Sustainable Strategies for Synthesizing Lignin-Incorporated Bio-Based Waterborne Polyurethane with Tunable Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CWPU-LX
2.3. Characterization
2.4. Determining the Hydroxyl Content of Castor Oil
2.5. Antibacterial Properties Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, H.K.; Wang, X.X.; Li, T.T.; Huang, S.Y.; Lin, Q.; Shiu, B.C.; Lou, C.W.; Lin, J.H. Effects of Hydrotalcite on Rigid Polyurethane Foam Composites Containing a Fire Retarding Agent: Compressive Stress, Combustion Resistance, Sound Absorption, and Electromagnetic Shielding Effectiveness. RSC Adv. 2018, 8, 33542–33550. [Google Scholar] [CrossRef] [PubMed]
- Czlonka, S.; Strakowska, A.; Strzelec, K.; Kairyte, A.; Kremensas, A. Bio-Based Polyurethane Composite Foams with Improved Mechanical, Thermal, and Antibacterial Properties. Materials 2020, 13, 1108. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and Characterization of Jatropha (Jatropha curcas L.) Oil-Based Polyurethane Wood Adhesive. Ind. Crops Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal Stability and Flame Retardancy of Polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Chaudhari, A.; Kuwar, A.; Mahulikar, P.; Hundiwale, D.; Kulkarni, R.; Gite, V. Development of Anticorrosive Two Pack Polyurethane Coatings Based on Modified Fatty Amide of Azadirachta Indica Juss Oil Cured at Room Temperature—A Sustainable Resource. RSC Adv. 2014, 4, 17866–17872. