Synergistic Effect of 4A Molecular Sieve on Intumescent Ternary H-Bonded Complex in Flame-Retarding of Polypropylene
Abstract
1. Introduction
2. Experimental
2.1. Materials
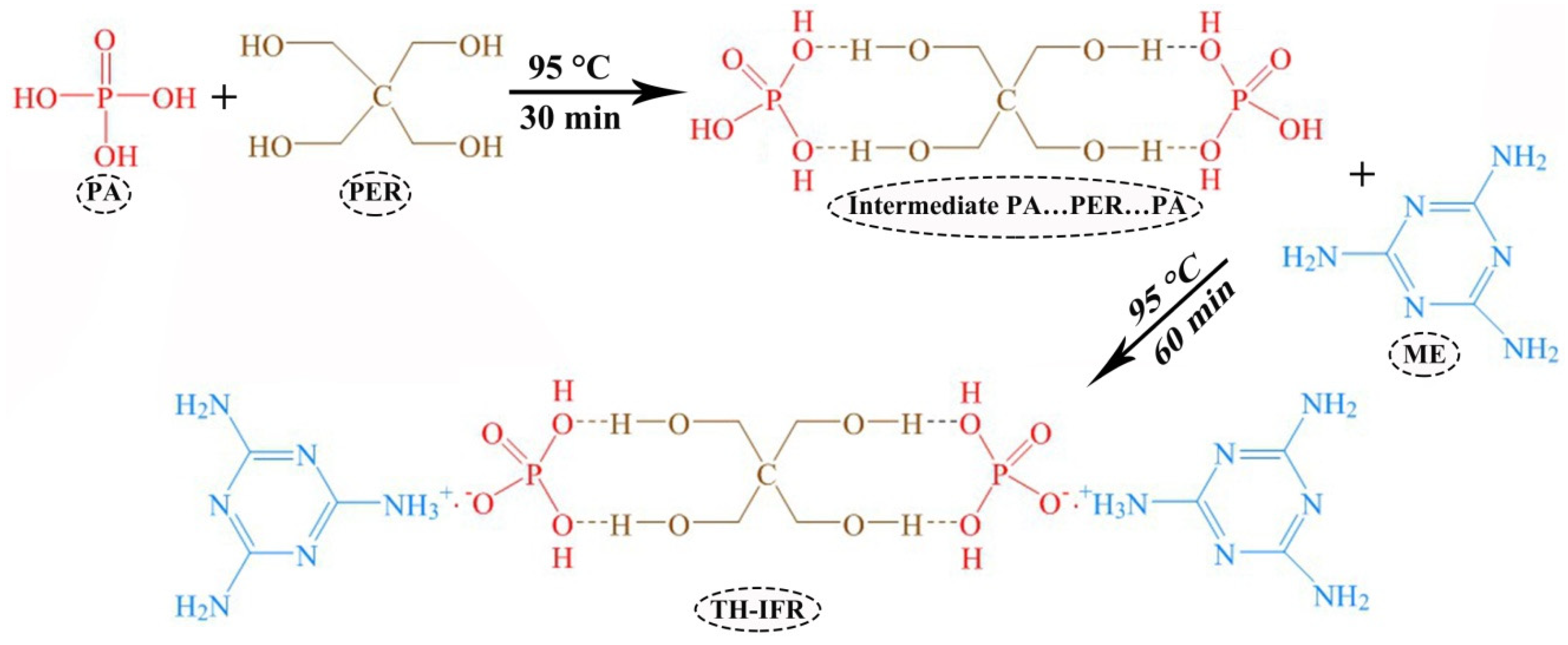
2.2. Synthesis of TH-IFR
2.3. Preparation of Flame-Retarded PP Composites
2.4. Characterization
3. Results and Discussion
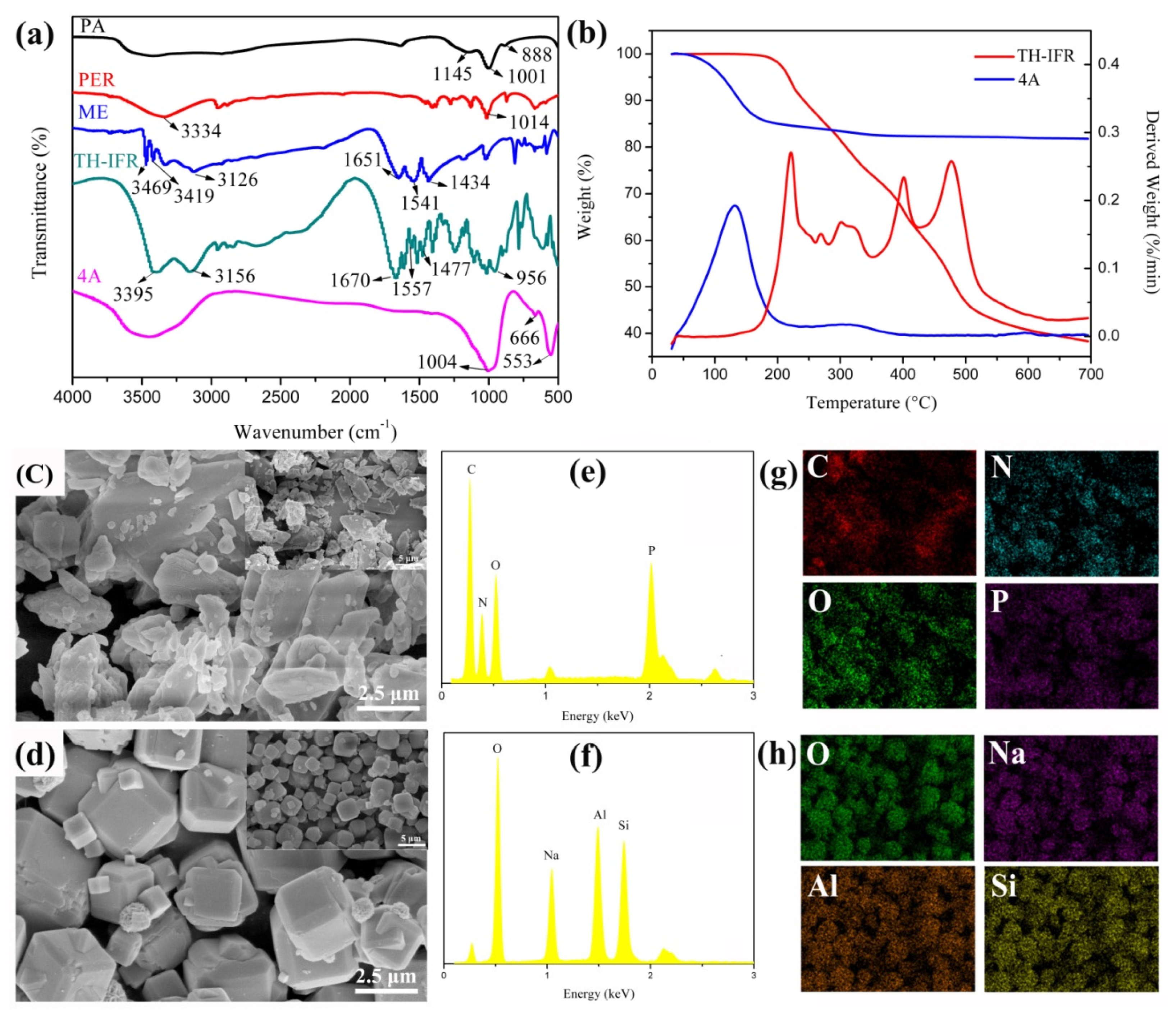
3.1. Synthesis and Characterization of TH-IFR
3.2. Characterization of Flame-Retarded PP Composities
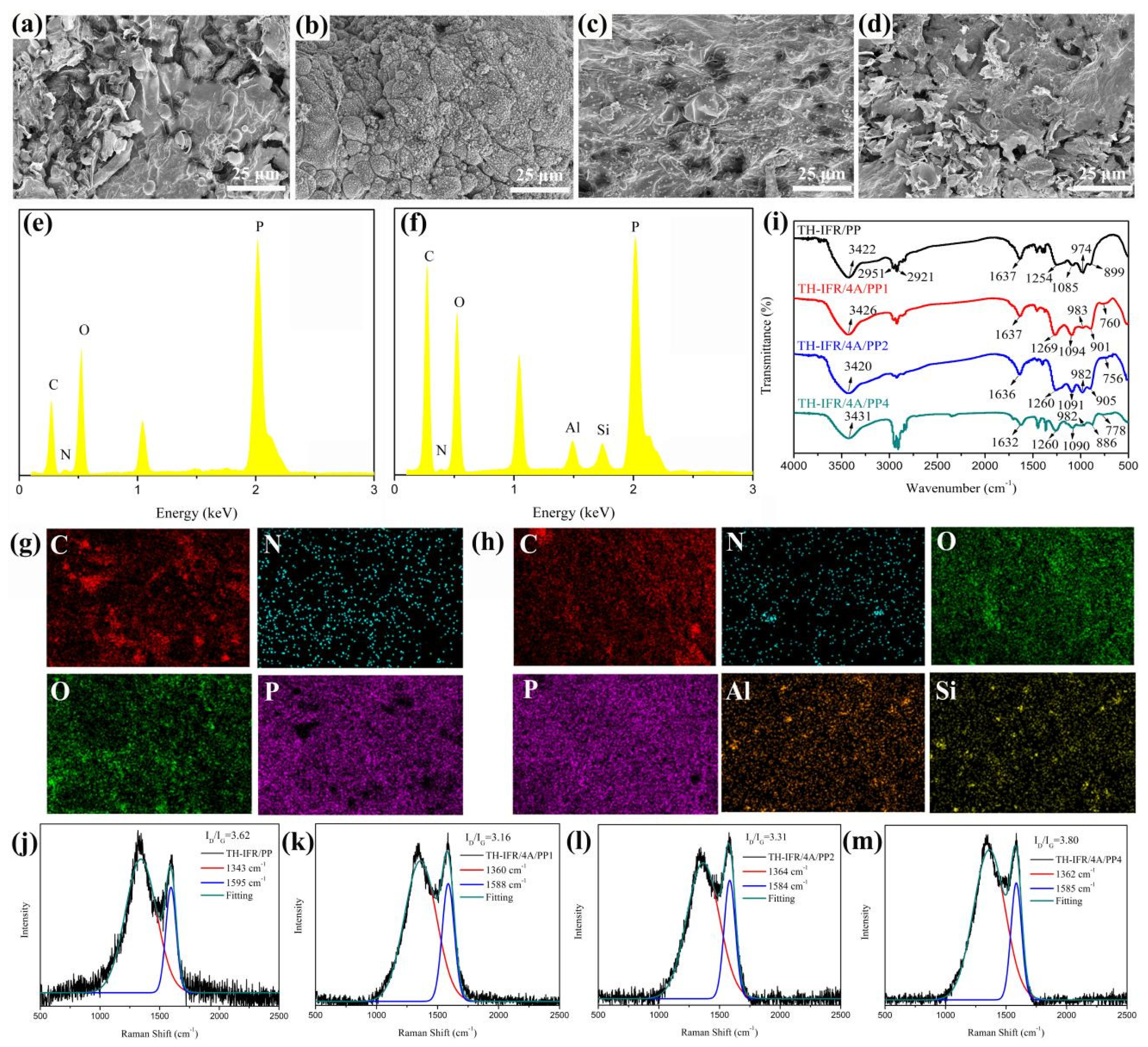
3.2.1. Morphology Analysis
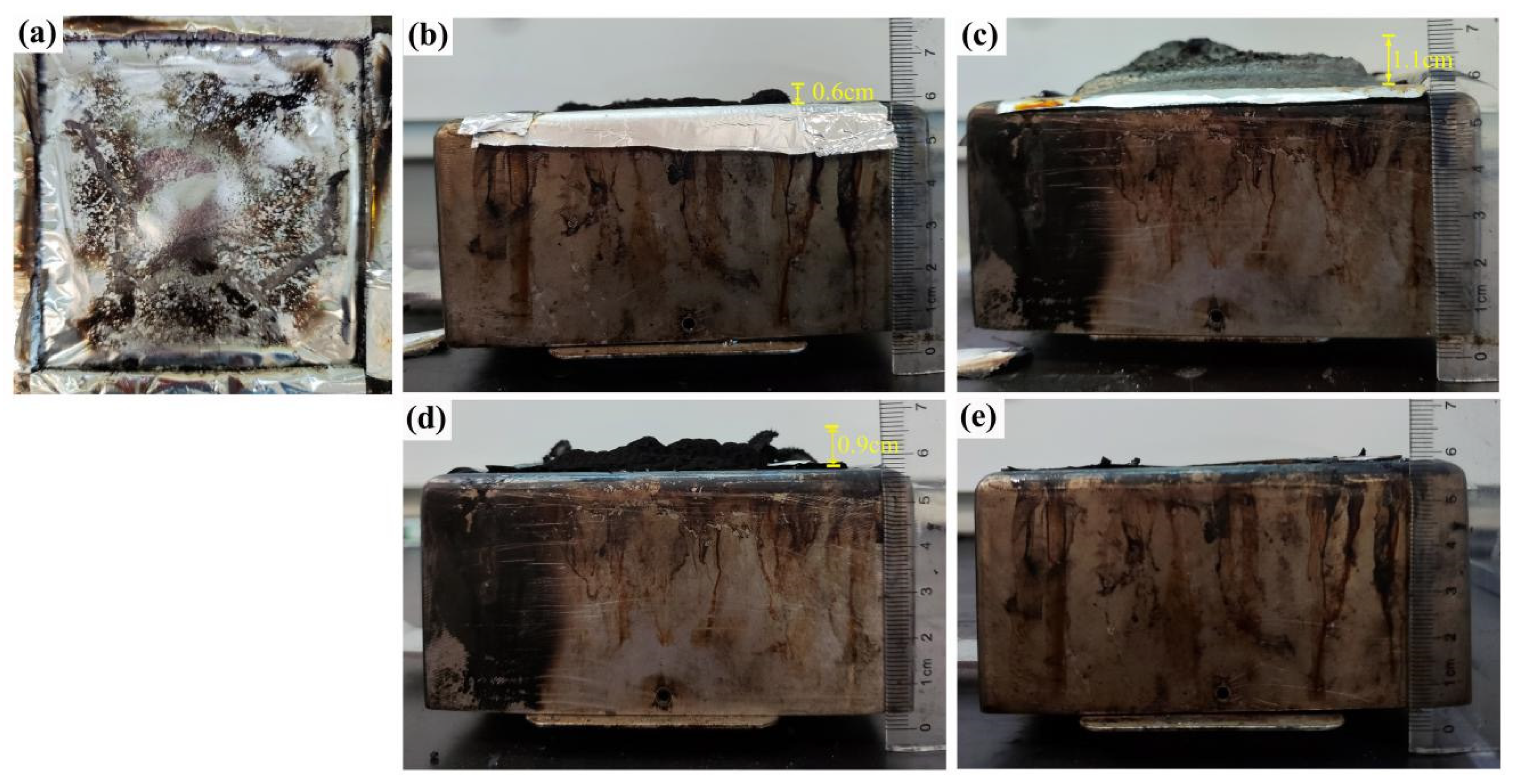
3.2.2. Flammability Performance
3.2.3. Thermal Performance
3.2.4. Carbon Residue Analysis
3.2.5. Synergistically Flame-Retarding Mechanism
3.2.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spoerk, M.; Holzer, C.; Gonzalez-Gutierrez, J. Material extrusion-based additive manufacturing of polypropylene: A review on how to improve dimensional inaccuracy and warpage. J. Appl. Polym. Sci. 2020, 137, 48545. [Google Scholar] [CrossRef]
- Xu, W.L.; Ma, P.B.; Jiang, G.M.; Wan, A.l. Mechanical properties of polypropylene warp-knitted hernia repair mesh with different pull densities. Polymers 2018, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Kundu, C.K.; Li, Z.W.; Li, X.H.; Zhang, Z.J. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. Part A-Appl. Sci. Technol. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Wang, N.; Chen, S.P.; Li, L.T.; Bai, Z.Y.; Guo, J.B.; Qin, J.; Chen, X.L.; Zhao, R.; Zhang, K.; Wu, H. An environmentally friendly nanohybrid flame retardant with outstanding flame-retardant efficiency for polypropylene. J. Phys. Chem. C 2021, 125, 5185–5196. [Google Scholar] [CrossRef]
