Photothermal Sensitive 3D Printed Biodegradable Polyester Scaffolds with Polydopamine Coating for Bone Tissue Engineering
Abstract
1. Introduction
2. Experimental
2.1. Materials
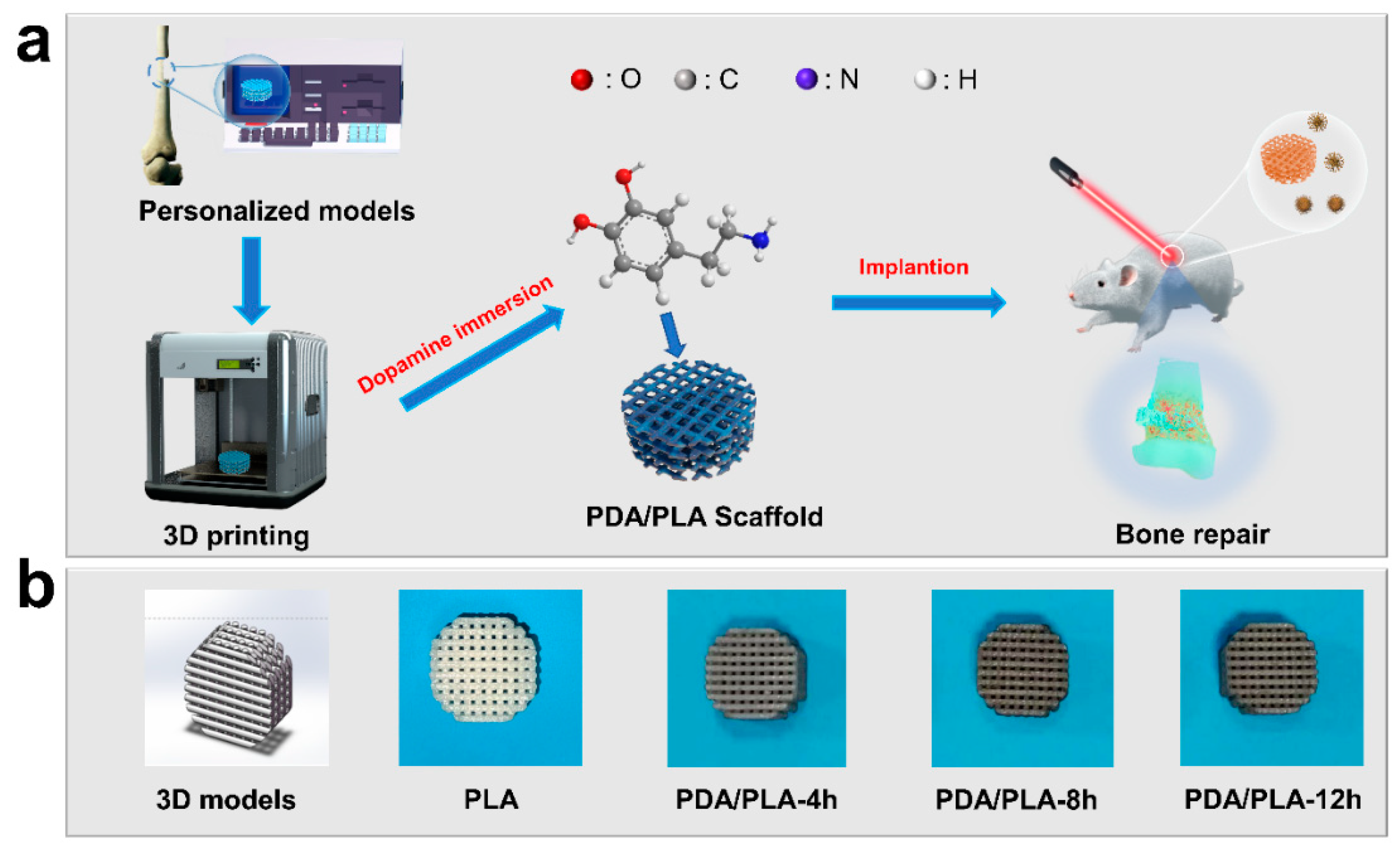
2.2. Preparation of PLA/PDA Scaffolds
2.3. Physio-Chemical Characterization
2.4. Degradation In Vitro
2.5. Mineralization In Vitro
2.6. Near Infrared Test
2.7. Statistical Analysis
3. Results and Discussion
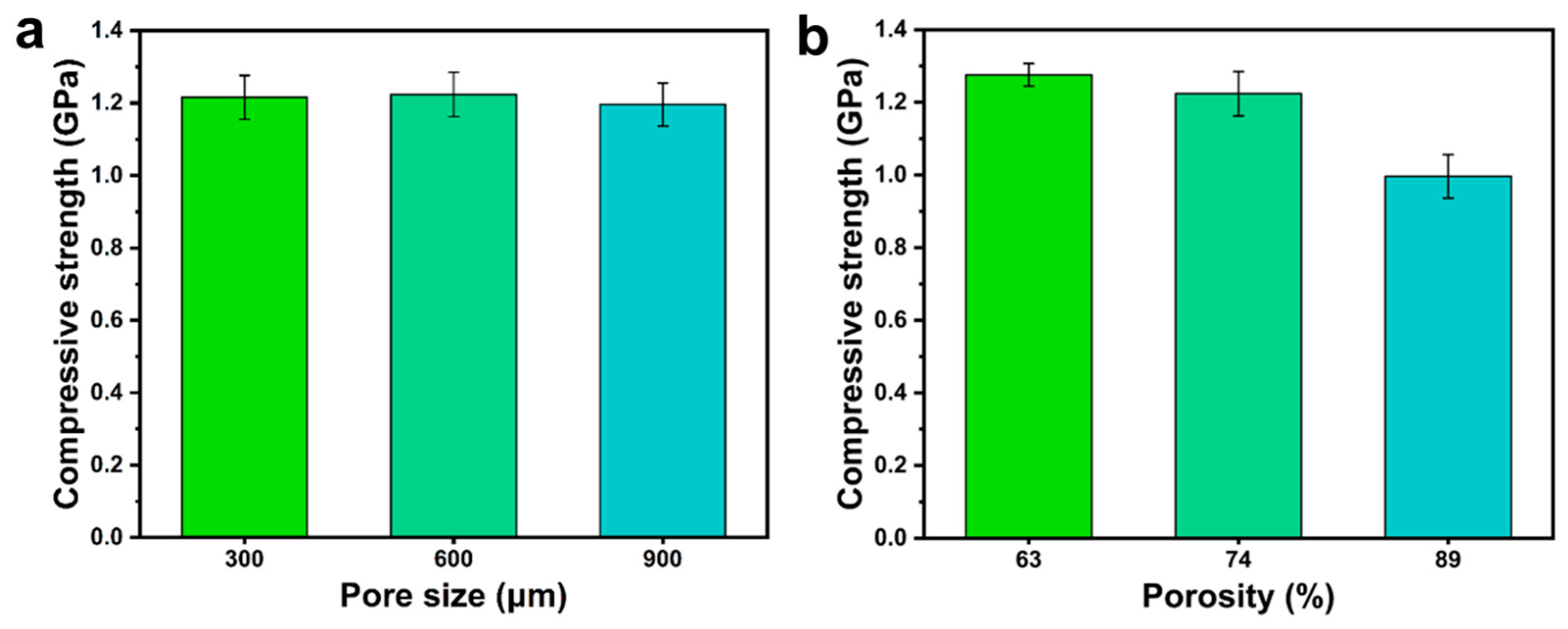
3.1. Analysis of the Optimal Pore Structure
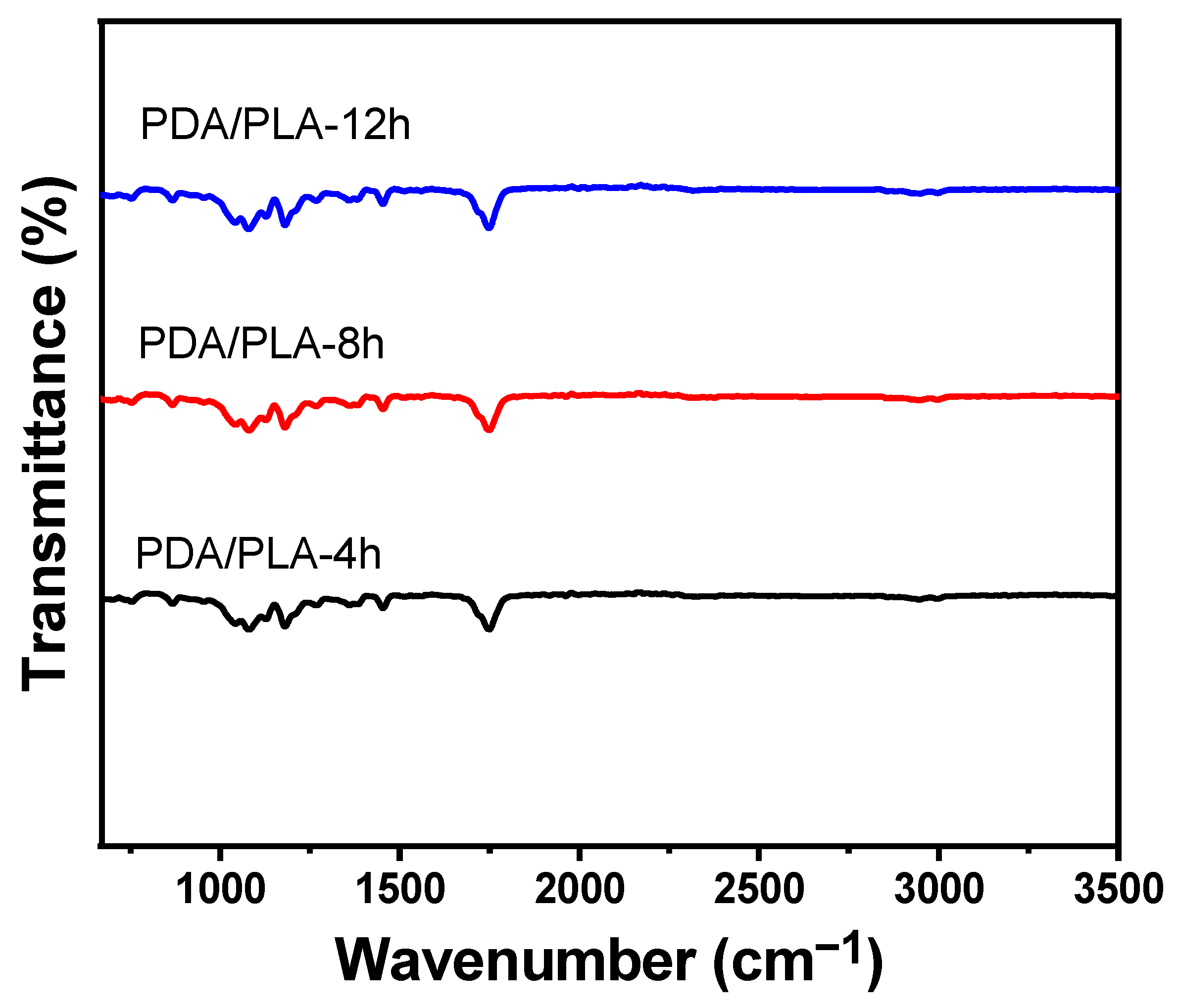
3.2. Analysis of Fourier Transform Infrared Spectroscopy
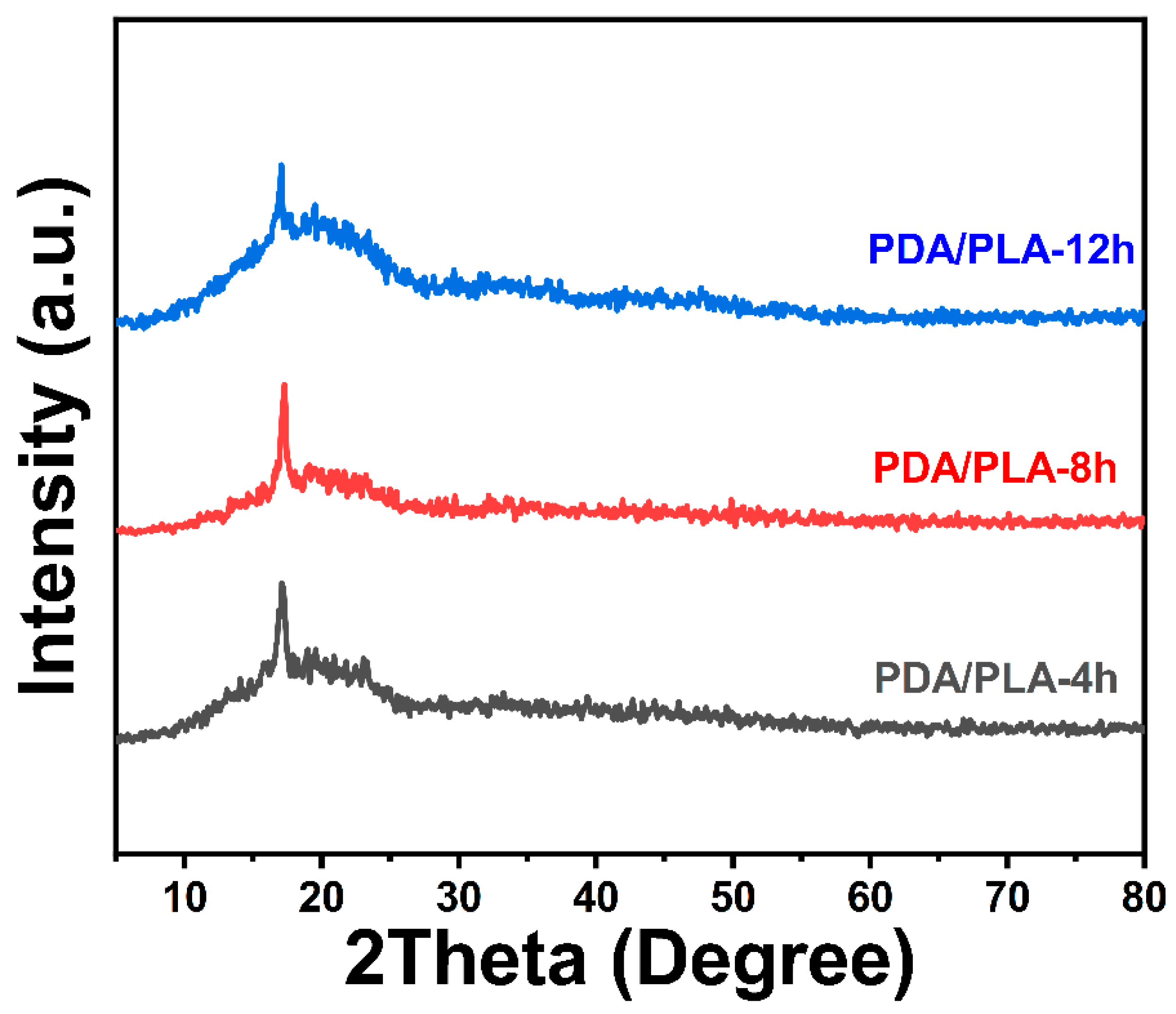
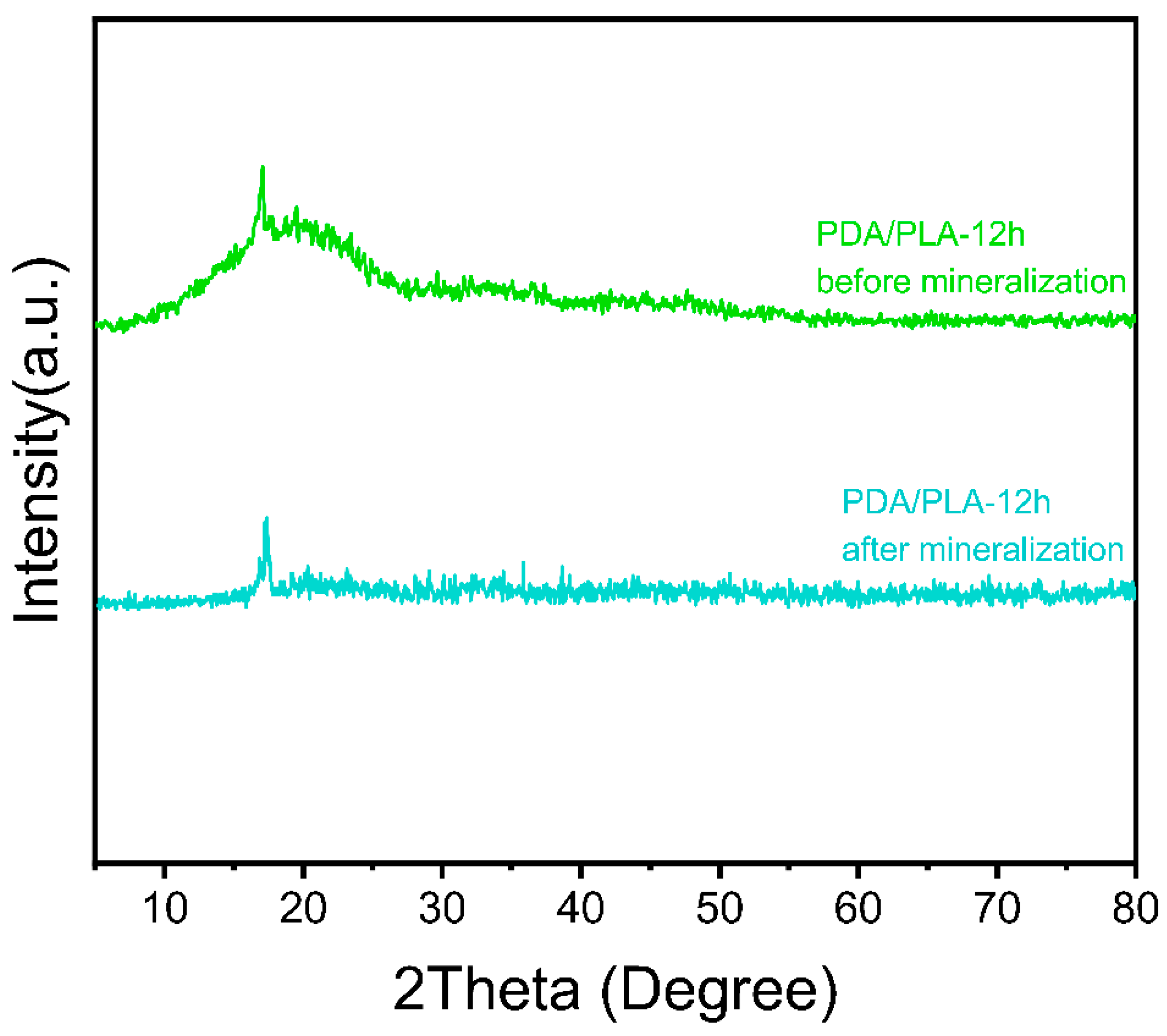
3.3. Analysis of Crystalline Structure
3.4. Hydrophilicity
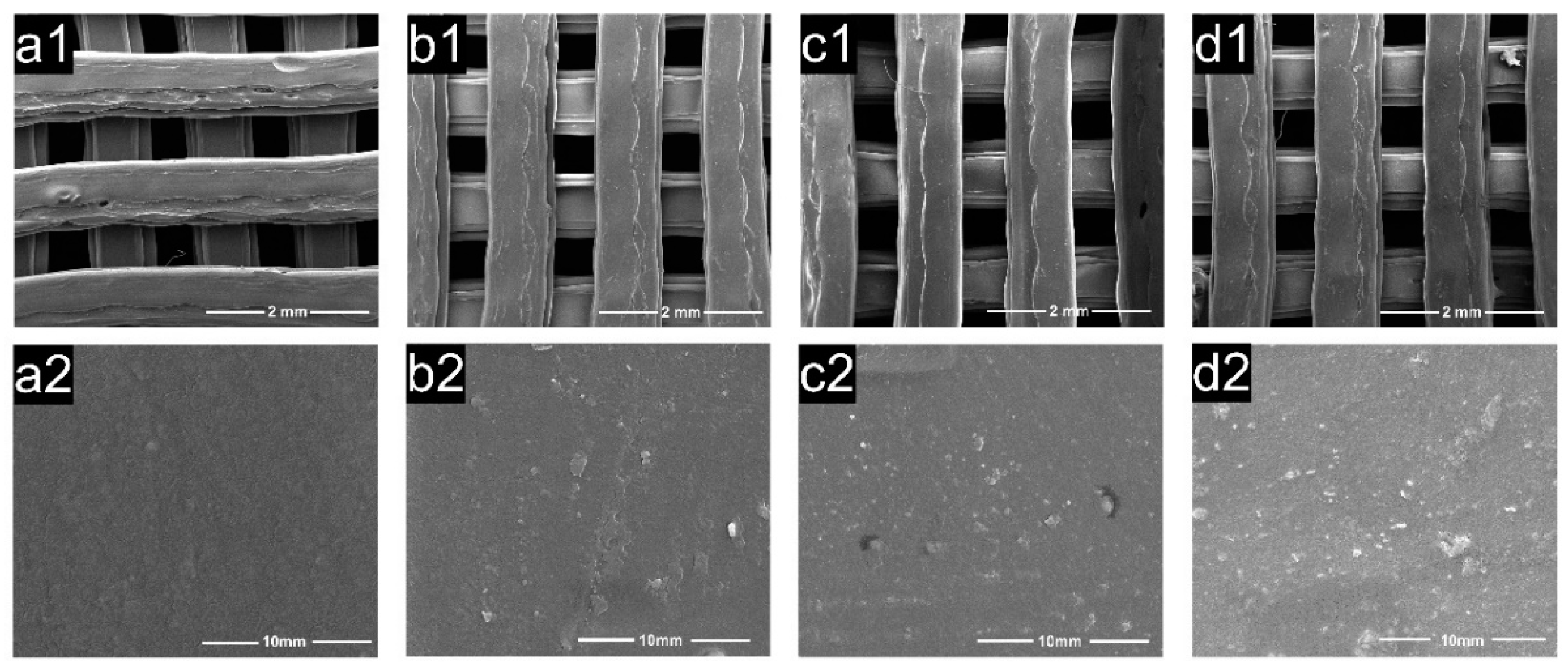
3.5. Micromorphology
3.6. Degradation In Vitro
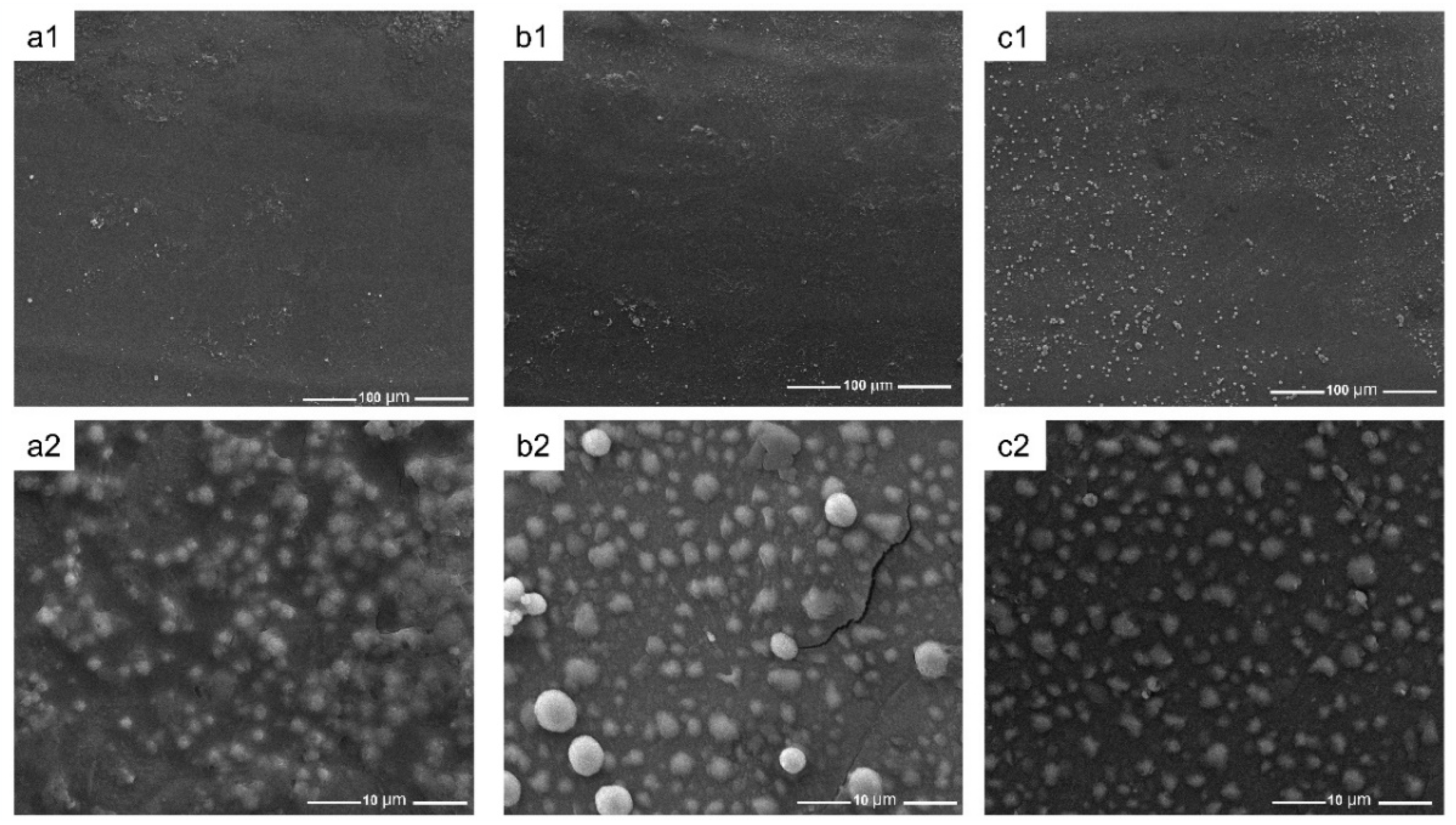
3.7. Mineralization In Vitro
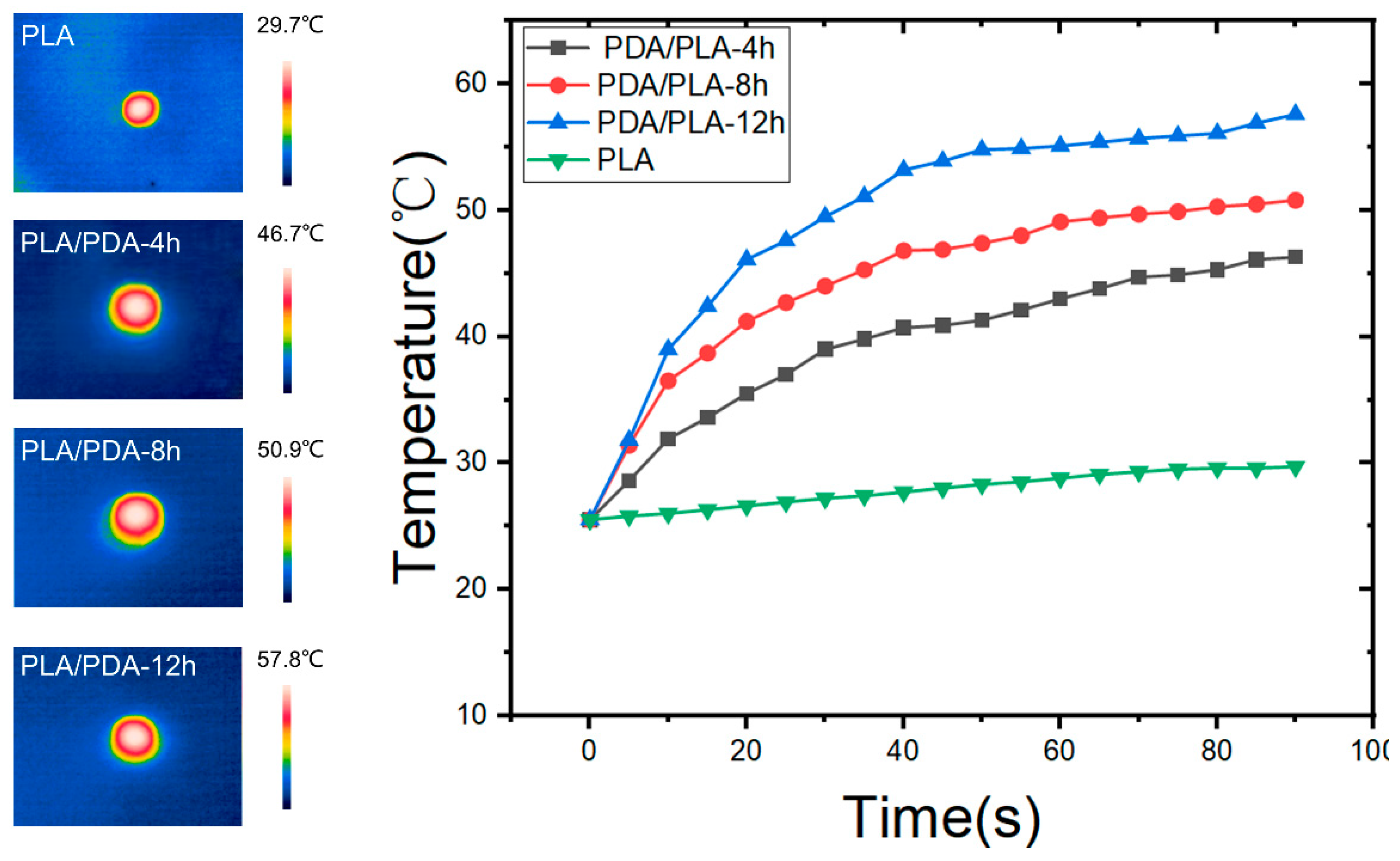
3.8. Near Infrared Response Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLA | Poly(lactic acid) |
| PDA | Poly(dopamine) |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscopy |
| EDS | Energy dispersive spectroscopy |
| WCA | Water contact angle |
| NIR | Near-infrared |
| FDM | Fused deposition modeling |
| FDA | Food and Drug Administration |
| PTT | Photothermal therapy |
| PTA | Photothermal agent |
| SBF | Simulated body fluid |
References
