Degradation of Structurally Modified Polylactide under the Controlled Composting of Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composting Conditions
2.3. Characterization of PLA Samples
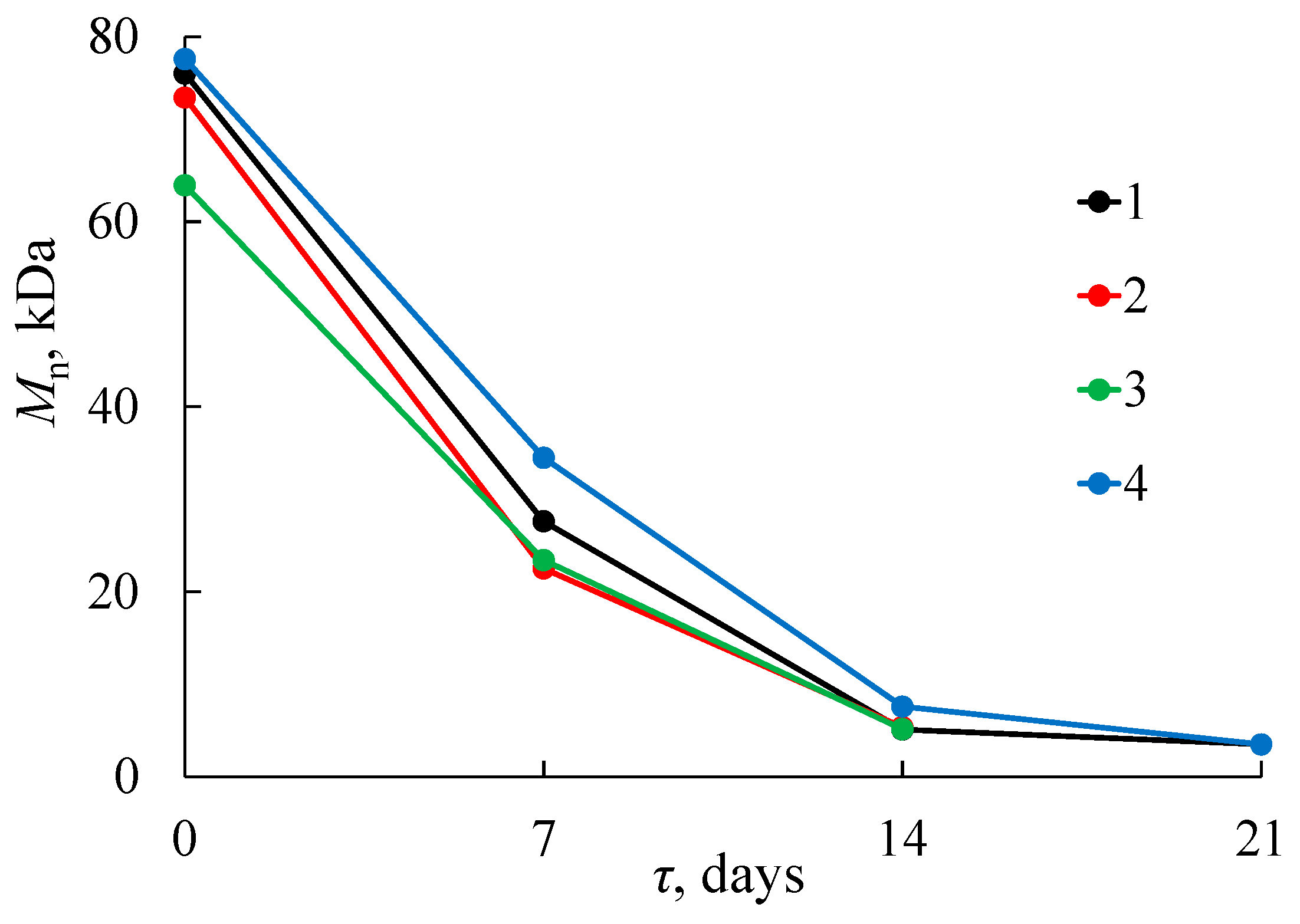
2.3.1. Gel Permeation Chromatography (GPC)
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Determination of Shape Stability
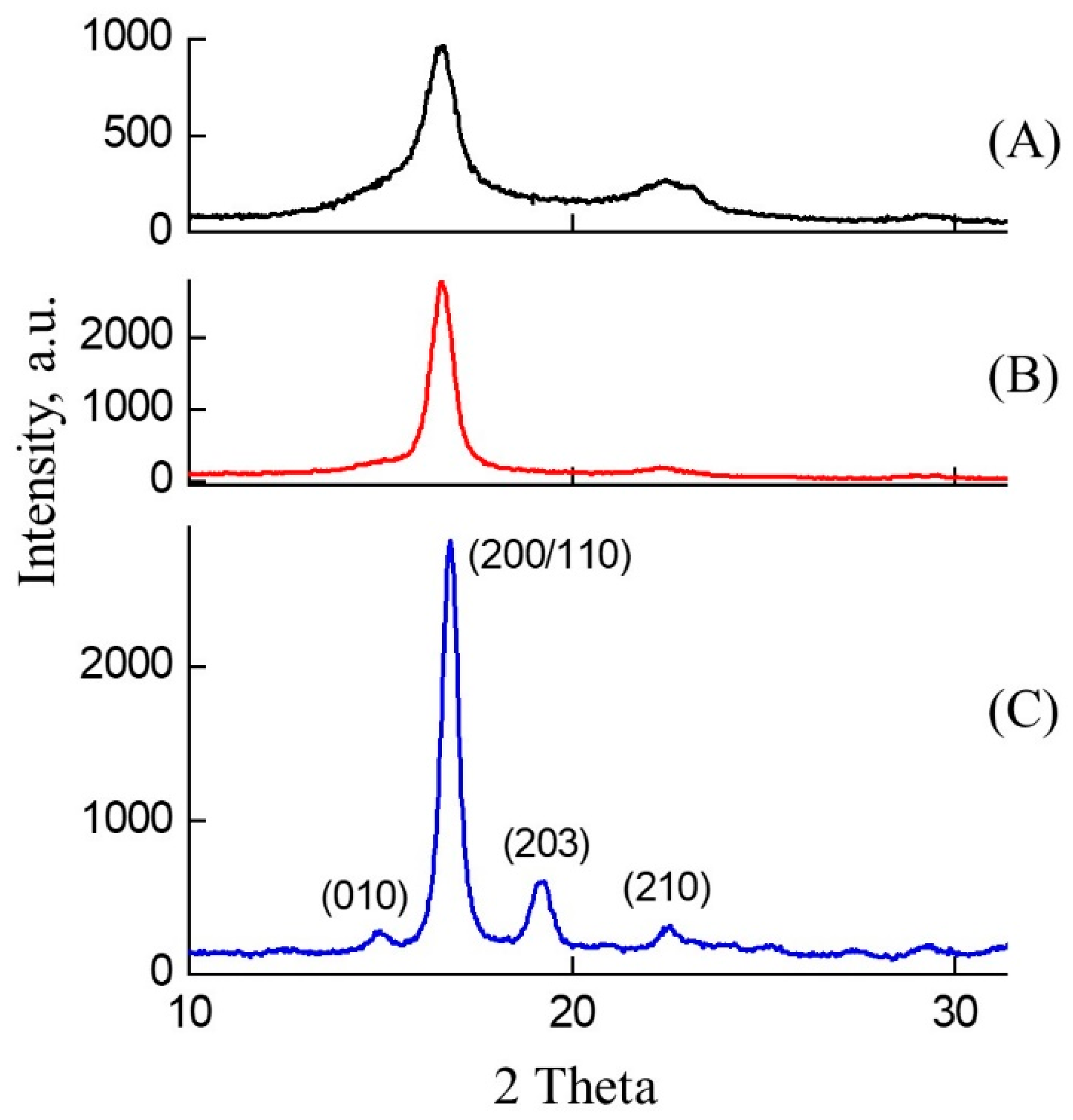
2.3.4. X-ray Diffraction Method (XRD)
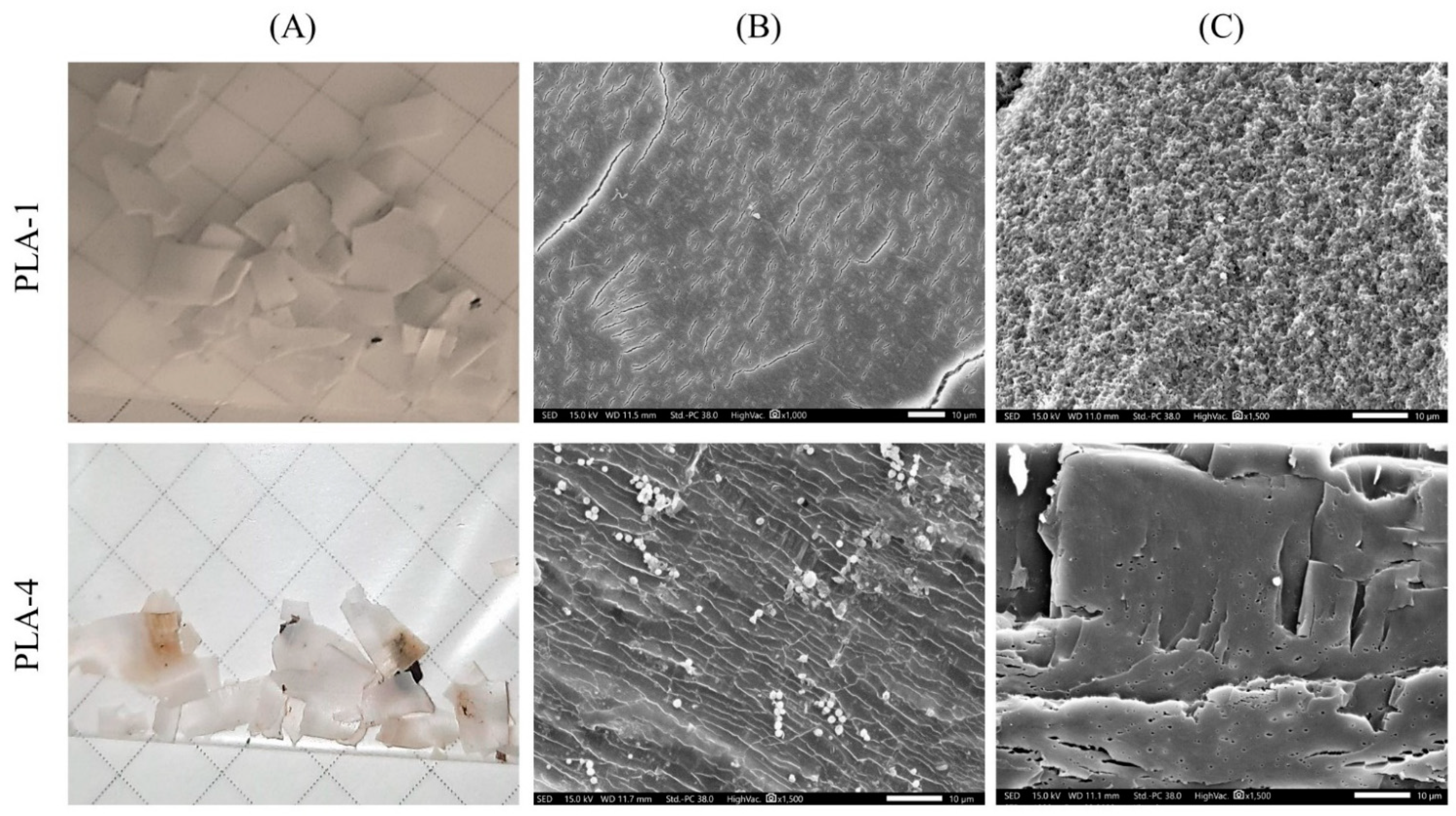
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Stress–Strain Test
2.3.7. Statistical Analysis
2.4. Isolation of Pure Cultures of Microorganisms-Destructors of PLA
3. Results
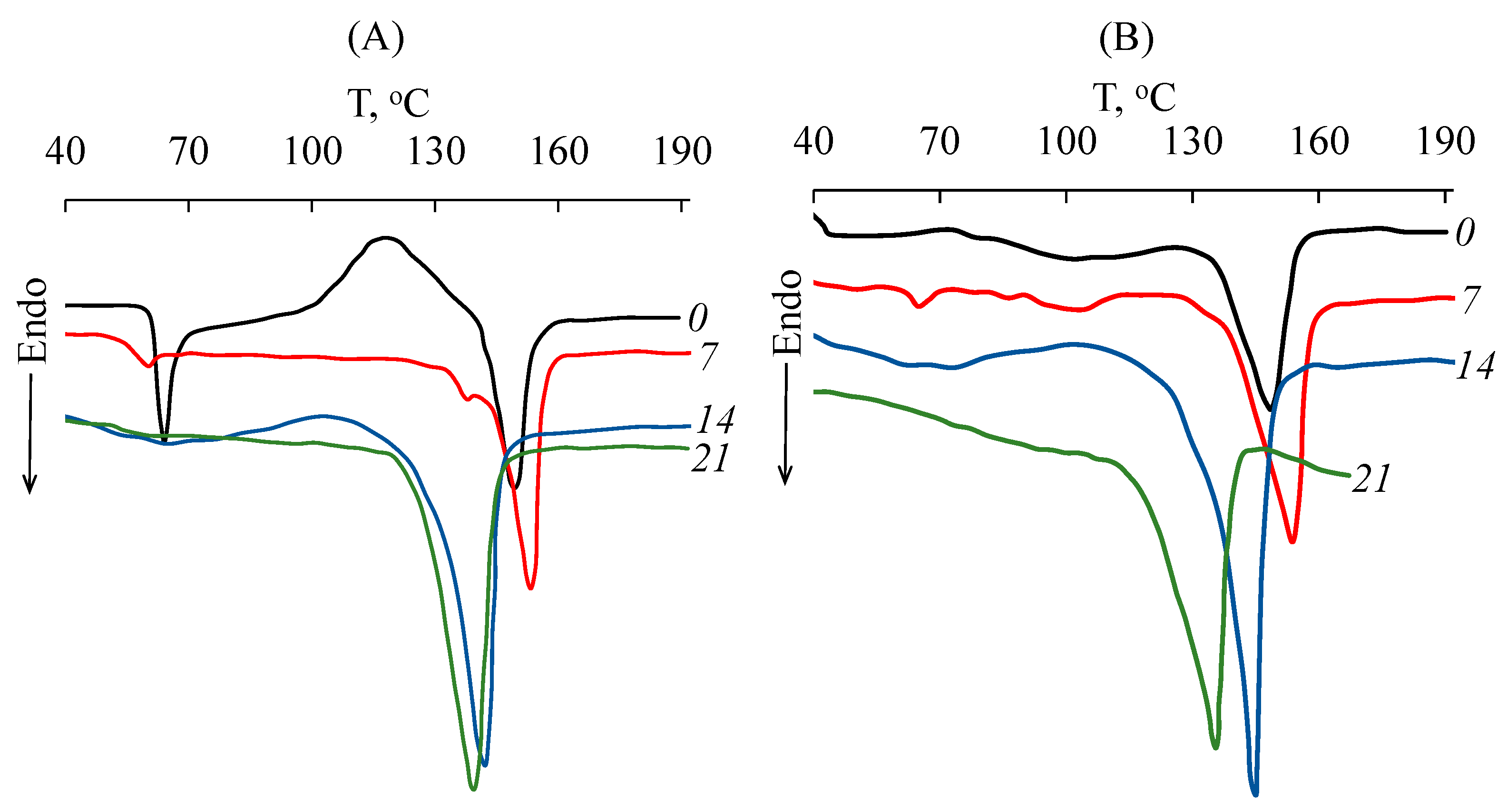
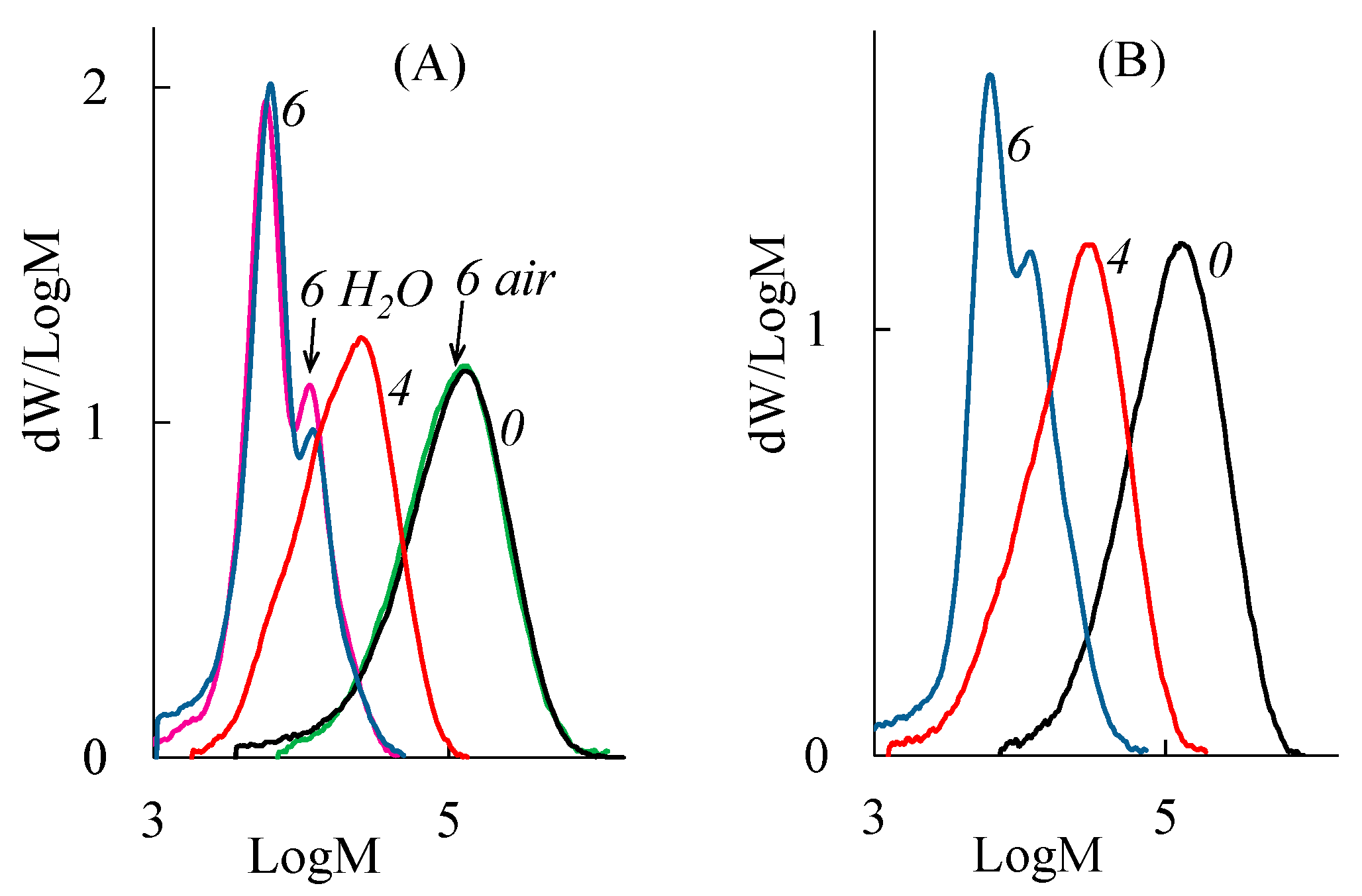
3.1. Composting of PLA in the SC Substrate
3.2. Composting of PLA in the AC Substrate
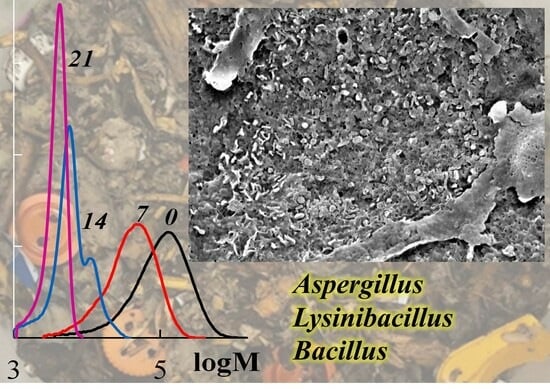
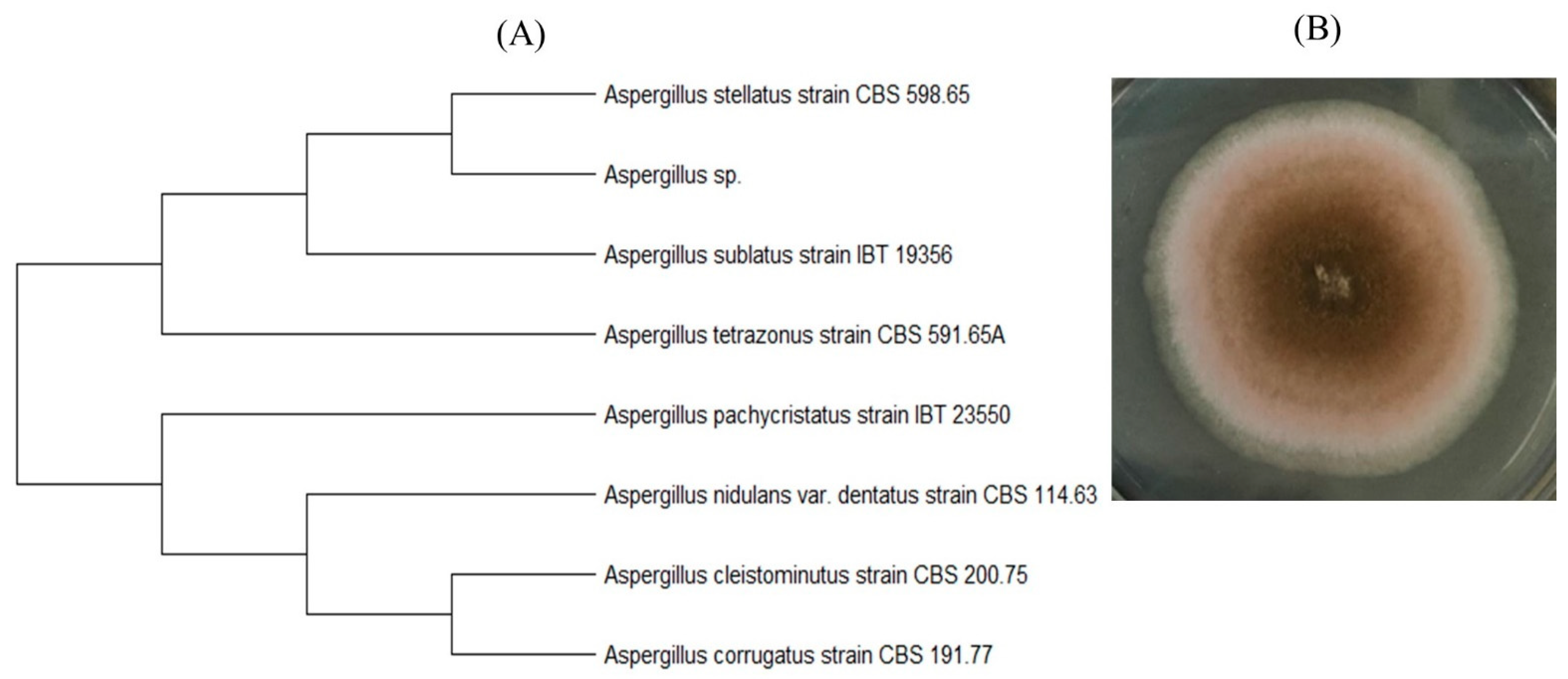
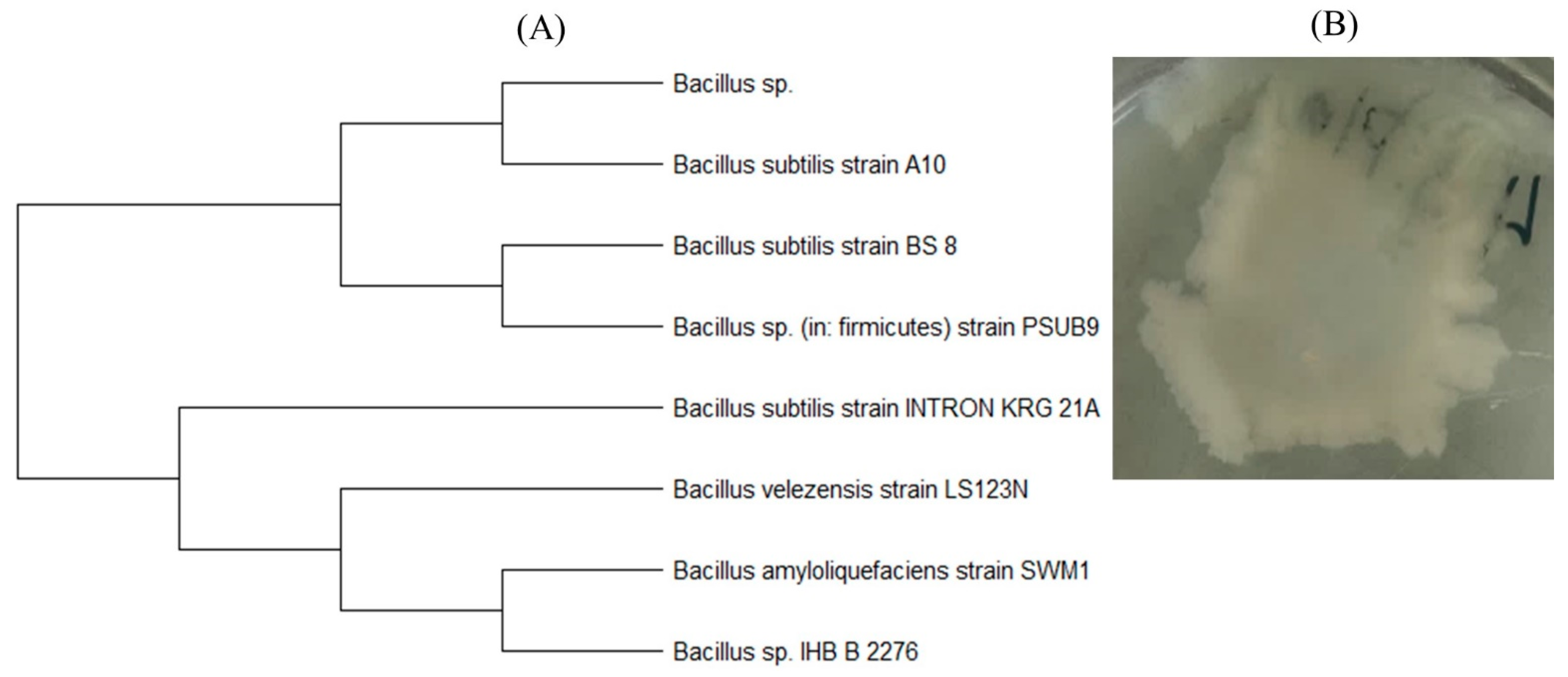
3.3. Identification of Microorganisms–Destructors of PLA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties-From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; de Nisi, P.; Tambone, F.; Adani, F. The role of waste management in reducing bioplastics’ leakage into the environment: A review. Bioresour. Technol. 2021, 337, 125459. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Taib, M.R.; Ghaleb, Z.A.; Mohd Ishak, Z.A. Thermal, mechanical, and morphological properties of polylactic acid toughened with an impact modifier. J. Appl. Polym. Sci. 2012, 123, 2715–2725. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Stefaniak, K.; Masek, A. Green Copolymers Based on Poly(Lactic Acid)-Short Review. Materials 2021, 14, 5254. [Google Scholar] [CrossRef]
- Ge, H.; Yang, F.; Hao, Y.; Wu, G.; Zhang, H.; Dong, L. Thermal, mechanical and rheological properties of plasticized poly (L-lactic acid). J. Appl. Polym. Sci. 2013, 127, 2832–2839. [Google Scholar] [CrossRef]