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Liu, G. Graft-Copolymer-Based Approach to Clear, Durable, and Anti-Smudge Polyurethane Coatings. Angew. Chem. Int. Ed. Engl. 2015, 54, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, X.X.; Yang, J. Development of Lignin-Based Waterborne Polyurethane Materials for Flame Retardant Leather Application. Polym. Bull. 2022, 80, 5553–5571. [Google Scholar] [CrossRef]
- Zoran, S.P.; Xianmei, W.; Olivera, B.; Alisa, Z.; Jian, H.; Ivan, J.; Mihail, I.; Jelena, M.; Darin, D. Polyols and Polyurethanes from Crude Algal Oil. J. Am. Oil Chem. Soc. 2013, 90, 1073–1078. [Google Scholar]
- Nubla, M.; Zhongshun, Y.; John, S.; Chunbao, X. Depolymerization of Lignins and Their Applications for the Preparation of Polyols and Rigid Polyurethane Foams: A Review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar]
- Pierre, F.; Luc, A. Renewable Polyols for Advanced Polyurethane Foams from Diverse Biomass Resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar]
- Stemmelen, M.; Pessel, F.; Lapinte, V.; Caillol, S.; Habas, J.P.; Robin, J.J. A Fully Biobased Epoxy Resin from Vegetable Oils: From the Synthesis of the Precursors by Thiol-Ene Reaction to the Study of the Final Material. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2434–2444. [Google Scholar] [CrossRef]
- Dieterich, D. Aqueous Emulsions, Dispersions and Solutions of Polyurethanes; Synthesis and Properties. Prog. Org. Coat. 1981, 9, 281–340. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R.; Mamat, S.; Osman Al Edrus, S.S. Chemical and Thermo-Mechanical Properties of Waterborne Polyurethane Dispersion Derived from Jatropha Oil. Polymers 2021, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Qian, Y.; Yang, D.; Qiu, X.; Zhou, M. Preparation and Performance of Lignin-Based Waterborne Polyurethane Emulsion. Ind. Crops Prod. 2021, 170, 113739. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Tavares, L.B.; Ito, N.M.; Salvadori, M.C.; dos Santos, D.J.; Rosa, D.S. Pbat/Kraft Lignin Blend in Flexible Laminated Food Packaging: Peeling Resistance and Thermal Degradability. Polym. Test. 2018, 67, 169–176. [Google Scholar] [CrossRef]
- Zhang, X.; Jeremic, D.; Kim, Y.; Street, J.; Shmulsky, R. Effects of Surface Functionalization of Lignin on Synthesis and Properties of Rigid Bio-Based Polyurethanes Foams. Polymers 2018, 10, 706. [Google Scholar] [CrossRef]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Lignin-Based Rigid Polyurethane Foam Reinforced with Pulp Fiber: Synthesis and Characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Zhang, S.; Yin, Y.; Wang, C. Uv-Resistant Transparent Lignin-Based Polyurethane Elastomer with Repeatable Processing Performance. Eur. Polym. J. 2021, 159, 110763. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, e2000492. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Lignin-Based Polyurethanes from Unmodified Kraft Lignin Fractionated by Sequential Precipitation. ACS Appl. Polym. Mater. 2019, 1, 1672–1679. [Google Scholar] [CrossRef]
- de Haro, J.C.; Allegretti, C.; Smit, A.T.; Turri, S.; D’Arrigo, P.; Griffini, G. Biobased Polyurethane Coatings with High Biomass Content: Tailored Properties by Lignin Selection. ACS Sustain. Chem. Eng. 2019, 7, 11700–11711. [Google Scholar] [CrossRef]
- Chee, P.L.; Sugiarto, S.; Yu, Y.; Tan, Y.C.; Ye, E.; Kai, D.; Loh, X.J. Antioxidative and Anti-Uv Lignin Carrier for Peptide Delivery. Macromol. Chem. Phys. 2021, 223, 2100364. [Google Scholar] [CrossRef]
