Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae
Abstract
:1. Introduction
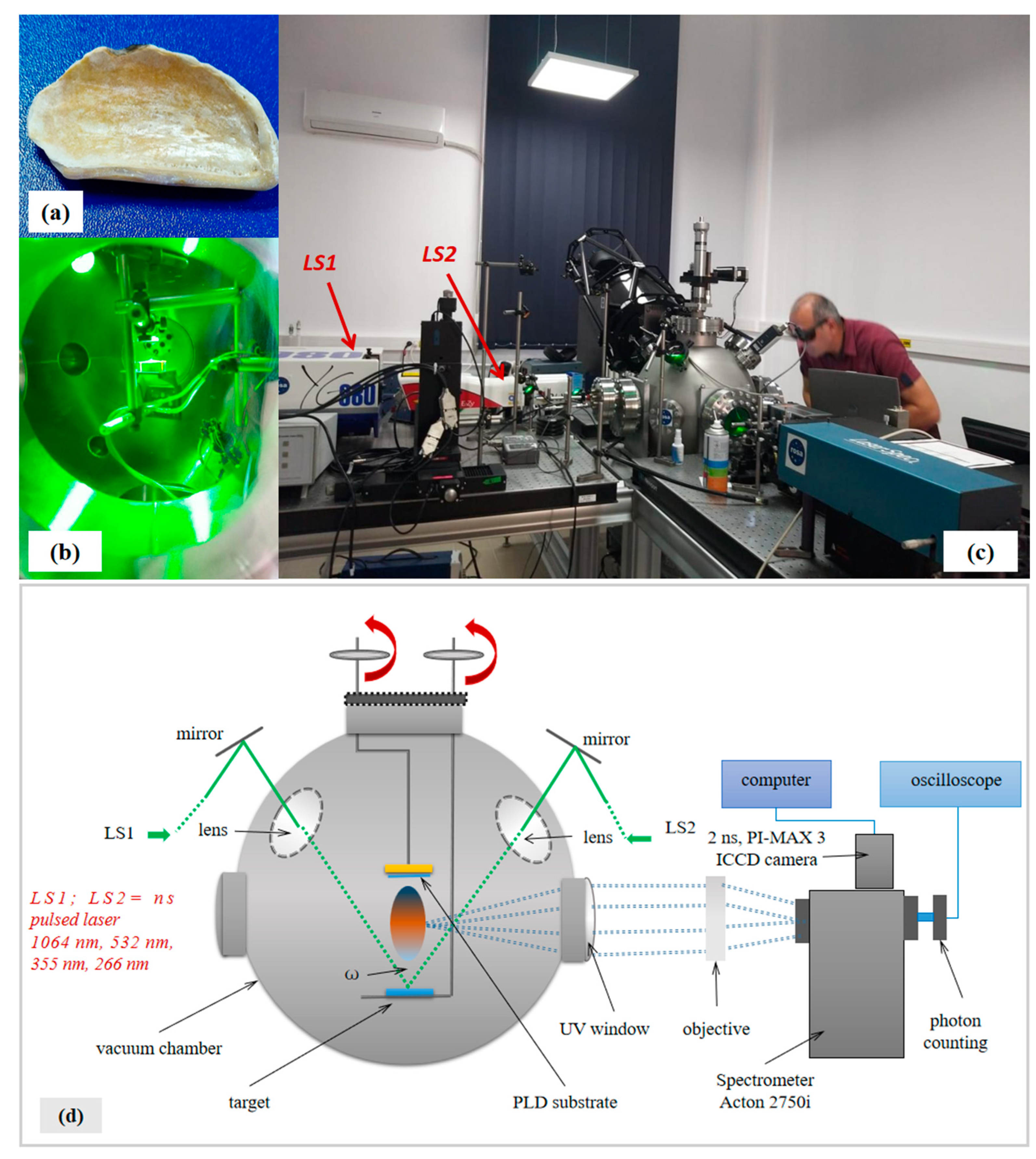
2. Materials and Methods
3. Results and Discussions