- Zych, A.; Perotto, G.; Trojanowska, D.; Tedeschi, G.; Bertolacci, L.; Francini, N.; Athanassiou, A. Super Tough Polylactic Acid Plasticized with Epoxidized Soybean Oil Methyl Ester for Flexible Food Packaging. ACS Appl. Polym. Mater. 2021, 3, 5087–5095. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Rodríguez, S.; González-Torres, M.; Ribas-Aparicio, R.M.; Del Prado-Audelo, M.L.; Leyva-Gómez, G.; Gürer, E.S.; Sharifi-Rad, J. Recent advances in modified poly (lactic acid) as tissue engineering materials. J. Biol. Eng. 2023, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Wang, S. Why Is Crystalline Poly(lactic acid) Brittle at Room Temperature? Macromolecules 2019, 52, 5429–5441. [Google Scholar] [CrossRef]
- Gao, X.R.; Li, Y.; Huang, H.D.; Jia-Zhuang, X.; Ling, X.; Xu, J.; Gan-Ji, Z.; Li, Z.M. Extensional Stress-Induced Orientation and Crystallization can Regulate the Balance of Toughness and Stiffness of Polylactide Films: Interplay of Oriented Amorphous Chains and Crystallites. Macromolecules 2019, 52, 5278–5288. [Google Scholar] [CrossRef]
- Khavpachev, M.; Trofimchuk, E.; Nikonorova, N.; Garina, E.; Moskvina, M.; Efimov, A.; Demina, V.; Bakirov, A.; Sedush, N.; Potseleev, V.; et al. Bioactive polylactide fibrous materials prepared by crazing mechanism. Macromol. Mater. Eng. 2020, 305, 2000163. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Shen, C.; Liu, C.; Wang, Z. Melt extension-induced shish-kebabs with heterogeneous spatial distribution of crystalline polymorphs in lightly crosslinked poly(lactic acid). Polymer 2020, 208, 122875. [Google Scholar] [CrossRef]
- Lyu, S.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and Time−Temperature Equivalence of Polymer Degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef]
- Kishore, G.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Upadhya, N.P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Beltrán-Sanahuja, A.; Benito-Kaesbach, A.; Sánchez-García, N.; Sanz-Lázaro, C. Degradation of conventional and biobased plastics in soil under contrasting environmental conditions. Sci. Total Environ. 2021, 787, 147678. [Google Scholar] [CrossRef]
