Advances in the Preparation of Tough Conductive Hydrogels for Flexible Sensors
Abstract
:1. Introduction
2. Requirements for Fabricating Tough Hydrogels
3. Design Elements for Conductive Hydrogels
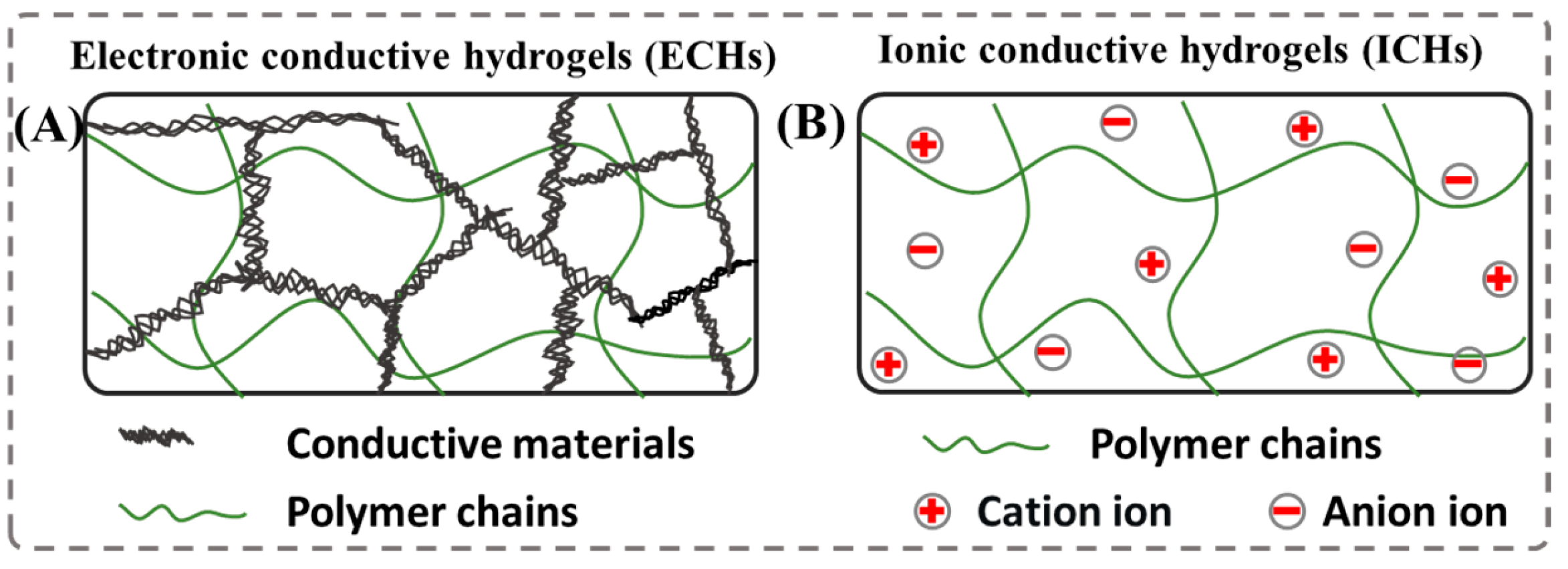
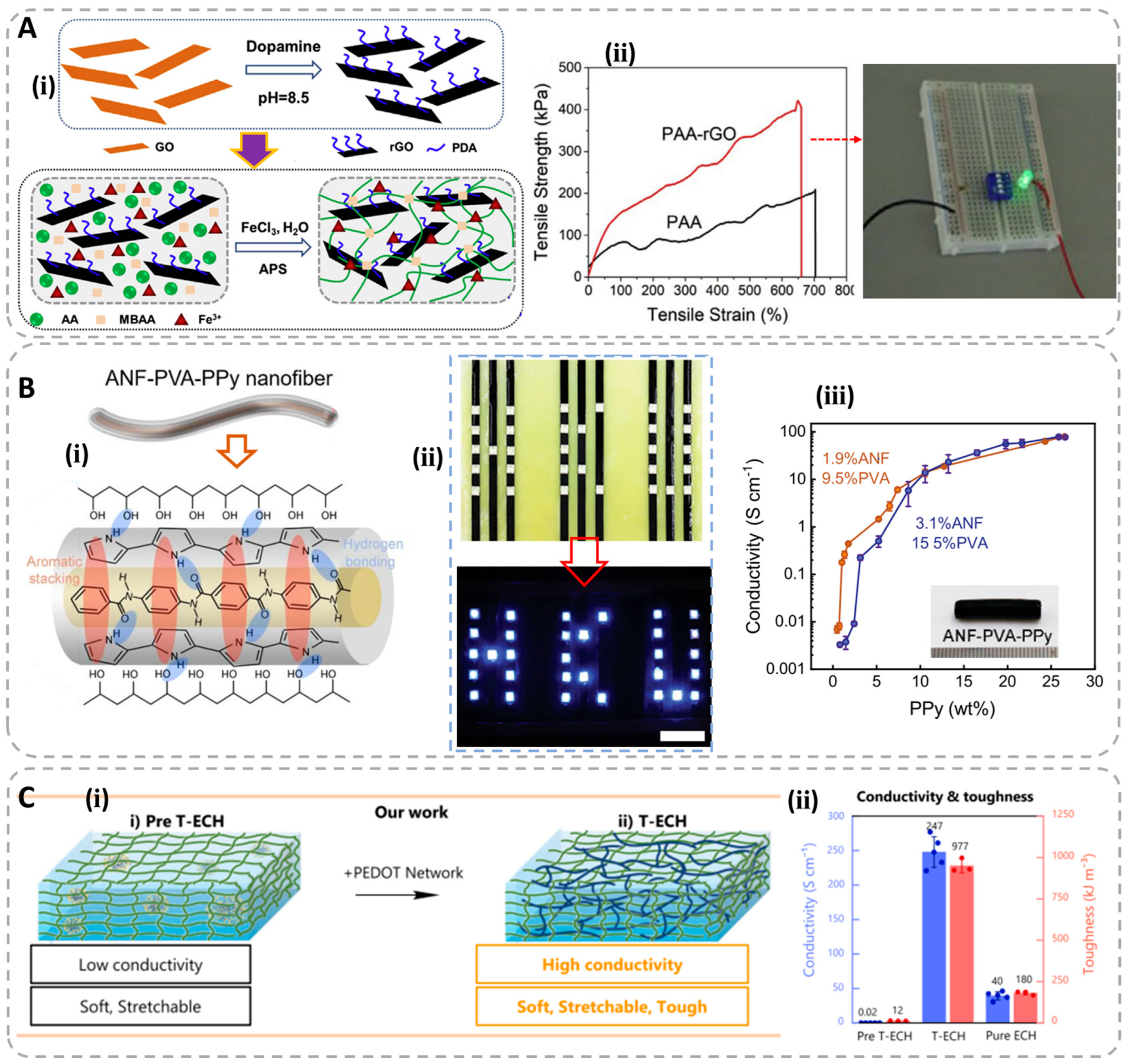
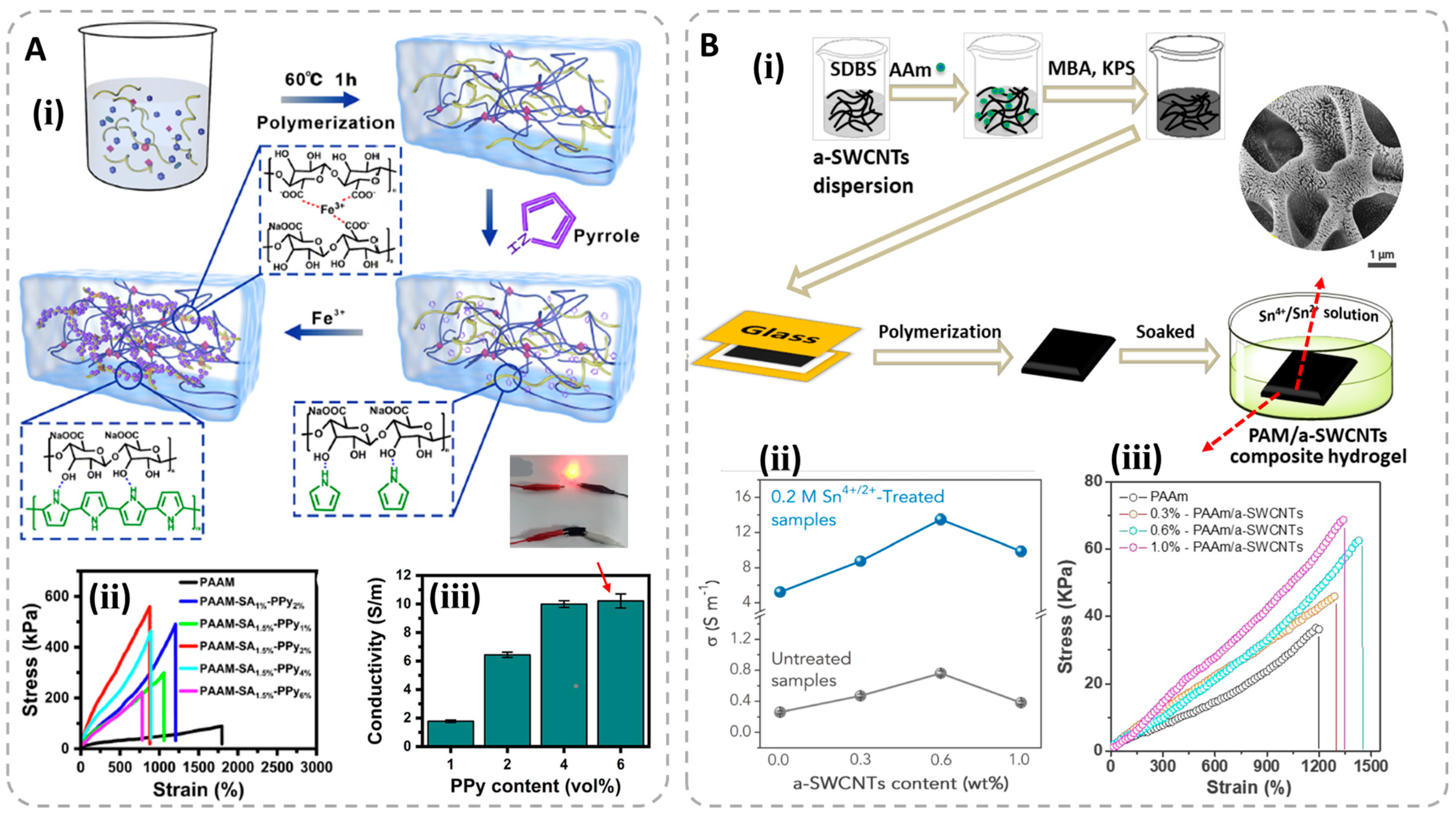
3.1. Electronic Conductive Hydrogels (ECHs)
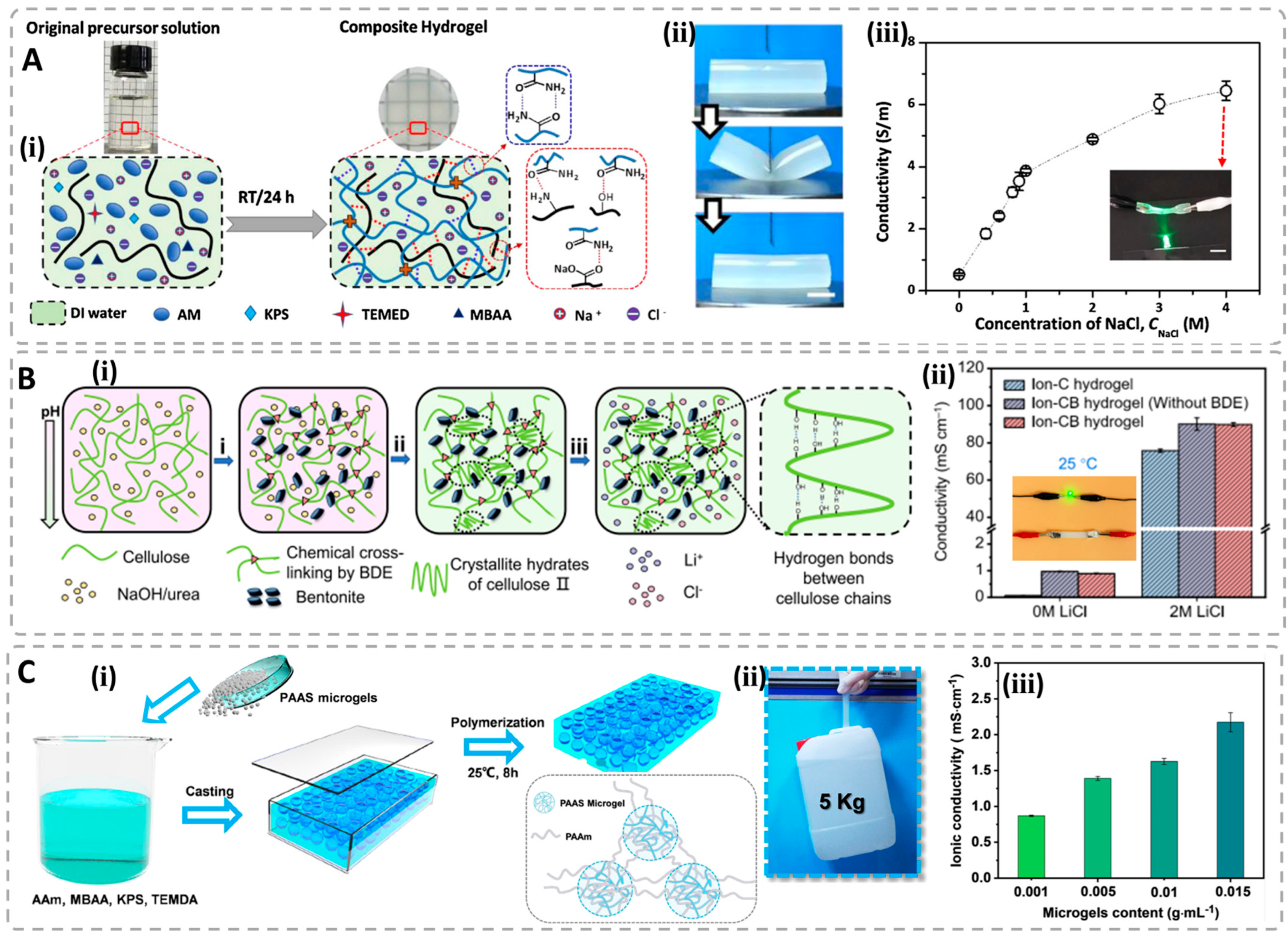
3.2. Ionic Conductive Hydrogels (ICHs)
4. Applications of Tough Conductive Hydrogels for Flexible Sensors
4.1. Resistive-Type Hydrogel Sensors
4.2. Capacitive-Type Hydrogel Sensors
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang, X.; Arıcan, M.O.; Zhou, T.; Zhao, X.; Zhang, Y.S. Functional Tough Hydrogels: Design, Processing, and Biomedical Applications. Acc. Mater. Res. 2022, 4, 101–114. [Google Scholar] [CrossRef]
- Means, A.K.; Grunlan, M.A. Modern Strategies to Achieve Tissue-Mimetic, Mechanically Robust Hydrogels. ACS Macro Lett. 2019, 8, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, e1902026. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Q.; Yang, Y.; Ji, X.; Sessler, J.L. Unlocking Chemically Encrypted Information Using Three Types of External Stimuli. J. Am. Chem. Soc. 2021, 143, 18635–18642. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Zheng, S.Y.; Wang, Q.; Li, Z.; Sun, G. Actuators assembled from hydrogel blocks of various shapes via condensation reactions. Mater. Chem. Phys. 2020, 253, 123332. [Google Scholar] [CrossRef]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef]
- Truby, R.L. Designing Soft Robots as Robotic Materials. Acc. Mater. Res. 2021, 2, 854–857. [Google Scholar] [CrossRef]
- Sachyani Keneth, E.; Kamyshny, A.; Totaro, M.; Beccai, L.; Magdassi, S. 3D Printing Materials for Soft Robotics. Adv. Mater. 2021, 33, e2003387. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.-Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, J.; Liu, X.; Zhao, X. Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. USA 2019, 116, 10244–10249. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yuk, H.; Zhang, T.; Parada, G.A.; Koo, H.; Yu, C.; Zhao, X. Stretchable Hydrogel Electronics and Devices. Adv. Mater. 2016, 28, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, C.; Cui, Y.; Li, A.; Qiao, Y.; Qiu, D. Conjoined-network rendered stiff and tough hydrogels from biogenic molecules. Sci. Adv. 2019, 5, eaau3442. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, J.; Dai, X.; Shao, Z.; Huang, X. Dual physically crosslinked healable polyacrylamide/cellulose nanofibers nanocomposite hydrogels with excellent mechanical properties. Carbohydr. Polym. 2018, 193, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Du, C.; Wang, Y.J.; Hou, L.X.; Du, M.; Zheng, Q.; Wu, Z.L. Influence of the a-Methyl Group on Elastic-To-Glassy Transition of Supramolecular Hydrogels with Hydrogen-Bond Associations. Macromolecules 2022, 55, 7512–7525. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Guan, Q.; Lv, J.; Wang, Z.; Ding, L.; Li, C.; Saiz, E.; Hou, X. Sweat-resistant bioelectronic skin sensor. Device 2023, 1, 100006. [Google Scholar] [CrossRef]
- Mo, F.; Liang, G.; Wang, D.; Tang, Z.; Li, H.; Zhi, C. Biomimetic organohydrogel electrolytes for high-environmental adaptive energy storage devices. EcoMat 2019, 1, e12008. [Google Scholar] [CrossRef]
