis, Thermal and Mechanical Properties of Fully Biobased Poly (hexamethylene succinate-co-2,5-furandicarboxylate) Copolyesters. Polymers, 15(2), 427. https://doi.org/10.3390/polym15020427