- Perez-Gavilan, A.; de Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Francone, A.; Kehagias, N.; Sotomayor-Torres, C.M.; Cocina, D.; et al. Antibacterial Activity Testing Methods for Hydrophobic Patterned Surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Liang, H.; Liang, D.; Cao, H.; Liu, C.; Qian, Y.; Lu, Q.; Zhang, C. High Bio-Content Castor Oil Based Waterborne Polyurethane/Sodium Lignosulfonate Composites for Environmental Friendly Uv Absorption Application. Ind. Crops Prod. 2019, 142, 111836. [Google Scholar] [CrossRef]
- Asif, A.; Hu, L.; Shi, W. Synthesis, Rheological, and Thermal Properties of Waterborne Hyperbranched Polyurethane Acrylate Dispersions for Uv Curable Coatings. Colloid Polym. Sci. 2009, 287, 1041–1049. [Google Scholar] [CrossRef]
- Wong, C.S.; Badri, K.H. Chemical Analyses of Palm Kernel Oil-Based Polyurethane Prepolymer. Mater. Sci. Appl. 2012, 03, 78–86. [Google Scholar] [CrossRef]
- Sun, N.; Di, M.; Liu, Y. Lignin-Containing Polyurethane Elastomers with Enhanced Mechanical Properties Via Hydrogen Bond Interactions. Int. J. Biol. Macromol. 2021, 184, 1–8. [Google Scholar] [CrossRef]
- Liang, H.; Liu, L.; Lu, J.; Chen, M.; Zhang, C. Castor Oil-Based Cationic Waterborne Polyurethane Dispersions: Storage Stability, Thermo-Physical Properties and Antibacterial Properties. Ind. Crops Prod. 2018, 117, 169–178. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Castor-Oil-Based Waterborne Polyurethane Dispersions Cured with an Aziridine-Based Crosslinker. Macromol. Mater. Eng. 2011, 296, 703–709. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Physicochemical Properties of Amino–Silane-Terminated Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-Based Polyurethane Prepared from Kraft Lignin and Modified Castor Oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, S.J.; Kim, B.K. Shape Memory Polyurethane Foams. Express Polym. Lett. 2012, 6, 63–69. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, M.; Verdolotti, L.; Di Maio, E.; Aurilia, M.; Iannace, S. Effect of Supramolecular Structures on Thermoplastic Zein-Lignin Bionanocomposites. J. Agric. Food Chem. 2011, 59, 10062–10070. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D. Lignin Nanocomposites. J. Macromol. Sci. Part A 2016, 53, 382–387. [Google Scholar] [CrossRef]
- Li, T.; Zheng, T.; Han, J.; Liu, Z.; Guo, Z.X.; Zhuang, Z.; Xu, J.; Guo, A.B. Effects of Diisocyanate Structure and Disulfide Chain Extender on Hard Segmental Packing and Self-Healing Property of Polyurea Elastomers. Polymers 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shang, Y.; He, J.; Li, T.; Li, Y.; Li, M.; Wang, M. Synthesis of Epoxy Resin-Based Aqueous Polyurethane and Application to Polyester-Cotton Fabric Finishing. Text. Res. J. 2022, 93, 2590–2603. [Google Scholar] [CrossRef]
- Liu, W.; Fang, C.; Wang, S.; Huang, J.; Qiu, X. High-Performance Lignin-Containing Polyurethane Elastomers with Dynamic Covalent Polymer Networks. Macromolecules 2019, 52, 6474–6484. [Google Scholar] [CrossRef]
- Ch’ng, S.Y.; Andriyana, A.; Tee, Y.L.; Verron, E. Effects of Carbon Black and the Presence of Static Mechanical Strain on the Swelling of Elastomers in Solvent. Materials 2015, 8, 884–898. [Google Scholar] [CrossRef]