- Huo, P.; Ding, H.; Tang, Z.; Liang, X.; Xu, J.; Wang, M.; Liang, R.; Sun, G. Conductive silk fibroin hydrogel with semi-interpenetrating network with high toughness and fast self-recovery for strain sensors. Int. J. Biol. Macromol. 2022, 212, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, J.; Zhou, G.; Wei, Y.; Wu, P.; Dong, A.; Yang, D. Gelation-Assisted Assembly of Large-Area, Highly Aligned, and Environmentally Stable MXene Films with an Excellent Trade-Off between Mechanical and Electrical Properties. Small 2022, 18, 2200829. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; He, W.; He, J.; Muhammad, Y.; Shi, Z.; Zhang, B.; Zhou, L.; Zhao, Z.; Zhao, Z. High strength, anti-freezing and conductive silkworm excrement cellulose-based ionic hydrogel with physical-chemical double cross-linked for pressure sensing. Int. J. Biol. Macromol. 2023, 236, 123936. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.N.; Zheng, S.Y.; Song, Y.; Wu, Z.L.; Zheng, Q. Hydrogen bond reinforced poly(1-vinylimidazole- co -acrylic acid) hydrogels with high toughness, fast self-recovery, and dual pH-responsiveness. Polymer 2017, 131, 95–103. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-Coordination Complexes Mediated Physical Hydrogels with High Toughness, Stick–Slip Tearing Behavior, and Good Processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakai, T.; Akagi, Y.; Chung, U.I.; Shibayama, M. SANS and SLS Studies on Tetra-Arm PEG Gels in As-Prepared and Swollen States. Macromolecules 2009, 42, 6245–6252. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, D.; Fu, C.; Guo, R.; Wu, C.-X.; Lin, Y. Recent Advances in Multi-mechanism Design of Crack-resistant Hydrogels. Soft Matter 2022, 18, 5153–5165. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liang, X.; Zhang, X.N.; Wu, Z.L.; Li, Z.; Sun, G. Tough supramolecular hydrogels with excellent self-recovery behavior mediated by metal-coordination interaction. Polymer 2019, 171, 201–210. [Google Scholar] [CrossRef]
- Yasui, T.; Zheng, Y.; Nakajima, T.; Kamio, E.; Matsuyama, H.; Gong, J.P. Rate-Independent Self-Healing Double Network Hydrogels Using a Thixotropic Sacrificial Network. Macromolecules 2022, 55, 9547–9557. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, Z.X.; Wu, Z.Y.; Liu, D.S.; Yang, X.X.; Wang, Y.X.; Jia, X.; Xu, X.; Jiang, P.; Wang, X.L. Strong and Ultra-Tough Supramolecular Hydrogel Enabled by Strain-Induced Microphase Separation. Adv. Funct. Mater. 2022, 33, 2210395. [Google Scholar] [CrossRef]
- Mu, Q.; Cui, K.; Wang, Z.J.; Matsuda, T.; Cui, W.; Kato, H.; Namiki, S.; Yamazaki, T.; Frauenlob, M.; Nonoyama, T.; et al. Force-triggered rapid microstructure growth on hydrogel surface for on-demand functions. Nat. Commun. 2022, 13, 6213. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Gong, J.P. Materials both Tough and Soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.Y.; Lin, Y.J.; Enriquez, E.; Peng, X.F.; Turng, L.S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Qi, P.; Zhang, X.; Wang, L.; Tan, Y.; Luan, Z.; Xia, Y.; Li, Y.; Sui, K. Multiple Weak H-Bonds Lead to Highly Sensitive, Stretchable, Self-Adhesive, and Self-Healing Ionic Sensors. ACS Appl. Mater. Interfaces 2019, 11, 7755–7763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent advances in 3D printing of tough hydrogels: A review. Compos. B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Wallin, T.J.; Pikul, J.; Shepherd, R.F. 3D printing of soft robotic systems. Nat. Rev. Mater. 