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, X.; Yan, G.; Chen, X.; Luo, K.; Zhou, S.; Zhang, P.; Li, J.; Wong, T.W. Biomimetic scaffolds with programmable pore structures for minimum invasive bone repair. Nanoscale 2021, 13, 16680–16689. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Kulkarni, K.; Kelderman, J.; Coleman, H.; Aguilar, M.I.; Parkington, H.; Del Borgo, M. Self-assembly of trifunctional tripeptides to form neural scaffolds. J. Mater. Chem. B 2021, 9, 4475–4479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xing, M.; Li, B. Biomimetic Layer-by-Layer Self-Assembly of Nanofilms, Nanocoatings, and 3D Scaffolds for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1641. [Google Scholar] [CrossRef]
- Dalton, P.D.; Klinkhammer, K.; Salber, J.; Klee, D.; Moller, M. Direct in vitro electrospinning with polymer melts. Biomacromolecules 2006, 7, 686–690. [Google Scholar] [CrossRef]
- Chen, T.; Zou, Q.; Du, C.; Wang, C.; Li, Y.; Fu, B. Biodegradable 3D printed HA/CMCS/PDA scaffold for repairing lacunar bone defect. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111148. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Piromalis, D. Fabricating Lattice Structures via 3D Printing: The Case of Porous Bio-Engineered Scaffolds. Appl. Mech. 2021, 2, 289–302. [Google Scholar] [CrossRef]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Z.; Geng, P.; Li, G.W.; Zhao, D.; Zhang, H.B.; Zhao, J. Influence of Layer Thickness and