- Liang, H.; Wang, S.; He, H.; Wang, M.; Liu, L.; Lu, J.; Zhang, Y.; Zhang, C. Aqueous Anionic Polyurethane Dispersions from Castor Oil. Ind. Crops Prod. 2018, 122, 182–189. [Google Scholar] [CrossRef]
- Bullermann, J.; Friebel, S.; Salthammer, T.; Spohnholz, R. Novel Polyurethane Dispersions Based on Renewable Raw Materials—Stability Studies by Variations of Dmpa Content and Degree of Neutralisation. Prog. Org. Coat. 2013, 76, 609–615. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, Z.; Wu, B.; Jiang, L.; Lei, J. Preparation and Properties of Cross-Linked Waterborne Polyurethane Based on Solvent-Free Route. Polym. Bull. 2019, 77, 3263–3275. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant Properties of Lignin in Polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Xing, Q.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.J. Biodegradable and High-Performance Poly(Butylene Adipate-Co-Terephthalate)–Lignin Uv-Blocking Films. ACS Sustain. Chem. Eng. 2017, 5, 10342–10351. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent Cellulose/Technical Lignin Composite Films for Advanced Packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of Cellulose and Lignin on Disintegration, Antimicrobial and Antioxidant Properties of Pla Active Films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Huwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, Y.; Li, Z.; Liang, Z.; Liu, W.; Yang, D.; Qiu, X. Near-Infrared-Activated Efficient Bacteria-Killing by Lignin-Based Copper Sulfide Nanocomposites with an Enhanced Photothermal Effect and Peroxidase-Like Activity. ACS Sustain. Chem. Eng. 2021, 9, 6479–6488. [Google Scholar] [CrossRef]