- Calmon, A.; Guillaume, S.; Bellon-Maurel, V.; Feuilloley, P.; Silvestre, F. An automated test for measuring polymer biodegradation. J. Polym. Environ. 1999, 7, 157–166. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.; Figueiredo, J.L. Towards Controlled Degradation of Poly(lactic) Acid in Technical Applications. C—J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Schindler, A. Aliphatic polyesters. I. The degradation of poly(ε-caprolactone) in 150 vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Limsukon, W.; Auras, R.; Smith, T. Effects of the Three-Phase Crystallization Behavior on the Hydrolysis of Amorphous and Semicrystalline Poly(lactic acid)s. ACS Appl. Polym. Mater. 2021, 3, 5920–5931. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, H.; Dong, Z.; Ouyang, Y.; Li, Y.; Huang, Q.; Wang, Z. Structural Evolution of Polyglycolide and Poly(glycolide-co-lactide) Fibers during In Vitro Degradation with Different Heat-Setting Temperatures. ACS Omega 2021, 6, 29254–29266. [Google Scholar] [CrossRef]
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable Polylactic Acid (PLA)-Based Sustainable Engineered Blends and Biocomposites: Recent Developments, Challenges, and Opportunities. ACS Eng. 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikarash, K.; Fukuda, N. Poly(l-lactide): XII. Formation, growth, and morphology of crystalline residues as extended-chain crystallites through hydrolysis of poly(l-lactide) films in phosphate-buffered solution. Polym. Degrad. Stab. 2004, 84, 515–523. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Moskvina, M.A.; Nikonorova, N.I.; Efimov, A.V.; Garina, E.S.; Grokhovskaya, T.E.; Ivanova, O.A.; Bakirov, A.V.; Sedush, N.G.; Chvalun, S.N. Hydrolytic degradation of polylactide films deformed by the environmental crazing mechanism. Eur. Polym. J. 2020, 139, 110000. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Mironov, V.V.; Bochkova, E.A.; Litti, Y.V.; Russkova, Y.I. The composition of the microbial community at different stages of composting, the prospect of obtaining compost from municipal organic waste (review). Appl. Biochem. Microbiol. 2019, 55, 211–221. [Google Scholar] [CrossRef]