Raster Angle on the Mechanical Properties of 3D-Printed PEEK and a Comparative Mechanical Study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.C.; Huang, J.H.; Xia, X.; Wei, J.W.; Zou, Q.; Zuo, Y.; Li, J.D.; Li, Y.B. A hierarchical scaffold with a highly pore-interconnective 3D printed PLGA/n-HA framework and an extracellular matrix like gelatin network filler for bone regeneration. J. Mater. Chem. B 2021, 9, 4488–4501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.J.; Johnson, B.N.; Jia, X.F. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.S.; Kjems, J.; Bunger, C.; Lysdahl, H. Improvement of Distribution and Osteogenic Differentiation of Human Mesenchymal Stem Cells by Hyaluronic Acid and beta-Tricalcium Phosphate-Coated Polymeric Scaffold In Vitro. BioRes. Open Access 2015, 4, 363–373. [Google Scholar] [CrossRef]
- Naghieh, S.; Ravari, M.R.K.; Badrossamay, M.; Foroozmehr, E.; Kadkhodaei, M. Numerical investigation of the mechanical properties of the additive manufactured bone scaffolds fabricated by FDM: The effect of layer penetration and post-heating. J. Mech. Behav. Biomed. Mater. 2016, 59, 241–250. [Google Scholar] [CrossRef]
- Sa, M.W.; Nguyen, B.N.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef]
- Sood, A.K.; Ohdar, R.K.; Mahapatra, S.S. Parametric appraisal of mechanical property of fused deposition modelling processed parts. Mater. Des. 2010, 31, 287–295. [Google Scholar] [CrossRef]