2018, 3, 84–100. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent advances of hydrogel electrolytes in flexible energy storage devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Shang, J.; Le, X.; Zhang, J.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef]
- Pinnaratip, R.; Bhuiyan, M.S.A.; Meyers, K.; Rajachar, R.M.; Lee, B.P. Multifunctional Biomedical Adhesives. Adv. Healthc. Mater. 2019, 8, 1801568. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, H.Y.; Hu, X.S.; Liang, X.X.; Wang, M.M.; Liu, Q.; Li, Z.J.; Sun, G.X. A dual-trigger-mode ionic hydrogel sensor for contact or contactless motion recognition. Mater. Horiz. 2020, 7, 2673–2682. [Google Scholar] [CrossRef]
- Su, G.; Yin, S.; Guo, Y.; Zhao, F.; Guo, Q.; Zhang, X.; Zhou, T.; Yu, G. Balancing the mechanical, electronic, and self-healing properties in conductive self-healing hydrogel for wearable sensor applications. Mater. Horiz. 2021, 8, 1795–1804. [Google Scholar] [CrossRef]
- Chun, K.Y.; Oh, Y.; Rho, J.; Ahn, J.H.; Kim, Y.J.; Choi, H.R.; Baik, S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat. Nanotechnol. 2010, 5, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Butt, H.; Volpatti, L.R.; Pavlichenko, I.; Humar, M.; Kwok, S.J.J.; Koo, H.; Kim, K.S.; Naydenova, I.; Khademhosseini, A.; et al. Photonic hydrogel sensors. Biotechnol. Adv. 2016, 34, 250–271. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Feig, V.R.; Bao, Z. A Low-Temperature Boost for Stretchable Conductors. Matter 2020, 3, 983–984. [Google Scholar] [CrossRef]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Mi, Y.; Zhao, S.; Qi, S.; Sun, M.; Peng, B.; Xu, Q.; Niu, Y.; Zhou, Y. Transparent stretchable hydrogel sensors: Materials, design and applications. J. Mater. Chem. C 2022, 10, 13351–13371. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent advances in conductive hydrogels: Classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Xu, J.; Tang, Z.; Li, Z.; Liang, R.; Sun, G. Hydrolyzed Hydrogels with Super Stretchability, High Strength, and Fast Self-Recovery for Flexible Sensors. ACS Appl. Mater. Interfaces 2021, 13, 22774–22784. [Google Scholar] [CrossRef]
- Zhu, F.; Lin, J.; Wu, Z.L.; Qu, S.; Yin, J.; Qian, J.; Zheng, Q. Tough and Conductive Hybrid Hydrogels Enabling Facile Patterning. ACS Appl. Mater. Interfaces 2018, 10, 13685–13692. [Google Scholar] [CrossRef]
- Guo, X.; Yang, F.; Liu, W.; Han, C.; Bai, Y.; Sun, X.; Hao, L.; Jiao, W.; Wang, R. Skin-inspired self-healing semiconductive touch panel based on novel transparent stretchable hydrogels. J. Mater. Chem. A 2021, 9, 14806–14817. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J. Conducting polymer hydrogels. Chem. Pap. 2016, 71, 269–291. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Zhu, W.; Guan, L.; Yang, X.; Zvyagin, A.V.; Zhao, Y.; Shen, C.; Yang, B.; Lin, Q. Muscle-Inspired MXene Conductive Hydrogels with Anisotropy and Low-Temperature Tolerance for Wearable Flexible Sensors and Arrays. Adv. Funct. Mater. 