- Mitchell, M.K.; Hirt, D.E. Degradation of PLA fibers at elevated temperature and humidity. Polym. Eng. Sci. 2014, 55, 1652–1660. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P. Comparison of the Degradability of Poly(lactide) Packages in Composting and Ambient Exposure Conditions. Packag. Technol. Sci. 2007, 20, 49–70. [Google Scholar] [CrossRef]
- Mironov, V.; Vanteeva, A.; Merkel, A. Microbiological activity during co-composting of food and agricultural waste for soil amendment. Agronomy 2021, 11, 928. [Google Scholar] [CrossRef]
- Mironov, V.V.; Trofimchuk, E.S.; Zagustina, N.A.; Ivanova, O.A.; Vanteeva, A.V.; Bochkova, E.A.; Ostrikova, V.V.; Zhang, S. Solid-Phase Biodegradation of Polylactides (Review). Appl. Biochem. Microbiol. 2022, 58, 665–676. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Kotova, I.B.; Taktarova, Y.V.; Tsavkelova, E.A.; Egorova, M.A.; Bubnov, I.A.; Malakhova, D.V.; Shirinkina, L.I.; Sokolova, T.G.; Bonch-Osmolovskaya, E.A. Microbial degradation of plastic and ways of its intensification. Microbiology 2021, 90, 627–659. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Efimov, A.V.; Grokhovskaya, T.E.; Nikonorova, N.I.; Moskvina, M.A.; Sedush, N.G.; Dorovatovskii, P.V.; Ivanova, O.A.; Rukhlya, E.G.; Volynskii, A.L.; et al. Cold crystallization of glassy polylactide during solvent crazing. ACS Appl. Mater. Interfaces 2017, 9, 34325–34336. [Google Scholar] [CrossRef] [PubMed]
- Trofimchuk, E.S.; Efimov, A.V.; Moskvina, M.A.; Ivanova, O.A.; Nikonorova, N.I.; Zezin, S.B.; Bakirov, A.V.; Volynskii, A.L. Nanocomposites based on porous polylactide, obtained by crazing mechanism in water-ethanol solution, and calcium phosphates. Polym. Sci. Ser. A 2018, 60, 845–853. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Gu, J.; Wang, G.; Li, Y.; Zhang, D. Influence of aeration on volatile sulfur compounds (VSCs) and NH3 emissions during aerobic composting of kitchen waste. Waste Manag. 2016, 58, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.A.; Mironov, V.V. Test results In-vessel composting system at the cattle farm located in the central part of Russia. Agric. Mech. Asia Afr. Lat. Am. 2018, 49, 86–90. [Google Scholar]
- Zorpas, A.A.; Loizidou, M. Sawdust and natural zeolite as a bulking agent for improving quality of a composting product from anaerobically stabilized sewage sludge. Bioresour. Technol. 2008, 99, 7545–7552. [Google Scholar] [CrossRef]
- Navarro, A.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between organic matter and carbon contents of organic wastes. Bioresour. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Arrigoni, J.P.; Paladino, G.; Garibaldi, L.A.; Laos, F. Inside the small-scale composting of kitchen and garden wastes: Thermal performance and stratification effect in vertical compost bins. Waste Manag. 2018, 76, 284–293. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. In Aktuelle Probleme der Polymer-Physik IV; Fischer, E.W., Müller, F.H., Kausch, H.H., Eds.; Steinkopff: Heidelberg, Germany, 1973; Volume 4, pp. 980–990. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chen, H.M.; Shen, Y.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y.; Zhou, Z.W. Molecular ordering and α′-form formation of poly(l-lactide) during the hydrolytic degradation. Polymer 2013, 54, 6644–6653. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Duan, J.; Yang, J.H.; Huang, T.; Qi, X.D.; Wang, Y. Comparison study of hydrolytic degradation behaviors between α′- and α-poly(l-lactide). Polym. Degrad. Stab. 2018, 148, 9. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Zhang, W.; Li, J.; Huang, S.; Meng, Y.; de Claville Christiansen, J.; Yu, D.; Wu, Z.; Jiang, S. Direct investigations on strain-induced cold crystallization behavior and structure evolutions in amorphous poly(lactic acid) with SAXS and WAXS measurements. Polymer 2016, 90, 111–121. [Google Scholar] [CrossRef]