- Alexandra, M.B.; Marta, F.G. Polymeric Materials with Antimicrobial Activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar]
- Xia, Y.; Zhang, Z.; Kessler, M.R.; Brehm-Stecher, B.; Larock, R.C. Antibacterial Soybean-Oil-Based Cationic Polyurethane Coatings Prepared from Different Amino Polyols. ChemSusChem 2012, 5, 2221–2227. [Google Scholar] [CrossRef]


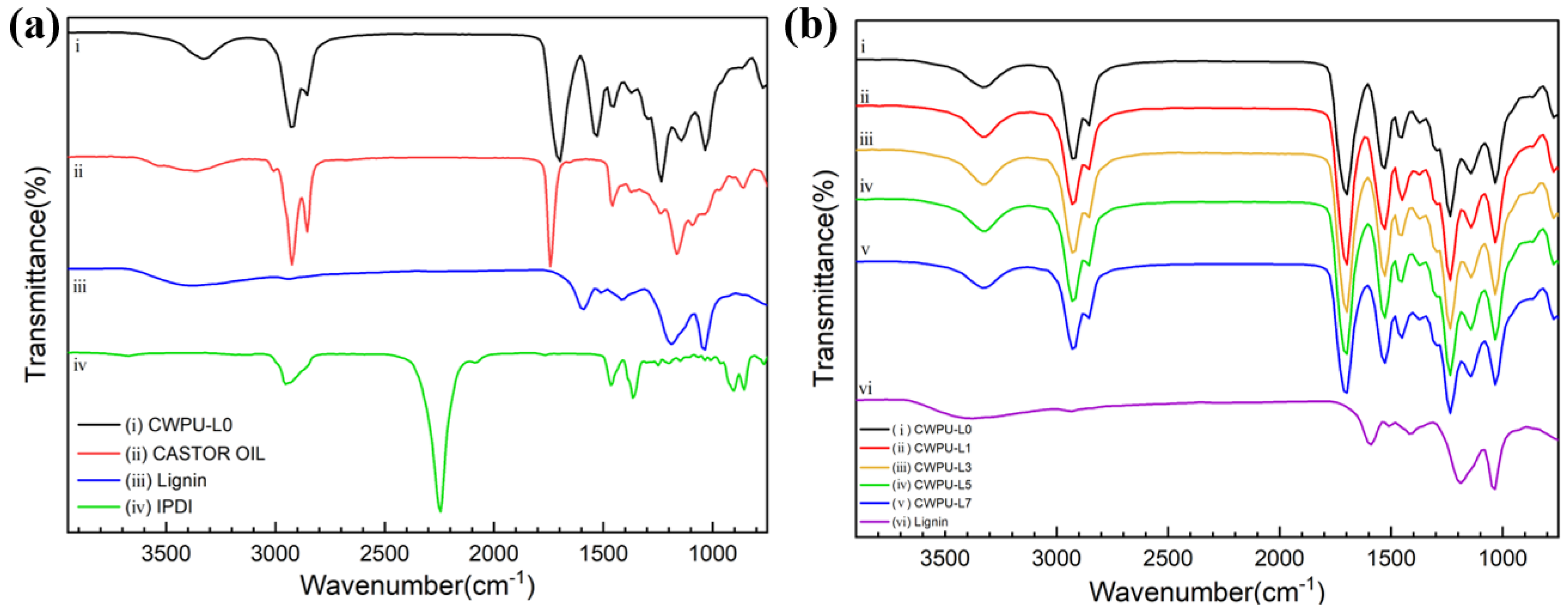
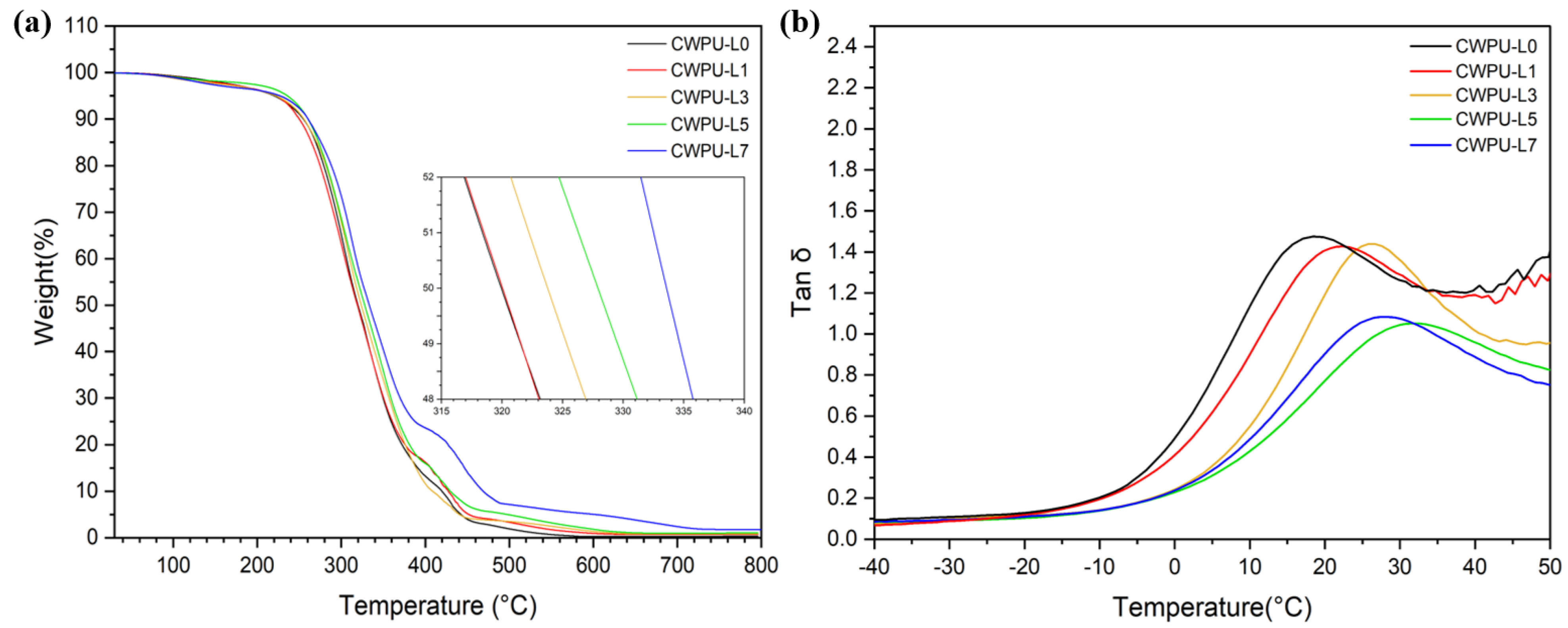
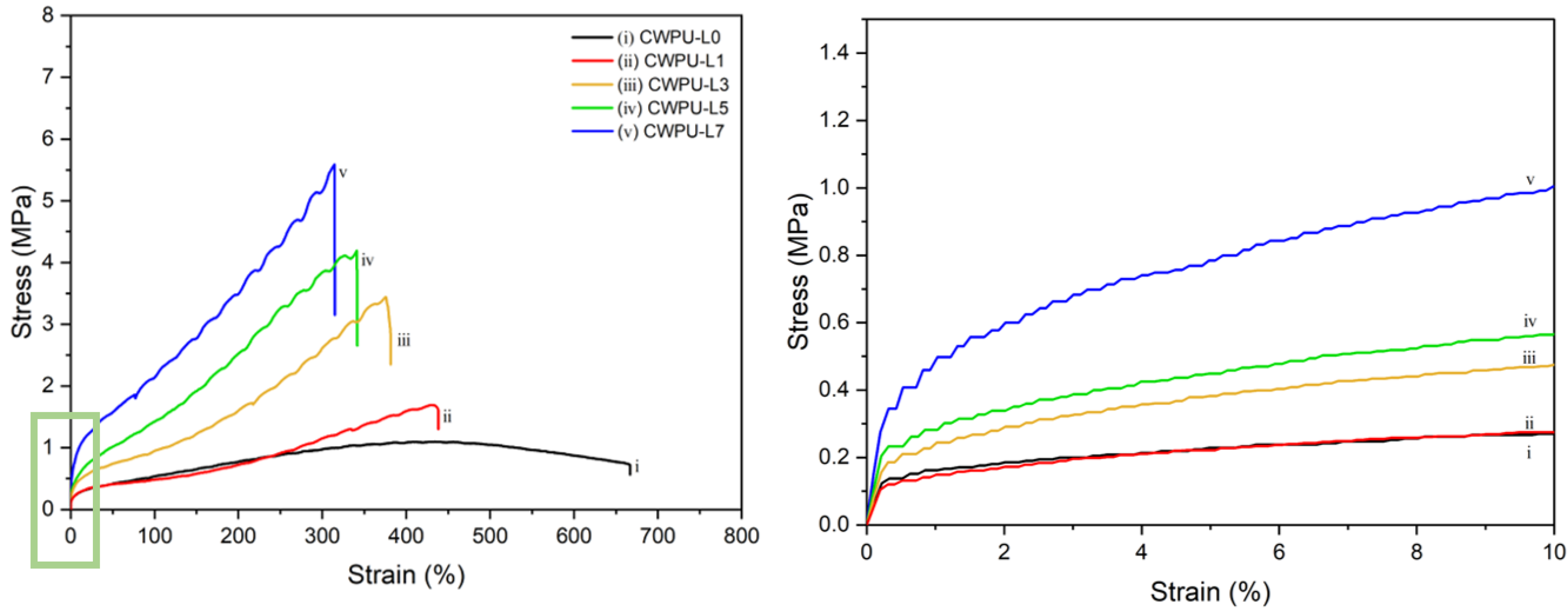

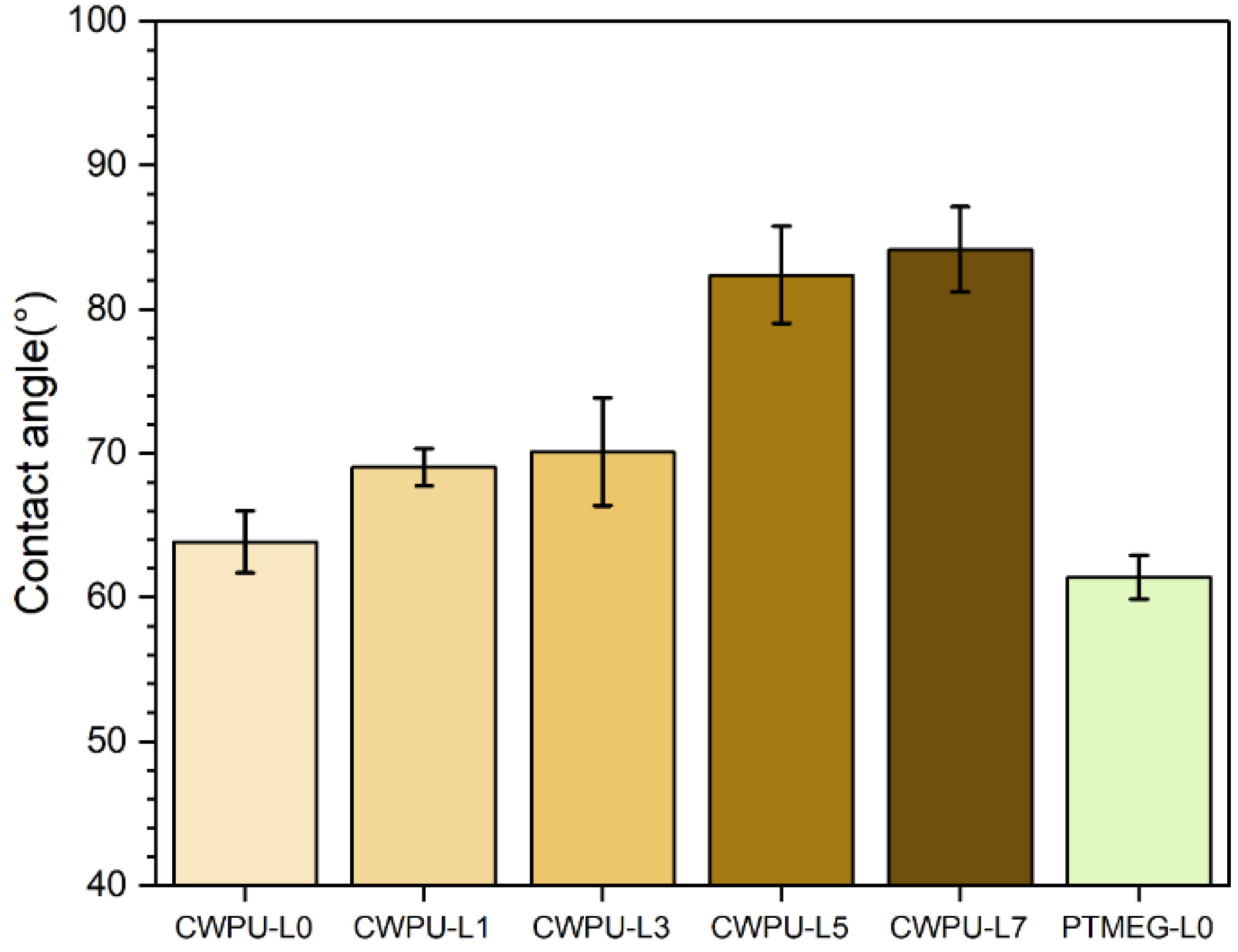
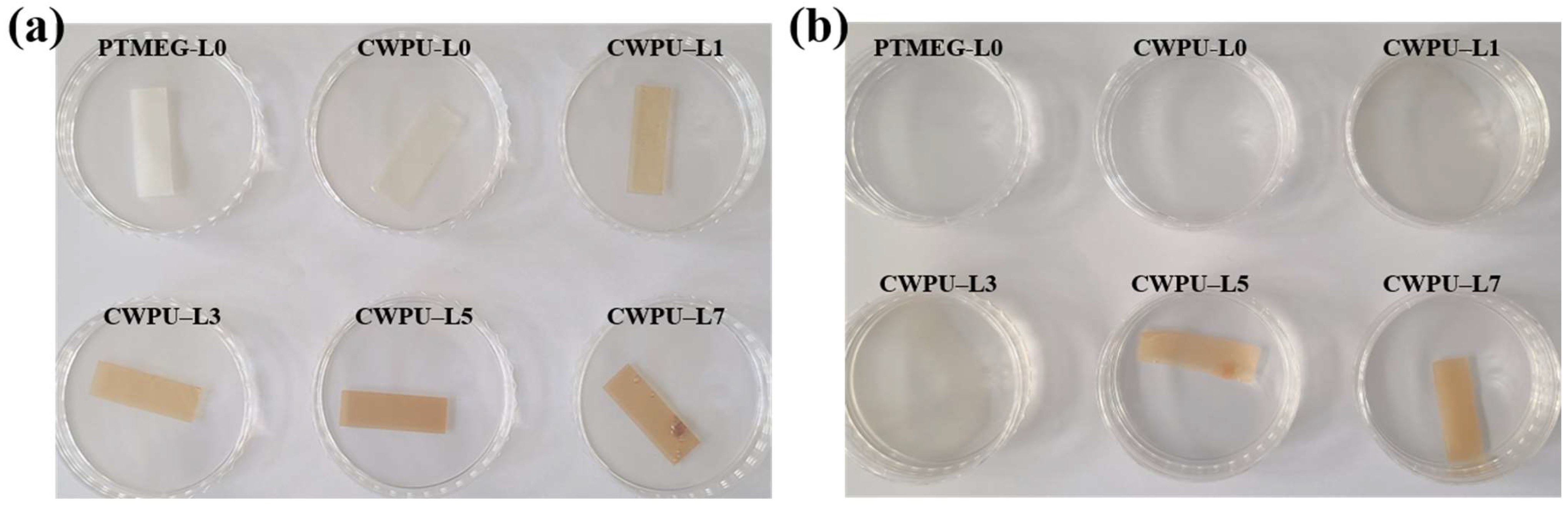

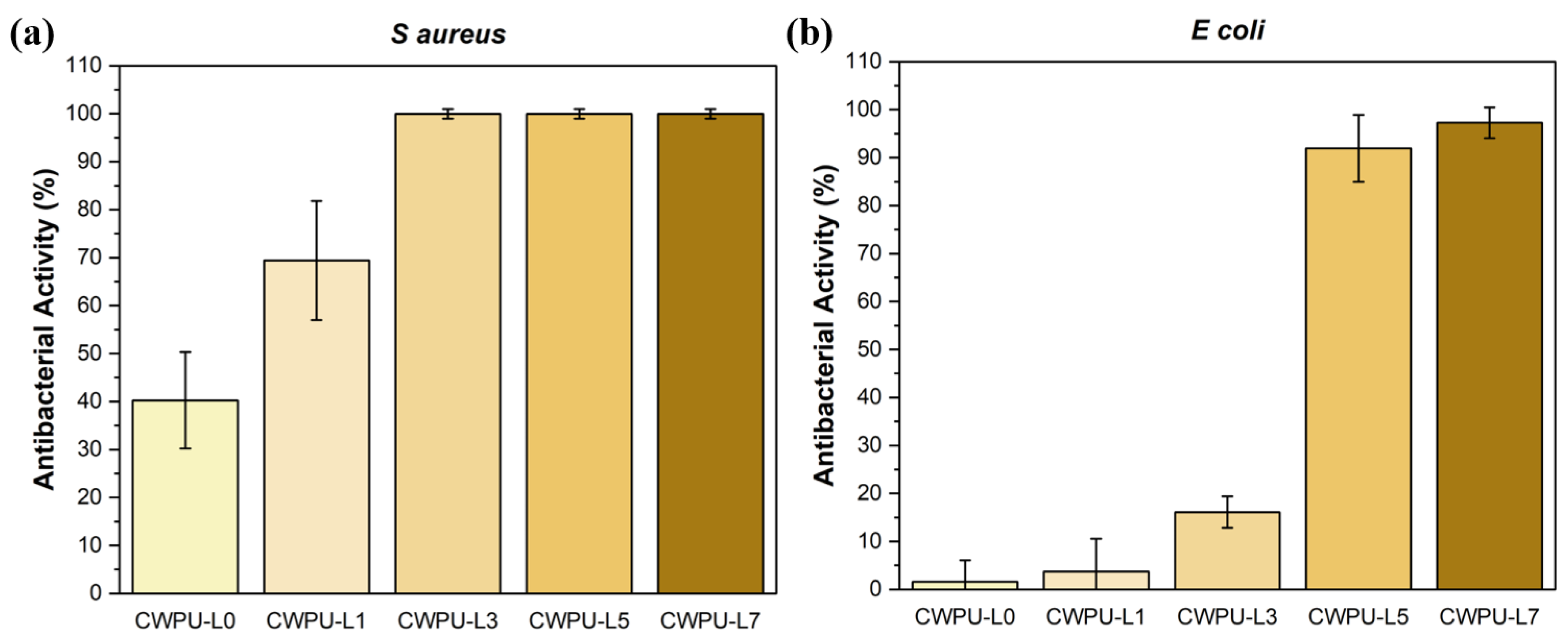
| Sample | Polyol (func. mol) | IPDI (func. mol) | Chain Extender (func. mol) | Lignin (wt%) | Content of Bio-Based Component (wt%) | ||
|---|---|---|---|---|---|---|---|
| CO | PTMEG | MDEA | DEA | ||||
| CWPU-L0 | 1 | - | 2.5 | 0.6 | 0.1 | 0 | 47.35% |
| CWPU-L1 | 1 | - | 1 | 47.89% | |||
| CWPU-L3 | 1 | - | 3 | 48.92% | |||
| CWPU-L5 | 1 | - | 5 | 49.85% | |||
| CWPU-L7 | 1 | - | 7 | 50.81% | |||
| PTMEG-L0 | - | 1 | 0 | 0.00% | |||
| Sample | Mw | Mn | PDI |
|---|---|---|---|
| CWPU-L0 | 21,781 | 7320 | 2.97 |
| CWPU-L1 | 22,980 | 7941 | 2.89 |
| CWPU-L3 | 32,604 | 14,716 | 2.22 |
| CWPU-L5 | 24,937 | 7107 | 3.5 |
| CWPU-L7 | 35,102 | 10,181 | 3.44 |
| PTMEG-L0 | 55,831 | 29,721 | 1.87 |
| Sample | Thermal Properties | |||
|---|---|---|---|---|
| TGA Analysis (°C) | DMA Analysis (°C) | |||
| Tonset | T50 | Tmax | Tg | |
| CWPU-L0 | 263.94 | 319.19 | 303.59 | 18.41 |
| CWPU-L1 | 256.37 | 319.92 | 300.69 | 22.44 |