2021, 31, 2105264. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, H.; Li, Z.; Dai, J.; Cong, H.-P.; Yu, S.-H. Highly compressible and environmentally adaptive conductors with high-tortuosity interconnected cellular architecture. Nat. Synth. 2022, 1, 975–986. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, L.-Y.; Jing, L.; Li, K.; Yang, H.; Li, Y.; Chen, P.-Y. Carbon nanotube-integrated conductive hydrogels as multifunctional robotic skin. Carbon 2020, 161, 784–793. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H.; Wang, Y.; Fan, X.; Li, Z.; Zhang, X.; Liu, T. Highly Stretchable, Ultra-Soft, and Fast Self-Healable Conductive Hydrogels Based on Polyaniline Nanoparticles for Sensitive Flexible Sensors. Adv. Funct. Mater. 2022, 32, 2204366. [Google Scholar] [CrossRef]
- Song, P.; Qin, H.L.; Gao, H.L.; Cong, H.P.; Yu, S.H. Self-healing and superstretchable conductors from hierarchical nanowire assemblies. Nat. Commun. 2018, 9, 2786. [Google Scholar] [CrossRef]
- Gan, D.; Han, L.; Wang, M.; Xing, W.; Xu, T.; Zhang, H.; Wang, K.; Fang, L.; Lu, X. Conductive and Tough Hydrogels Based on Biopolymer Molecular Templates for Controlling in Situ Formation of Polypyrrole Nanorods. ACS Appl. Mater. Interfaces 2018, 10, 36218–36228. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Sung, C.; Nam, K.S.; Kang, T.; Kim, H.; Lee, H.; Park, H.; Park, S.; Kang, J. Highly conductive tissue-like hydrogel interface through template-directed assembly. Nat. Commun. 2023, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Cao, Y.; Yang, Y.; Yang, Y.; Gao, Y.; Ma, Z.; Wang, J.; Wang, W.; Wu, D. Freezing-Tolerant, Highly Sensitive Strain and Pressure Sensors Assembled from Ionic Conductive Hydrogels with Dynamic Cross-Links. ACS Appl. Mater. Interfaces 2020, 12, 25334–25344. [Google Scholar] [CrossRef]
- Dechiraju, H.; Jia, M.; Luo, L.; Rolandi, M. Ion-Conducting Hydrogels and Their Applications in Bioelectronics. Adv. Sustain. Syst. 2021, 6, 2100173. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, G.; Ren, X. Graphene assisted ion-conductive hydrogel with super sensitivity for strain sensor. Polymer 2021, 215, 123340. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic Salts Induce Thermally Reversible and Anti-Freezing Cellulose Hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Wu, G.; Jin, K.; Liu, L.; Zhang, H. A rapid self-healing hydrogel based on PVA and sodium alginate with conductive and cold-resistant properties. Soft Matter 2020, 16, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mu, Y.; Luo, Y.; Menon, C.; Zhou, Z.; Chu, P.K.; Feng, S.P. Hofmeister Effect and Electrostatic Interaction Enhanced Ionic Conductive Organohydrogels for Electronic Applications. Adv. Funct. Mater. 2021, 32, 2110859. [Google Scholar] [CrossRef]
- Ding, H.; Liang, X.; Wang, Q.; Wang, M.; Li, Z.; Sun, G. A semi-interpenetrating network ionic composite hydrogel with low modulus, fast self-recoverability and high conductivity as flexible sensor. Carbohydr. Polym. 2020, 248, 116797. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Mao, S.; Yuan, J.; Wang, S.; He, X.; Zhang, X.; Du, C.; Zhang, D.; Wu, Z.L.; Yang, J. Molecularly Engineered Zwitterionic Hydrogels with High Toughness and Self-Healing Capacity for Soft Electronics Applications. Chem. Mater. 2021, 33, 8418–8429. [Google Scholar] [CrossRef]