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsubé, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.-B.; Houbraken, J.; Samson, R.A. Aspergillus section Nidulantes (formerly Emericella): Polyphasic taxonomy, chemistry and biology. Stud. Mycol. 2016, 84, 1–118. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; López-González, J.A.; Arcos-Nievas, M.A.; Suárez-Estrella, F.; Jurado, M.M.; Estrella-González, M.J.; López, M.J. Revisiting the succession of microbial populations throughout composting: A matter of thermotolerance. Sci. Total Environ. 2021, 773, 145587. [Google Scholar] [CrossRef]
- Kumar, A. Aspergillus nidulans: A Potential Resource of the Production of the Native and Heterologous Enzymes for Industrial Applications. Int. J. Microbiol. 2020, 2020, 8894215. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess 2022, 9, 42. [Google Scholar] [CrossRef]
- Sanchez, J.G.; Tsuchii, A.; Tokiwa, Y. Degradation of polycaprolactone at 50 °C by a thermotolerant Aspergillus sp. Biotechnol. Lett. 2000, 22, 849–853. [Google Scholar] [CrossRef]
- Karimi-Avargani, M.; Bazooyar, F.; Biria, D.; Zamani, A.; Skrifvars, M. The special effect of the Aspergillus flavus and its enzymes on biological degradation of the intact polylactic acid (PLA) and PLA-Jute composite. Polym. Degrad. Stab. 2020, 179, 109295. [Google Scholar] [CrossRef]
- Antipova, T.V.; Zhelifonova, V.P.; Zaitsev, K.V.; Nedorezova, P.M.; Aladyshev, A.M.; Klyamkina, A.N.; Kostyuk, S.V.; Danilogorskaya, A.A.; Kozlovsky, A.G. Biodegradation of Poly-ε-caprolactones and Poly-L-lactides by Fungi. J. Polym. Environ. 2018, 26, 4350–4359. [Google Scholar] [CrossRef]
- Khadka, S.; Adhikari, S.; Thapa, A.; Panday, R.; Adhikari, M.; Sapkota, S.; Regmi, R.S.; Adhikari, N.P.; Proshad, R.; Koirala, N. Screening and Optimization of Newly Isolated Thermotolerant Lysinibacillus fusiformis Strain SK for Protease and Antifungal Activity. Curr. Microbiol. 2020, 77, 1558–1568. [Google Scholar] [CrossRef]