- Sood, A.K.; Ohdar, R.K.; Mahapatra, S.S. Improving dimensional accuracy of Fused Deposition Modelling processed part using grey Taguchi method. Mater. Des. 2009, 30, 4243–4252. [Google Scholar] [CrossRef]
- Es-Said, O.S.; Foyos, J.; Noorani, R.; Mendelson, M.; Marloth, R.; Pregger, B.A. Effect of layer orientation on mechanical properties of rapid prototyped samples. Mater. Manuf. Process. 2000, 15, 107–122. [Google Scholar] [CrossRef]
- Feng, J. Preparation and properties of poly(lactic acid) melt spun fiber aligned and disordered scaffolds. Mater. Lett. 2017, 192, 153–156. [Google Scholar] [CrossRef]
- Kantaros, A. Bio-Inspired Materials: Exhibited Characteristics and Integration Degree in Bio-Printing Operations. Am. J. Eng. Appl. Sci. 2022, 15, 255–263. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Esposti, M.D.; Chiellini, F.; Aparicio, C.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Polylactic acid-based porous scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Gremare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’Heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 659–670. [Google Scholar] [CrossRef]
- Zhang, Y. Post-printing surface modification and functionalization of 3D-printed biomedical device. Int. J. Bioprint. 2017, 3, 001. [Google Scholar] [CrossRef]