- Cui, J.; Chen, J.; Ni, Z.; Dong, W.; Chen, M.; Shi, D. High-Sensitivity Flexible Sensor Based on Biomimetic Strain-Stiffening Hydrogel. ACS Appl. Mater. Interfaces 2022, 14, 47148–47156. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, L.; Demir, B.; An, M.; Wu, Z.L.; Yin, J.; Xiao, R.; Zheng, Q.; Qian, J. Accelerating solar desalination in brine through ion activated hierarchically porous polyion complex hydrogels. Mater. Horiz. 2020, 7, 3187–3195. [Google Scholar] [CrossRef]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Cao, J.; Cai, Y.; Yu, L.; Zhou, J. Dual physically crosslinked hydrogels based on the synergistic effects of electrostatic and dipole–dipole interactions. J. Mater. Chem. B 2019, 7, 676–683. [Google Scholar] [CrossRef]
- Aldebert, P.; Gebel, G.; Loppinet, B.; Nakamura, N. Polyelectrolyte Effect in Perfluorosulfonated Ionomer Solutions. Polymer 1995, 36, 431–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Gao, Z.; Bi, D.; Qu, N.; Huang, S.; Zhao, X.; Li, R. Highly conductive and tough polyacrylamide/sodium alginate hydrogel with uniformly distributed polypyrrole nanospheres for wearable strain sensors. Carbohydr. Polym. 2023, 315, 120953. [Google Scholar] [CrossRef]
- Liang, L.; Lv, H.; Shi, X.-L.; Liu, Z.; Chen, G.; Chen, Z.-G.; Sun, G. A flexible quasi-solid-state thermoelectrochemical cell with high stretchability as an energy-autonomous strain sensor. Mater. Horiz. 2021, 8, 2750–2760. [Google Scholar] [CrossRef]
- Yu, Q.; Zheng, Z.; Dong, X.; Cao, R.; Zhang, S.; Wu, X.; Zhang, X. Mussel-inspired hydrogels as tough, self-adhesive and conductive bioelectronics: A review. Soft Matter. 2021, 17, 8786–8804. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, T.; Xie, X.; Song, G.; Ma, X.; Mu, Q.; Huang, Z.; Liu, X.; Sun, C.; Xu, W. Advances and challenges in conductive hydrogels: From properties to applications. Eur. Polym. J. 2022, 177, 111454. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of Conductive Hydrogels for Fabricating Flexible Strain Sensors. Small 2021, 18, 2101518. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.; Brancart, J.; Terryn, S.; Bosman, A.W.; Norvez, S.; Van Assche, G.; Iida, F.; Vanderborght, B.; Clemens, F. Soft self-healing resistive-based sensors inspired by sensory transduction in biological systems. Appl. Mater. Today 2022, 29, 101638. [Google Scholar] [CrossRef]
- Yi, F.-L.; Guo, F.-L.; Li, Y.-Q.; Wang, D.-Y.; Huang, P.; Fu, S.-Y. Polyacrylamide Hydrogel Composite E-skin Fully Mimicking Human Skin. ACS Appl. Mater. Interfaces 2021, 13, 32084–32093. [Google Scholar] [CrossRef]
- Liu, C.W.; Zhang, R.; Wang, Y.; Wei, C.M.; Li, F.; Qing, N.; Tang, L.Y. Highly adhesive chitosan/poly(vinyl alcohol) hydrogels via the synergy of phytic acid and boric acid and their application as highly sensitive and widely linear strain sensors. Mater. Horiz. 2023, 10, 3488–3498. [Google Scholar] [CrossRef]