- Shang, S.; Yuan, B.H.; Sun, Y.R.; Chen, G.Q.; Huang, C.Y.; Yu, B.; He, S.; Dai, H.M.; Chen, X.F. Facile preparation of layered melamine-phytate flame retardant via supramolecular self-assembly technology. J. Colloid. Interf. Sci. 2019, 553, 364–371. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Jin, Q.; Zhang, N.E.; Guo, X.R.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Yu, G.X.; Ma, C.; Li, J. Flame retardant effect of cytosine pyrophosphate and pentaerythritol on polypropylene. Compos. Part B-Eng. 2020, 180, 107520. [Google Scholar] [CrossRef]
- De Juan, S.; Zhang, J.H.; Acuña, P.; Nie, S.B.; Liu, Z.Q.; Zhang, W.; Luisa Puertas, M.; Esteban-Cubillo, A.; Santarén, J.; Wang, D.Y. An efficient approach to improving fire retardancy and smoke suppression for intumescent flame-retardant polypropylene composites via incorporating organo-modified sepiolite. Fire. Mater. 2019, 43, 961–970. [Google Scholar] [CrossRef]
- Yang, W.; Dong, X.; Nie, S.B.; Yang, J.N.; Zhang, X.F.; Wu, X.Q.; Fang, C.Y.; Su, H.L. Flame-retardant and thermal properties of highly efficient water-resistant intumescent flame-retardant polypropylene composites. J. Therm. Anal. Calorim. 2022, 147, 7323–7336. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, H.; Wang, Y.D.; Ma, Y.M.; Zhu, Z.M.; Lin, X.B. A novel highly efficient intumescent flame-retardant polypropylene: Thermal degradation, flame retardance and mechanism. J. Polym. Res. 2022, 29, 205. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G.S. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2017, 138, 106–114. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.H.; Zhang, M.D.; Yuan, G.W. Synergistic and compatibilizing effect of octavinyl polyhedral oligomeric silsesquioxane nanoparticles in polypropylene/intumescent flame retardant composite system. Compos. Part A-Appl. Sci. Technol. 2019, 123, 46–58. [Google Scholar] [CrossRef]