- Suryia Prabha, M.; Divakar, K.; Deepa Arul Priya, J.; Panneer Selvam, G.; Balasubramanian, N.; Gautam, P. Statistical analysis of production of protease and esterase by a newly isolated Lysinibacillus fusiformis AU01: Purification and application of protease in sub-culturing cell lines. Ann. Microbiol. 2015, 65, 33–46. [Google Scholar] [CrossRef]
- Jeon, J.M.; Park, S.J.; Choi, T.R.; Park, J.H.; Yang, Y.H.; Yoon, J.J. Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym. Degrad. Stab. 2021, 191, 109662. [Google Scholar] [CrossRef]
- Esmaeili, A.; Pourbabaee, A.A.; Alikhani, H.A.; Shabani, F.; Esmaeil, E. Biodegradation of Low-Density Polyethylene (LDPE) by Mixed Culture of Lysinibacillus xylanilyticus and Aspergillus niger in Soil. PLoS ONE 2013, 8, 71720. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Jung, Y.T.; Park, S.; Oh, T.-K.; Yoon, J.H. Lysinibacillus xylanilyticus sp. nov., a xylan-degrading bacterium isolated from forest humus. Int. J. Syst. Evol. Microbiol. 2010, 60, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhan, Z.; Ye, H.; Lin, X.; Yan, Y.; Zhang, Y. Accelerated biodegradation of PLA/PHB-blended nonwovens by a microbial community. RSC Adv. 2019, 9, 10386–10394. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]


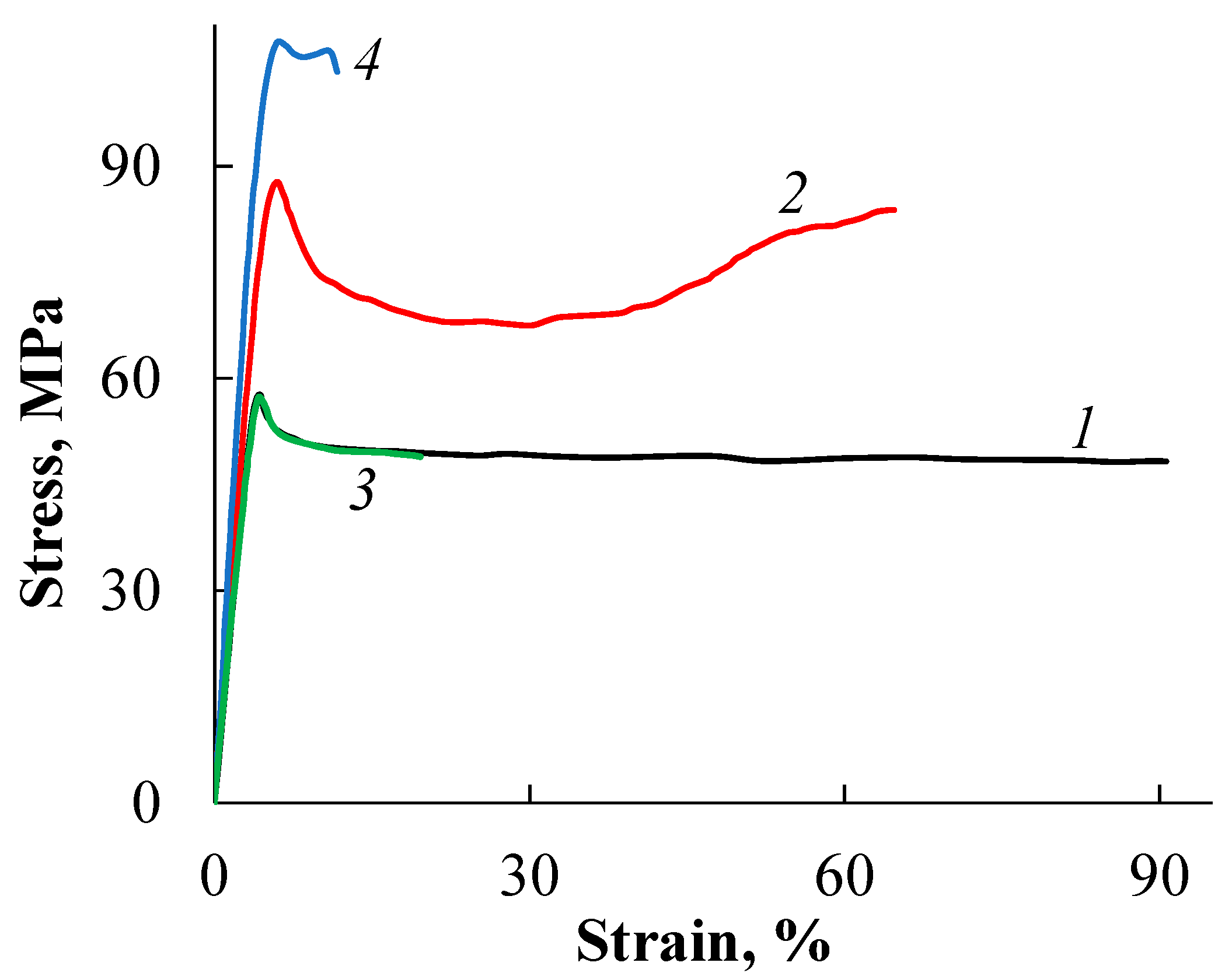
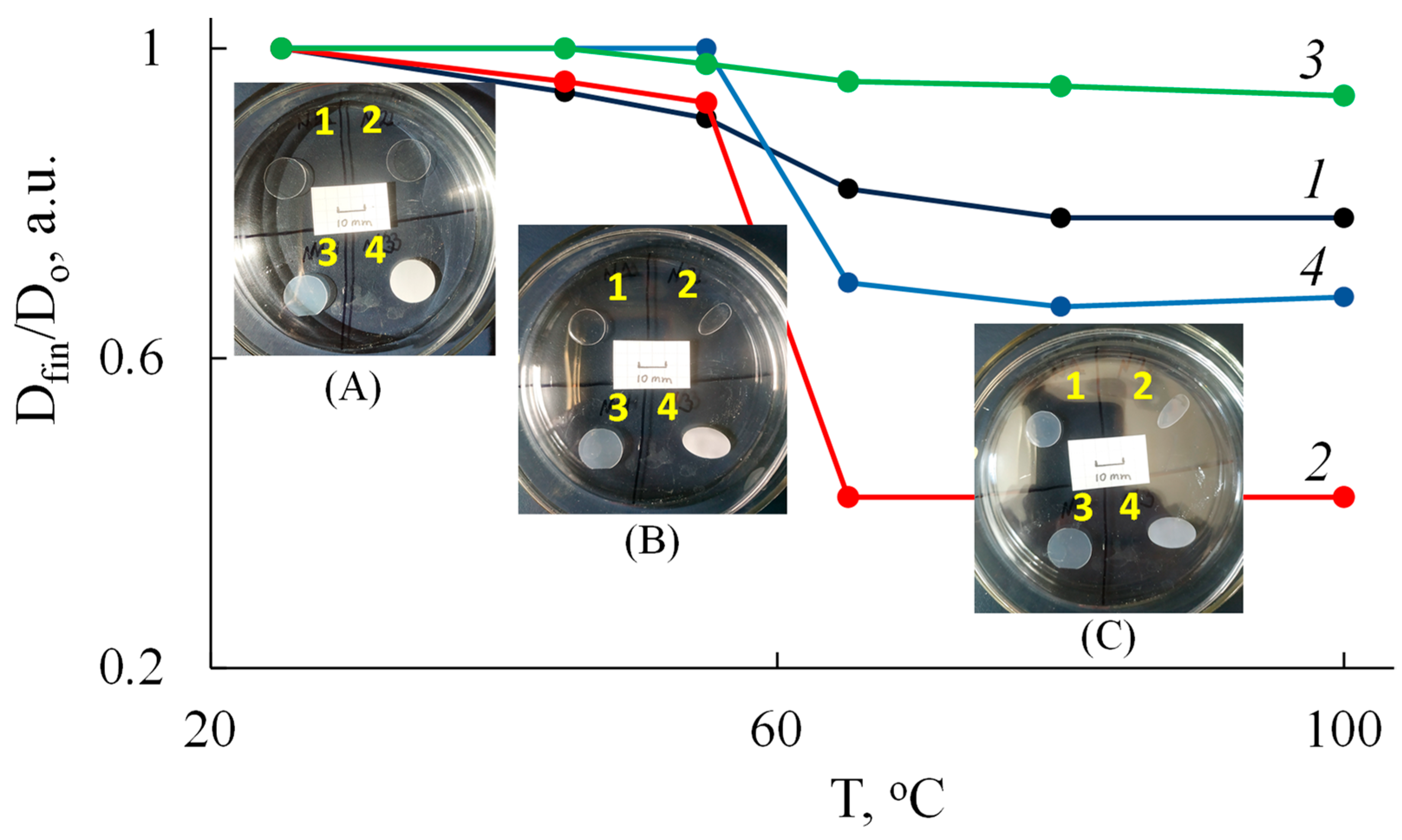


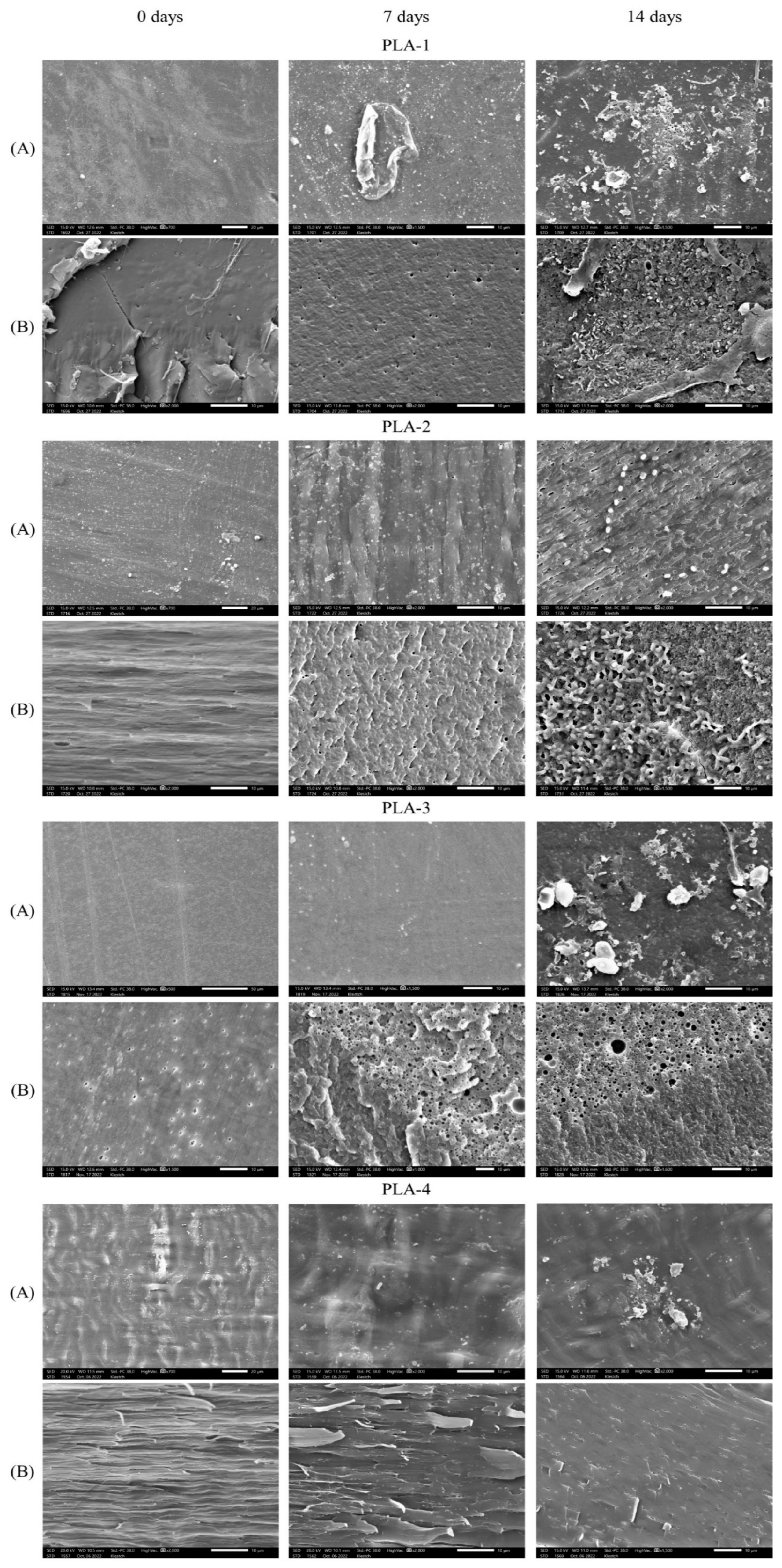


| Parameter | Value * | |
|---|---|---|
| SC | AC | |
| pH | 6.5 ± 0.5 | 6.9 ± 0.4 |
| Electrical conductivity (EC), mS cm−1 | 0.40 ± 0.03 | 0.42 ± 0.05 |
| Moisture content, wt.% | 69.8 ± 1.2 | 71.4 ± 1.3 |
| C/N | 39.5 | 31.2 |
| Type | Sample Description | h, µm | α, % | Mn, kDa/ĐM |
|---|---|---|---|---|
| PLA–1 | Amorphous, isotropic, transparent | 195 ± 5 | 0 | 76/2.18 |
| PLA–2 | Amorphous, oriented (λ = 2.1), transparent | 95 ± 3 | 0 | 73/1.96 |
| PLA–3 | Semi-crystalline, isotropic, white and opaque | 215 ± 5 | 20 ± 3 | 64/2.07 |