- Pi, M.H.; Qin, S.H.; Wen, S.H.; Wang, Z.S.; Wang, X.Y.; Li, M.; Lu, H.L.; Meng, Q.D.; Cui, W.; Ran, R. Rapid Gelation of Tough and Anti-Swelling Hydrogels under Mild Conditions for Underwater Communication. Adv. Funct. Mater. 2023, 33, 2210188. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Zhang, N.; Li, J.; Zhou, P.; Hu, F.; Rong, Y.; Lu, B.; Gu, G. High-Stretchability, Ultralow-Hysteresis Conducting Polymer Hydrogel Strain Sensors for Soft Machines. Adv. Mater. 2022, 34, e2203650. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lv, Y.; Qiu, D.; Zhou, Y.; Zeng, H.; Chu, Y. An ultra-stretchable, highly sensitive and biocompatible capacitive strain sensor from an ionic nanocomposite for on-skin monitoring. Nanoscale 2019, 11, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Huang, Y.; Li, Q.; Wang, Z.; Jiang, R.; Gai, W.; Zhi, C. A Highly Stable and Durable Capacitive Strain Sensor Based on Dynamically Super-Tough Hydro/Organo-Gels. Adv. Funct. Mater. 2021, 31, 2010830. [Google Scholar] [CrossRef]
- Jiang, P.-P.; Qin, H.; Dai, J.; Yu, S.-H.; Cong, H.-P. Ultrastretchable and Self-Healing Conductors with Double Dynamic Network for Omni-Healable Capacitive Strain Sensors. Nano Lett. 2021, 22, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, F.; Yao, C.; Li, L. Low Hysteresis Hydrogel Induced by Spatial Confinement. Adv. Funct. Mater. 2023, 33, 2214935. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Jin, X.; Liu, H.; Lai, J.; Du, H.; Chen, W.; Ma, A. Capacitive Pressure Sensors Containing Reliefs on Solution-Processable Hydrogel Electrodes. ACS Appl. Mater. Interfaces 2021, 13, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A Review of Conductive Hydrogel Used in Flexible Strain Sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, X.; Dong, Y.; Li, J. Flexible Self-Repairing Materials for Wearable Sensing Applications: Elastomers and Hydrogels. Macromol. Rapid Commun. 2020, 41, 2000444. [Google Scholar] [CrossRef]
- Kaniewska, K.; Karbarz, M.; Katz, E. Nanocomposite hydrogel films and coatings—Features and applications. Appl. Mater. Today 2020, 20, 100776. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Cui, H.; Liu, C.; Li, Y.; Xia, Y.; Sui, K. Direct Current-Powered High-Performance Ionic Hydrogel Strain Sensor Based on Electrochemical Redox Reaction. ACS Appl. Mater. Interfaces 2019, 11, 24289–24297. [Google Scholar] [CrossRef]
- Shu, L.; Wang, Z.; Zhang, X.-F.; Yao, J. Highly conductive and anti-freezing cellulose hydrogel for flexible sensors. Int. J. Biol. Macromol. 2023, 230, 123425. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Lv, X.; Sun, S. A highly sensitive strain sensor based on a silica@polyaniline core–shell particle reinforced hydrogel with excellent flexibility, stretchability, toughness and conductivity. Soft Matter. 2021, 17, 2142–2150. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Qin, Y.; Mo, J.; Liu, Y.; Zhang, S.; Wang, J.; Fu, Q.; Wang, S.; Nie, S. Stretchable Triboelectric Self-Powered Sweat Sensor Fabricated from Self-Healing Nanocellulose Hydrogels. Adv. Funct. Mater. 2022, 32, 2201846. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L. Light Management with Patterned Micro- and Nanostructure Arrays for Photocatalysis, Photovoltaics, and Optoelectronic and Optical Devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Stanisz, M.; Klapiszewski, Ł.; Jesionowski, T. Recent advances in the fabrication and application of biopolymer-based micro- and nanostructures: A comprehensive review. Chem. Eng. J. 2020, 397, 125409. [Google Scholar] [CrossRef]