- Wu, K.; Shen, M.M.; Hu, Y. Synthesis of a novel intumescent flame retardant and its flame retardancy in polypropylene. J. Polym. Res. 2011, 18, 425–433. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, J.H.; Ding, A.X.; Ni, A.Q.; Chen, H.D. Flame retardancy and mechanical properties of glass fibre reinforced polyethylene composites filled with novel intumescent flame retardant. Compos. Part B-Eng. 2019, 179, 107555. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Makhlouf, G.; Hassan, M.A. Manufacturing, thermal stability, and flammability properties of polypropylene containing new single molecule intumescent flame retardant. Polym. Adv. Technol. 2019, 30, 1403–1414. [Google Scholar] [CrossRef]
- Lai, X.J.; Tang, S.; Li, H.Q.; Zeng, X.R. Flame-retardant mechanism of a novel polymeric intumescent flame retardant containing caged bicyclic phosphate for polypropylene. Polym. Degrad. Stab. 2015, 113, 22–31. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiao, C.M.; Li, S.X.; Hu, Y. Preparation and properties of a single molecule intumescent flame retardant. Fire. Saf. J. 2013, 58, 208–212. [Google Scholar] [CrossRef]
- Zhu, C.J.; He, M.S.; Liu, Y.; Cui, J.G.; Tai, Q.L.; Song, L.; Hu, Y. Synthesis and application of a mono-component intumescent flame retardant for polypropylene. Polym. Degrad. Stab. 2018, 151, 144–151. [Google Scholar] [CrossRef]
- Qi, H.S.; Liu, S.W.; Chen, X.L.; Shen, C.H.; Gao, S.J. The flame retardant and thermal performances of polypropylene with a novel intumescent flame retardant. J. Appl. Polym. Sci. 2020, 137, e49047. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Liu, Y.H.; Dai, B.Y.; Meng, C.Y.; Guo, Z.X. Fabrication of cellulose-based halogen-free flame retardant and its synergistic effect with expandable graphite in polypropylene. Carbohyd. Polym. 2019, 213, 257–265. [Google Scholar] [CrossRef]