- Tsai, W.B.; Chen, W.T.; Chien, H.W.; Kuo, W.H.; Wang, M.J. Poly(dopamine) coating to biodegradable polymers for bone tissue engineering. J. Biomater. Appl. 2014, 28, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.M.; Xiao, M.; Cao, J.F.; Zhao, L.Y.; Xu, N.; Long, S.R.; Fan, J.L.; Shao, K.; Sun, W.; Yan, X.H.; et al. NIR Light-Driving Barrier-Free Group Rotation in Nanoparticles with an 88.3% Photothermal Conversion Efficiency for Photothermal Therapy. Adv. Mater. 2020, 32, 1907855. [Google Scholar] [CrossRef]
- Dong, W.J.; Li, Y.S.; Niu, D.C.; Ma, Z.; Gu, J.L.; Chen, Y.; Zhao, W.R.; Liu, X.H.; Liu, C.S.; Shi, J.L. Facile Synthesis of Monodisperse Superparamagnetic Fe3O4 Core@hybrid@Au Shell Nanocomposite for Bimodal Imaging and Photothermal Therapy. Adv. Mater. 2011, 23, 5392–5397. [Google Scholar] [CrossRef]
- Huang, X.Q.; Tang, S.H.; Mu, X.L.; Dai, Y.; Chen, G.X.; Zhou, Z.Y.; Ruan, F.X.; Yang, Z.L.; Zheng, N.F. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.W.; Tang, M.H.; Sun, Y.G.; Zou, R.J.; Chen, Z.G.; Zhu, M.F.; Yang, S.P.; Wang, J.L.; Wang, J.H.; Hu, J.Q. Hydrophilic Flower-Like CuS Superstructures as an Efficient 980 nm Laser-Driven Photothermal Agent for Ablation of Cancer Cells. Adv. Mater. 2011, 23, 3542. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.A.; Zhang, G.X.; Sun, X.M.; Lee, S.T.; Liu, Z.A. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Boissonnault, J.A.; Dau, P.V.; Cohen, S.M. Metal-Organic Framework Regioisomers Based on Bifunctional Ligands. Angew. Chem.-Int. Ed. 2011, 50, 12193–12196. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Avolio, R.; Alfe, M.; Errico, M.E.; Napolitano, A.; d’Ischia, M. Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ai, K.L.; Liu, J.H.; Deng, M.; He, Y.Y.; Lu, L.H. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Gomez, S.; Vlad, M.D.; Lopez, J.; Fernandez, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Simpson, R.L.; Wiria, F.E.; Amis, A.A.; Chua, C.K.; Leong, K.F.; Hansen, U.N.; Chandraselkaran, M.; Lee, M.W. Development of a 95/5 poly(L-lactide-co-glycolide)/hydroxylapatite and beta-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 17–25. [Google Scholar] [CrossRef]
- Cavo, M.; Scaglione, S. Scaffold microstructure effects on functional and mechanical performance: Integration of theoretical and experimental approaches for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 872–879. [Google Scholar] [CrossRef]
- Roy, T.D.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J. Biomed. Mater. Res. Part A 2003, 66A, 283–291. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, H.; Nowicki, M.; Miao, S.; Lee, S.J.; Masood, F.; Harris, B.T.; Zhang, L.G. Three-Dimensional-Bioprinted Dopamine-Based Matrix for Promoting Neural Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 8993–9001. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, Y.; Chao, D.; Guan, C.; Zhang, Y.; Li, L.; Ge, X.; Bacho, I.M.; Tu, J.; Fan, H.J. Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale 2014, 6, 5008–5048. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, D.; Qiu, H.; Ju, Y.; Zhang, T.; Zhang, L.; Meng, Y.; Wei, Y.; Chen, G. Improved electrochemical properties of tavorite LiFeSO4F by surface coating with hydrophilic poly-dopamine via a self-polymerization process. RSC Adv. 2016, 6, 6523–6527. [Google Scholar] [CrossRef]
- Wang, G. Influence of polydopamine/polylactic acid coating on mechanical properties and cell behavior of 3D-printed calcium silicate scaffolds. Mater. Lett. 2020, 275, 128131. [Google Scholar] [CrossRef]
- Meng, C.J.; Zhao, J.M.; Yin, Y.X.; Luo, J.; Zhao, L.Y.; Jiang, W.B.; Feng, J.Y. Preparation and Characterization of PLA Film/3D Printing Composite Scaffold for Tissue Engineering Application. Fibers Polym. 2020, 21, 709–716. [Google Scholar] [CrossRef]
- Luo, K.; Wang, L.; Chen, X.H.; Zeng, X.Y.; Zhou, S.Y.; Zhang, P.C.; Li, J.F. Biocompatible Poly(epsilon-caprolactone)-based Shape-memory Polyurethane Composite Scaffold with Bone-induced Activity. J. Bionic Eng. 2022, 19, 167–178. [Google Scholar] [CrossRef]
- Promnil, S.; Ruksakulpiwat, C.; Numpaisal, P.O.; Ruksakulpiwat, Y. Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering. Polymers 2022, 14, 2435. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Rodrigues, E.D.; Wessling, M.; Stamatialis, D. Insights into the role of material surface topography and wettability on cell-material interactions. Soft Matter 2010, 6, 4377–4388. [Google Scholar] [CrossRef]