- Dong, M.; Han, Y.; Hao, X.P.; Yu, H.C.; Yin, J.; Du, M.; Zheng, Q.; Wu, Z.L. Digital Light Processing 3D Printing of Tough Supramolecular Hydrogels with Sophisticated Architectures as Impact-Absorption Elements. Adv. Mater. 2022, 34, e2204333. [Google Scholar] [CrossRef]
- Tang, W.; Fu, C.; Xia, L.; Lyu, P.; Li, L.; Fu, Z.; Pan, H.; Zhang, C.; Xu, W. A flexible and sensitive strain sensor with three-dimensional reticular structure using biomass Juncus effusus for monitoring human motions. Chem. Eng. J. 2022, 438, 135600. [Google Scholar] [CrossRef]
- Ding, H.; Dong, M.; Zheng, Q.; Wu, Z.L. Digital light processing 3D printing of hydrogels: A minireview. Mol. Syst. Des. Eng. 2022, 7, 1017–1029. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Chen, Y.; Liu, P.; Duan, H.; Cheng, P. 3D Printed Ultrastretchable, Hyper-Antifreezing Conductive Hydrogel for Sensitive Motion and Electrophysiological Signal Monitoring. Research 2020, 2020, 1426078. [Google Scholar] [CrossRef]
- Kaur, M.; Kim, T.H.; Kim, W.S. New Frontiers in 3D Structural Sensing Robots. Adv. Mater. 2021, 33, 2002534. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Wang, Q.; Zhang, Y.Z.; Alshareef, H.N.; Dong, X. 3D Printing of Hydrogels for Stretchable Ionotronic Devices. Adv. Funct. Mater. 2021, 31, 2107437. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, C.; Li, S.; Deng, K.; Tang, J.; Luo, Q.; Zhang, S.; Chang, Y.; Pan, T. Aquatic Skin Enabled by Multi-Modality Iontronic Sensing. Adv. Funct. Mater. 2022, 32, 2205947. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.-C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.-C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2022, 41, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ali, M.A.; Rai, P.; Ghori, I.; Sharma, A.; Malhotra, B.D.; John, R. Dual-modality microfluidic biosensor based on nanoengineered mesoporous graphene hydrogels. Lab Chip 2020, 20, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Wu, Z.; Gao, F.-L.; Gao, X.-Z.; Zhao, H.-Y.; Li, X.; Yu, Z.-Z. Self-adhesive, self-healing, biocompatible and conductive polyacrylamide nanocomposite hydrogels for reliable strain and pressure sensors. Nano Energy 2023, 109, 108324. [Google Scholar] [CrossRef]
- Kim, C.-C.; Lee, H.-H.; Oh, K.H.; Sun, J.-Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wu, P. Zwitterionic Skins with a Wide Scope of Customizable Functionalities. ACS Nano 2018, 12, 12860–12868. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Song, H.; Nguyen, D.H.; Zhang, C.; Liu, T. Strong–Weak Response Network-Enabled Ionic Conductive Hydrogels with High Stretchability, Self-Healability, and Self-Adhesion for Ionic Sensors. ACS Appl. Mater. Interfaces 2022, 14, 32551–32560. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Yin, R.; Yin, A.; Feng, Q.; Liu, F.; Shao, J.; Su, T.; Wang, H.; Chen, G.; et al. Environmentally adaptive and durable hydrogels toward multi-sensory application. Chem. Eng. J. 2022, 449, 137907. [Google Scholar] [CrossRef]