- Liu, H.C.; Li, S.; Zhang, Z.Y.; Li, B.; Xu, M.J. An efficient and convenient strategy toward fire safety and water resistance of polypropylene composites through design and synthesis of a novel mono-component intumescent flame retardant. Polym. Adv. Technol. 2019, 30, 1543–1554. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.S.; Wang, X.H.; Deng, N.; Chu, Z.Y. Preparation of a novel mono-component intumescent flame retardant for enhancing the flame retardancy and smoke suppression properties of epoxy resin. J. Therm. Anal. Calorim. 2018, 134, 1505–1519. [Google Scholar] [CrossRef]
- Zhao, W.J.; Cheng, Y.M.; Li, Z.W.; Li, X.H.; Zhang, Z.J. Improvement in fire-retardant properties of polypropylene filled with intumescent flame retardants, using flower-like nickel cobaltate as synergist. J. Mater. Sci. 2021, 56, 2702–2716. [Google Scholar] [CrossRef]
- Yuan, B.H.; Fan, A.; Yang, M.; Chen, X.F.; Hu, Y.; Bao, C.L.; Jiang, S.H.; Niu, Y.; Zhang, Y.; He, S.; et al. The effects of graphene on the flammability and fire behavior of intumescent flame retardant polypropylene composites at different flame scenarios. Polym. Degrad. Stab. 2017, 143, 42–56. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Intumescent flame retardant-montmorillonite synergism in ABS nanocomposites. Appl. Clay. Sci. 2008, 42, 238–425. [Google Scholar] [CrossRef]
- Xu, Z.S.; Jia, H.Y.; Yan, L.; Chu, Z.Y.; Zhou, H. Synergistic effect of bismuth oxide and mono-component intumescent flame retardant on the flammability and smoke suppression properties of epoxy resins. Polym. Adv. Technol. 2019, 31, 25–35. [Google Scholar] [CrossRef]
- Feng, C.M.; Liang, M.Y.; Jiang, J.L.; Huang, J.G.; Liu, H.B. Flame retardancy and thermal degradation behavior of efficient intumescent flame retardant LDPE composite containing 4A zeotile. J. Anal. Appl. Pyrol. 2016, 118, 9–19. [Google Scholar] [CrossRef]
- Han, L.J.; Wu, W.H.; Qi, Y.X.; Qu, H.Q.; Xu, J.Z. Synergistic flame retardant effect of BiFeO3 in intumescent flame-retardant polypropylene composites. Polym. Compos. 2017, 38, 2771–2778. [Google Scholar] [CrossRef]