- Liu, X.S.; Cao, J.M.; Li, H.; Li, J.Y.; Jin, Q.; Ren, K.F.; Ji, J. Mussel-Inspired Polydopamine: A Biocompatible and Ultrastable Coating for Nanoparticles in Vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 2: Hyperthermia techniques. Crit. Rev. Biomed. Eng. 2006, 34, 491–542. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Li, J.; Chen, X.; Yang, Q.; Zeng, X.; Bai, R.; Wang, L. Photothermal Sensitive 3D Printed Biodegradable Polyester Scaffolds with Polydopamine Coating for Bone Tissue Engineering. Polymers 2023, 15, 381. https://doi.org/10.3390/polym15020381
Huang Z, Li J, Chen X, Yang Q, Zeng X, Bai R, Wang L. Photothermal Sensitive 3D Printed Biodegradable Polyester Scaffolds with Polydopamine Coating for Bone Tissue Engineering. Polymers. 2023; 15(2):381. https://doi.org/10.3390/polym15020381
Chicago/Turabian StyleHuang, Zuoxun, Junfeng Li, Xiaohu Chen, Qing Yang, Xiyang Zeng, Ruqing Bai, and Li Wang. 2023. "Photothermal Sensitive 3D Printed Biodegradable Polyester Scaffolds with Polydopamine Coating for Bone Tissue Engineering" Polymers 15, no. 2: 381. https://doi.org/10.3390/polym15020381
APA StyleHuang, Z., Li, J., Chen, X., Yang, Q., Zeng, X., Bai, R., & Wang, L. (2023). Photothermal Sensitive 3D Printed Biodegradable Polyester Scaffolds with Polydopamine Coating for Bone Tissue Engineering. Polymers, 15(2), 381. https://doi.org/10.3390/polym15020381