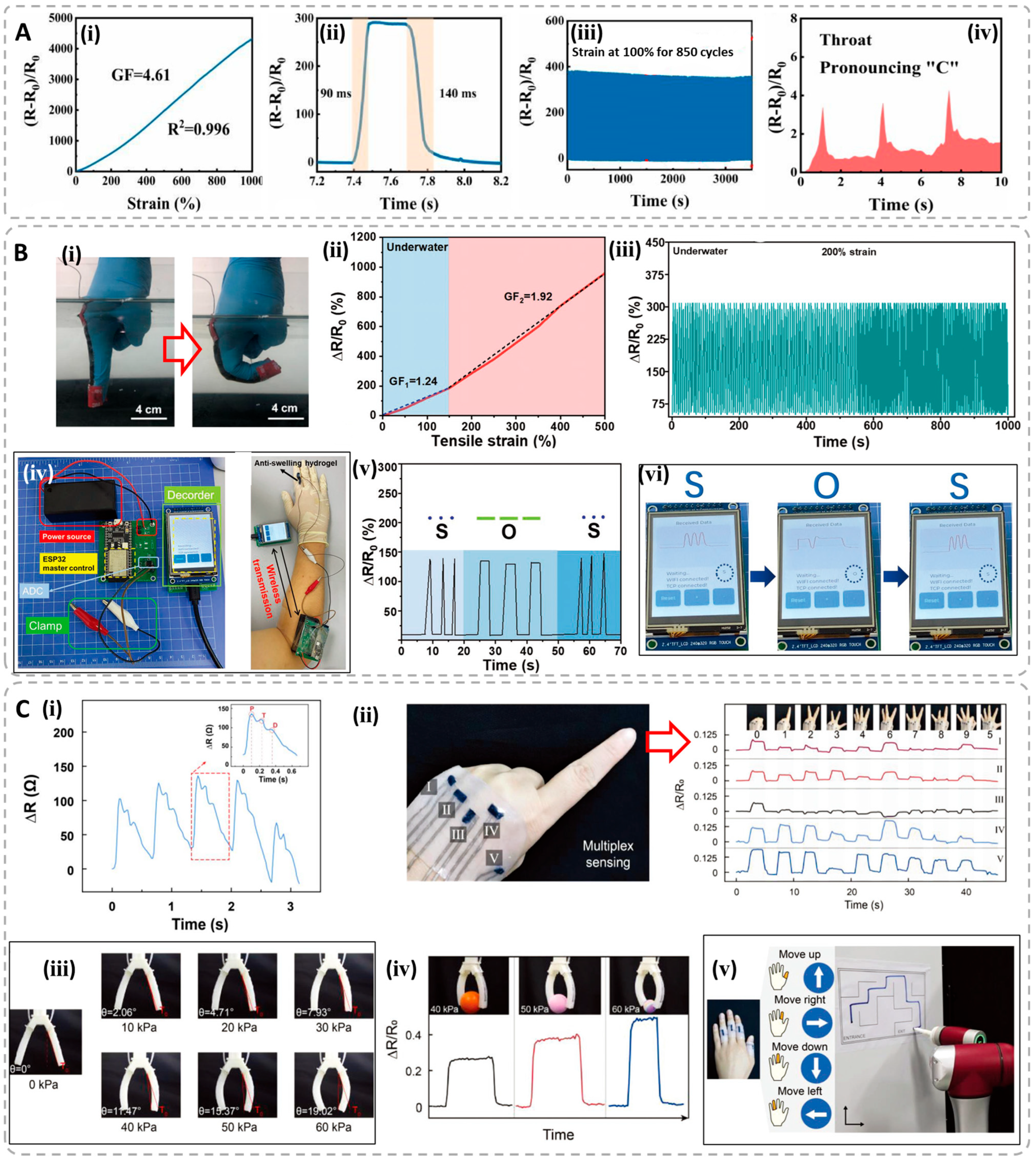
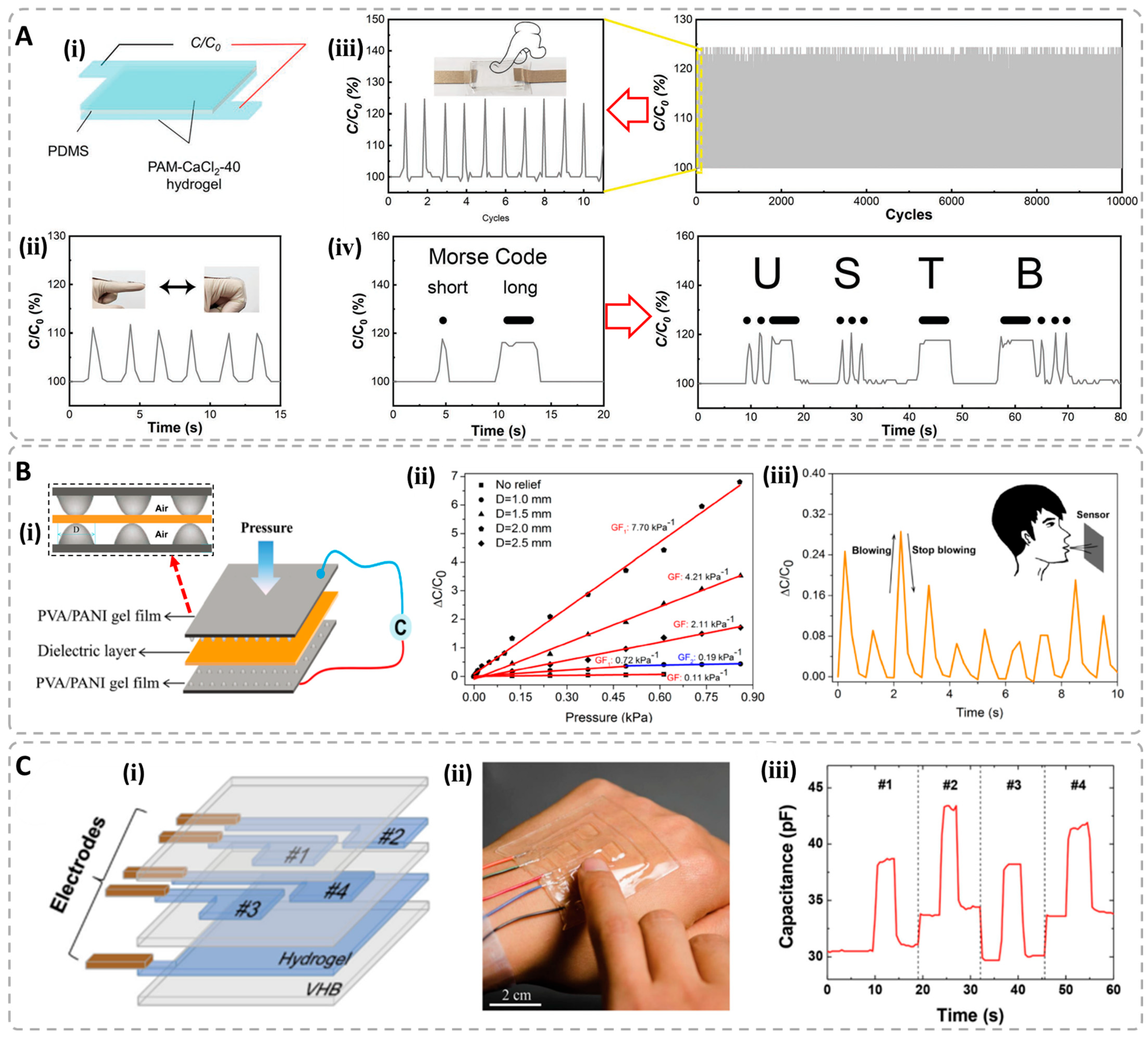
| Hydrogel Materials | Conduction Type | Conductivity | Sensing Type | Gauge Factor | Sensing Range | Fitting Relation | Application Field | Ref. |
|---|---|---|---|---|---|---|---|---|
| ANF−PVA | Electronic | 80 S/cm | Resistive | N/A | N/A | N/A | Bioelectronics | [74] |
| PAA/PEDOT:PSS | Electronic | 247 S/cm | Resistive | N/A | N/A | N/A | Bioelectronics | [75] |
| CMC/PAM/NaCl | Ionic | 6.44 S/m | Resistive | 0.104–0.214 | 0.5–800% | Piecewise linearity | Wearable skin | [84] |
| Cellulose/bentonite | Ionic | 89.9 mS/cm | Resistive | N/A | N/A | N/A | Wearable skin | [85] |
| MRH | Ionic | 2.2 mS/cm | Resistive | 2.98–6.78 | 0–300% | Piecewise linearity | Wearable skin | [87] |
| PAAM−SA−PPy NSs | Ionic/Electronic | 10 S/m | Resistive | 1.89–4.53 | 0–800% | Piecewise linearity | Wearable skin | [92] |
| PAAm/a−SWCNT/Sn | Ionic/Electronic | 13.47 S/m | Resistive | 0.3–0.8 | 0–100% | N/A | Wearable skin | [93] |
| CS/PVA−PA−BA | Ionic | 5.3 S/m | Resistive | 4.61 | 0–1000% | Linearity | Wearable skin | [94] |
| GPMZr | Ionic/Electronic | 1.76 S/m | Resistive | 1.24–1.92 | 0–500% | Piecewise linearity | Wearable skin/Underwater communicator | [95] |
| PEDOT:PSS−PVA | Electronic | N/A | N/A | 4.07 | 0–300% | Linearity | Robotic skin | [96] |
| PAM−CaCl2 | Ionic | N/A | N/A | N/A | N/A | N/A | Wearable skin/Bionic muscle | [97] |
| PVA/PANI | Electronic | N/A | Capacitive | 1.26–7.7 kPa−1 | 0–7.4 kPa | Piecewise linearity | Wearable skin | [98] |
| PAM/NaCl | Ionic | N/A | Capacitive | N/A | N/A | N/A | Wearable skin/Location detecting | [55] |
| PDA@CNT/PAAm | Electronic | 2 mS/m | Resistive | 1.99–3.93 | 0–400% | Piecewise linearity | Wearable skin/Smart ring device | [99] |
| HAPAA/PANI | Ionic/Electronic | 3.35 S/m | Resistive | 2.6–17.9 | 0–1500% | Piecewise linearity | Wearable skin/Touch screen device | [50] |
| PAAm/LiCl | Ionic | 1 S/m | N/A | N/A | N/A | N/A | Touch panel | [100] |
| TiO2/PDMAA | Electronic | N/A | N/A | N/A | N/A | N/A | Touch panel | [60] |
| P(AAc−co−SBMA) | Ionic | N/A | Capacitive | 0.9 kPa−1 | 0–5 kPa | Linearity | Wearable skin | [101] |
| PMVIC−r−SAMS | Ionic | 0.55 S/m | Capacitive | 1.12–1.14 | 0–200% | Piecewise linearity | Wearable skin | [102] |
| PSCL | Ionic | 17.1 mS/cm | Capacitive | 0.14 °C−1 | 12–95 °C | Nonlinearity | Temperature sensor | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Liu, J.; Shen, X.; Li, H. Advances in the Preparation of Tough Conductive Hydrogels for Flexible Sensors. Polymers 2023, 15, 4001. https://doi.org/10.3390/polym15194001
Ding H, Liu J, Shen X, Li H. Advances in the Preparation of Tough Conductive Hydrogels for Flexible Sensors. Polymers. 2023; 15(19):4001. https://doi.org/10.3390/polym15194001
Chicago/Turabian StyleDing, Hongyao, Jie Liu, Xiaodong Shen, and Hui Li. 2023. "Advances in the Preparation of Tough Conductive Hydrogels for Flexible Sensors" Polymers 15, no. 19: 4001. https://doi.org/10.3390/polym15194001
APA StyleDing, H., Liu, J., Shen, X., & Li, H. (2023). Advances in the Preparation of Tough Conductive Hydrogels for Flexible Sensors. Polymers, 15(19), 4001. https://doi.org/10.3390/polym15194001