- Chen, X.Y.; Tao, T. Analysis of application of molecular sieve on the woody Fire-Retardant research. Appl. Mech. Mater. 2014, 585–586, 1395–1399. [Google Scholar] [CrossRef]
- Jiao, C.M.; Jiang, H.Z.; Chen, X.L. Reutilization of abandoned molecular sieve as flame retardant and smoke suppressant for thermoplastic polyurethane elastomer. J. Therm. Anal. Calorim. 2019, 138, 3905–3913. [Google Scholar] [CrossRef]
- Jiao, C.M.; Jiang, H.Z.; Chen, X.L. Properties of fire agent integrated with molecular sieve and tetrafluoroborate ionic liquid in thermoplastic polyurethane elastomer. Polym. Adv. Technol. 2019, 30, 2159–2167. [Google Scholar] [CrossRef]
- Luo, T.T.; Jiao, C.M.; Chen, X.L.; Jiang, H.Z. Flame-retardant effect of modified molecular sieve by ionic liquid in TPU. J. Therm. Anal. Calorim. 2021, 147, 4141–4150. [Google Scholar] [CrossRef]
- Yang, G.C.; Liu, J.; Xu, B.B.; Liu, Z.J.; Ma, F.; Zhang, Q.H. Effect of silicon-containing nitrogen and phosphorus flame-retardant system on the mechanical properties and thermal and flame-retardant behaviors of corrugated cardboard. J. Therm. Anal. Calorim. 2020, 145, 2321–2334. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, Y.H. Synthesis of a hydrogen-bonded complex intumescent flame retardant through supramolecular complexation and its application in LDPE foam. RSC Adv. 2017, 7, 31298–31309. [Google Scholar] [CrossRef]
- Pasban, S.; Raissi, H.; Mollania, F. Solvent effects on the structural, electronic properties and intramolecular N–H O hydrogen bond strength of 5-aminomethylene-pyrimidine-2,4,6 trion with DFT calculations. J. Mol. Liq. 2016, 215, 77–87. [Google Scholar] [CrossRef]
- Pan, Y.T.; Luo, Z.L.; Wang, B.B. Cross-linking modification of ammonium polyphosphate via ionic exchange and self-assembly for enhancing the fire safety properties of polypropylene. Polymers 2020, 12, 2761. [Google Scholar] [CrossRef]