| PLA–4 | Semi-crystalline, oriented (λ = 4.5), whitish and opaque | 100 ± 7 | 30 ± 3 | 78/1.83 |
| Sample Type | Composting Time, Days | Tmelt, °C | α, % |
|---|---|---|---|
| SC | |||
| PLA–1 | 0 | – | 0 |
| 7 | 153 | 39 | |
| 14 | 142 | 74 | |
| 21 | 139 | 74 | |
| PLA–2 | 0 | – | 0 |
| 7 | 154 | 41 | |
| 14 | 144 | 71 | |
| PLA–3 | 0 | 153 | 20 |
| 7 | 154 | 43 | |
| 14 | 144 | 65 | |
| PLA–4 | 0 | 149 | 30 |
| 7 | 154 | 47 | |
| 14 | 145 | 74 | |
| 21 | 135 | 67 | |
| AC | |||
| PLA–1 | 0 | – | 0 |
| 4 | 151 | 55 | |
| 6 | 144 | 84 | |
| PLA–4 | 0 | 149 | 30 |
| 4 | 152 | 48 | |
| 6 | 144 | 78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimchuk, E.; Ostrikova, V.; Ivanova, O.; Moskvina, M.; Plutalova, A.; Grokhovskaya, T.; Shchelushkina, A.; Efimov, A.; Chernikova, E.; Zhang, S.; et al. Degradation of Structurally Modified Polylactide under the Controlled Composting of Food Waste. Polymers 2023, 15, 4017. https://doi.org/10.3390/polym15194017
Trofimchuk E, Ostrikova V, Ivanova O, Moskvina M, Plutalova A, Grokhovskaya T, Shchelushkina A, Efimov A, Chernikova E, Zhang S, et al. Degradation of Structurally Modified Polylactide under the Controlled Composting of Food Waste. Polymers. 2023; 15(19):4017. https://doi.org/10.3390/polym15194017
Chicago/Turabian StyleTrofimchuk, Elena, Valeria Ostrikova, Olga Ivanova, Marina Moskvina, Anna Plutalova, Tatyana Grokhovskaya, Anna Shchelushkina, Alexander Efimov, Elena Chernikova, Shenghua Zhang, and et al. 2023. "Degradation of Structurally Modified Polylactide under the Controlled Composting of Food Waste" Polymers 15, no. 19: 4017. https://doi.org/10.3390/polym15194017
APA StyleTrofimchuk, E., Ostrikova, V., Ivanova, O., Moskvina, M., Plutalova, A., Grokhovskaya, T., Shchelushkina, A., Efimov, A., Chernikova, E., Zhang, S., & Mironov, V. (2023). Degradation of Structurally Modified Polylactide under the Controlled Composting of Food Waste. Polymers, 15(19), 4017. https://doi.org/10.3390/polym15194017