| CWPU-L3 | 256.27 | 323.77 | 305.86 | 25.76 |
| CWPU-L5 | 260.07 | 327.95 | 304.11 | 31.67 |
| CWPU-L7 | 261.04 | 333.78 | 297.94 | 27.44 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| CWPU-L0 | 1.10 | 666.9 |
| CWPU-L1 | 1.69 | 438.2 |
| CWPU-L3 | 3.43 | 375.2 |
| CWPU-L5 | 4.18 | 340.9 |
| CWPU-L7 | 5.57 | 314.3 |
| Samples | FWHM a |
|---|---|
| CWPU-L0 | 6.5504 0.03 |
| CWPU-L1 | 6.5573 0.03 |
| CWPU-L3 | 6.6449 0.03 |
| CWPU-L5 | 6.6445 0.05 |
| CWPU-L7 | 6.7008 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.M.; Choi, J.S.; Jang, S.; Park, H.; Lee, S.Y.; Jung, J.; Park, J. Sustainable Strategies for Synthesizing Lignin-Incorporated Bio-Based Waterborne Polyurethane with Tunable Characteristics. Polymers 2023, 15, 3987. https://doi.org/10.3390/polym15193987
Kim BM, Choi JS, Jang S, Park H, Lee SY, Jung J, Park J. Sustainable Strategies for Synthesizing Lignin-Incorporated Bio-Based Waterborne Polyurethane with Tunable Characteristics. Polymers. 2023; 15(19):3987. https://doi.org/10.3390/polym15193987
Chicago/Turabian StyleKim, Bo Min, Jin Sil Choi, Sunjin Jang, Hyeji Park, Seung Yeol Lee, Joonhoo Jung, and Jaehyeung Park. 2023. "Sustainable Strategies for Synthesizing Lignin-Incorporated Bio-Based Waterborne Polyurethane with Tunable Characteristics" Polymers 15, no. 19: 3987. https://doi.org/10.3390/polym15193987
APA StyleKim, B. M., Choi, J. S., Jang, S., Park, H., Lee, S. Y., Jung, J., & Park, J. (2023). Sustainable Strategies for Synthesizing Lignin-Incorporated Bio-Based Waterborne Polyurethane with Tunable Characteristics. Polymers, 15(19), 3987. https://doi.org/10.3390/polym15193987