- Demirhan, Y.; Yurtseven, R.; Usta, N. The effect of boric acid on flame retardancy of intumescent flame retardant polypropylene composites including nanoclay. J. Thermoplastic. Compos. 2021, 08927057211052327. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Bréant, P.; Tremillon, J.M. 4A zeolite synergistic agent in new flame retardant intumescent formulations of polyethylenic polymers-study of the effect of the constituent monomers. Polym. Degrad. Stab. 1996, 54, 275–287. [Google Scholar] [CrossRef]
- Demir, H.; Arkış, E.; Balköse, D.; Ülkü, S. Synergistic effect of natural zeolites on flame retardant additives. Polym. Degrad. Stab. 2005, 89, 478–483. [Google Scholar] [CrossRef]
- Feng, C.M.; Zhang, Y.; Liu, S.W.; Chi, Z.G.; Xu, J.R. Synergistic effects of 4A zeolite on the flame retardant properties and thermal stability of a novel halogen-free PP/IFR composite. Polym. Adv. Technol. 2013, 24, 478–486. [Google Scholar] [CrossRef]
- Zhang, M.D.; Zhang, W.C.; Chen, Y.H.; Yang, B. Preparation of efficiently intumescent-flame-retarded polypropylene composite: Synergistic effect of novel phosphorus-containing polyhedral oligomeric silsesquioxane. Plast. Rubber. Compos. 2021, 50, 464–476. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.S.; Deng, N.; Chu, Z.Y. Synergistic effects of mono-component intumescent flame retardant grafted with carbon black on flame retardancy and smoke suppression properties of epoxy resins. J. Therm. Anal. Calorim. 2019, 138, 915–927. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, H.; Liu, Y.; Wang, Q. Synergistic effect between piperazine pyrophosphate and melamine polyphosphate in flame retarded glass fiber reinforced polypropylene. Polym. Degrad. Stab. 2021, 184, 109477. [Google Scholar] [CrossRef]
- Liu, L.B.; Xu, Y.; He, Y.T.; Xu, M.J.; Shi, Z.X.; Hu, H.C.; Yang, Z.C.; Li, B. An effective mono-component intumescent flame retardant for the enhancement of water resistance and fire safety of thermoplastic polyurethane composites. Polym. Degrad. Stab. 2019, 167, 146–157. [Google Scholar] [CrossRef]
- Ding, H.Y.; Wang, J.F.; Wang, C.P.; Chu, F.X. Synthesis of a novel phosphorus and nitrogen-containing bio-based polyols and its application in flame retardant polyurethane sealant. Polym. Degrad. Stab. 2016, 124, 43–50. [Google Scholar] [CrossRef]
- Yuan, B.H.; Hu, Y.; Chen, X.F.; Shi, Y.Q.; Niu, Y.; Zhang, Y.; He, S.; Dai, H.M. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A-Appl. Sci. Technol. 2017, 100, 106–117. [Google Scholar] [CrossRef]





| Sample | Composition (%) | Flammability | ||||
|---|---|---|---|---|---|---|
| PP | TH-IFR | 4A | LOI (%) | UL-94 | Drpping | |
| PP | 100.0 | - | - | 19.0 (±0.6) | No rating | Yes |
| TH-IFR/PP | 78.0 | 22.0 | - | 27.6 (±0.7) | V-1 | No |
| TH-IFR/4A/PP1 | 78.0 | 21.0 | 1.0 | 30.1 (±0.3) | V-0 | No |
| TH-IFR/4A/PP2 | 78.0 | 20.0 | 2.0 | 28.5 (±0.6) | V-1 | No |
| TH-IFR/4A/PP3 | 78.0 | 19.0 | 3.0 | 27.0 (±0.5) | V-2 | Yes |
| TH-IFR/4A/PP4 | 78.0 | 18.0 | 4.0 | 25.9 (±0.4) | No rating | Yes |
| Sample | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | pSPR (m2/s) | pMLR (g/s) | av-EHC (MJ/kg) | FPI (s·m2/kW) | Residue (%) |
|---|---|---|---|---|---|---|---|---|
| PP | 28 | 850.19 | 108.52 | 0.129 | 0.181 | 40.44 | 0.033 | 0 |
| TH-IFR/PP | 21 | 390.28 | 99.16 | 0.066 | 0.097 | 32.82 | 0.054 | 5.64 |
| TH-IFR/4A/PP1 | 21 | 212.42 | 90.04 | 0.045 | 0.056 | 30.26 | 0.099 | 9.08 |
| TH-IFR/4A/PP2 | 22 | 318.76 | 97.20 | 0.054 | 0.096 | 32.66 | 0.069 | 6.38 |
| TH-IFR/4A/PP4 | 23 | 474.54 | 101.34 | 0.074 | 0.115 | 33.80 | 0.048 | 2.21 |
| Sample | Tonset (°C) | Tmax (°C) | MDR (%/min) | C700 °C (%) | T700 °C (%) | Variation (%) |
|---|---|---|---|---|---|---|
| PP | 400 | 449 | 2.61 | 0 | - | - |
| TH-IFR/PP | 302 | 446 | 2.50 | 9.61 | 8.42 | 14.13 |
| TH-IFR/4A/PP1 | 290 | 448 | 2.16 | 12.75 | 8.86 | 43.39 |
| TH-IFR/4A/PP2 | 303 | 446 | 2.42 | 10.67 | 9.29 | 14.85 |
| TH-IFR/4A/PP3 | 314 | 447 | 2.53 | 10.34 | 9.73 | 6.27 |
| TH-IFR/4A/PP4 | 333 | 446 | 2.61 | 9.72 | 10.16 | −4.33 |
| Sample | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|
| PP | 114.55 | 116.2 | 166.54 | 99.50 | 47.61 |
| TH-IFR/PP | 122.99 | 77.42 | 166.80 | 61.71 | 37.85 |
| TH-IFR/4A/PP1 | 120.80 | 82.40 | 167.32 | 74.27 | 45.56 |
| TH-IFR/4A/PP2 | 121.16 | 89.51 | 166.75 | 78.47 | 48.14 |
| TH-IFR/4A/PP3 | 122.49 | 89.90 | 166.19 | 79.49 | 48.76 |
| TH-IFR/4A/PP4 | 122.75 | 90.27 | 165.49 | 82.99 | 50.91 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PP | 30.3 (±0.3) | 77.5 (±2.1) |
| TH-IFR/PP | 27.6 (±0.4) | 47.7 (±1.0) |
| TH-IFR/4A/PP1 | 28.9 (±0.2) | 31.6 (±1.8) |
| TH-IFR/4A/PP2 | 27.8 (±0.1) | 43.3 (±2.8) |
| TH-IFR/4A/PP3 | 27.6 (±0.6) | 44.0 (±3.0) |
| TH-IFR/4A/PP4 | 27.5 (±0.2) | 44.6 (±2.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.; Chen, Y.; Wang, X.; Pei, H. Synergistic Effect of 4A Molecular Sieve on Intumescent Ternary H-Bonded Complex in Flame-Retarding of Polypropylene. Polymers 2023, 15, 374. https://doi.org/10.3390/polym15020374
Wen Q, Chen Y, Wang X, Pei H. Synergistic Effect of 4A Molecular Sieve on Intumescent Ternary H-Bonded Complex in Flame-Retarding of Polypropylene. Polymers. 2023; 15(2):374. https://doi.org/10.3390/polym15020374
Chicago/Turabian StyleWen, Qilin, Yinghong Chen, Xin Wang, and Haoran Pei. 2023. "Synergistic Effect of 4A Molecular Sieve on Intumescent Ternary H-Bonded Complex in Flame-Retarding of Polypropylene" Polymers 15, no. 2: 374. https://doi.org/10.3390/polym15020374
APA StyleWen, Q., Chen, Y., Wang, X., & Pei, H. (2023). Synergistic Effect of 4A Molecular Sieve on Intumescent Ternary H-Bonded Complex in Flame-Retarding of Polypropylene. Polymers, 15(2), 374. https://doi.org/10.3390/polym15020374