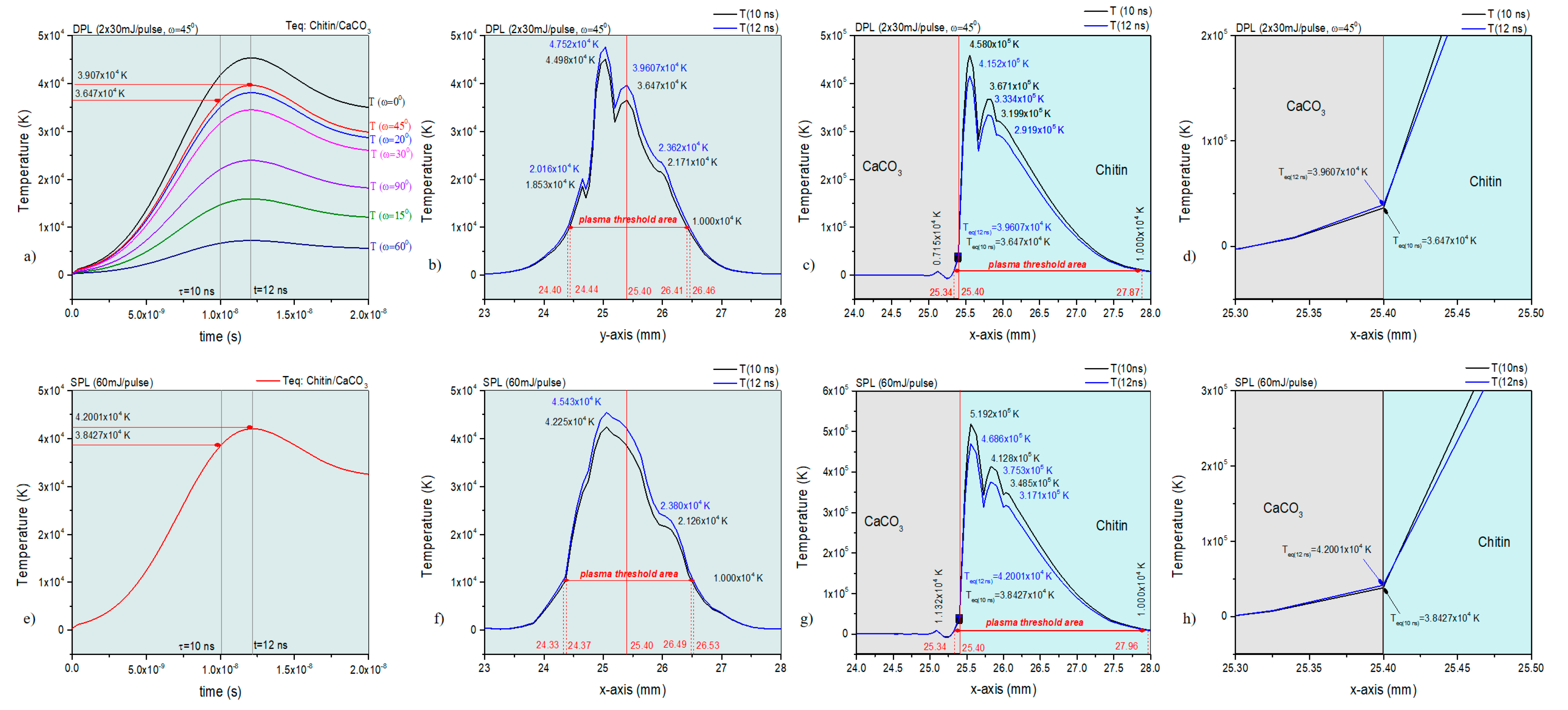
3.1. Experiment Optimization with Numerical Analysis in COMSOL V5.6
3.2. Morphological and Chemical Analysis of the Thin Film Obtained in Dual-Pulsed Lasers Regim
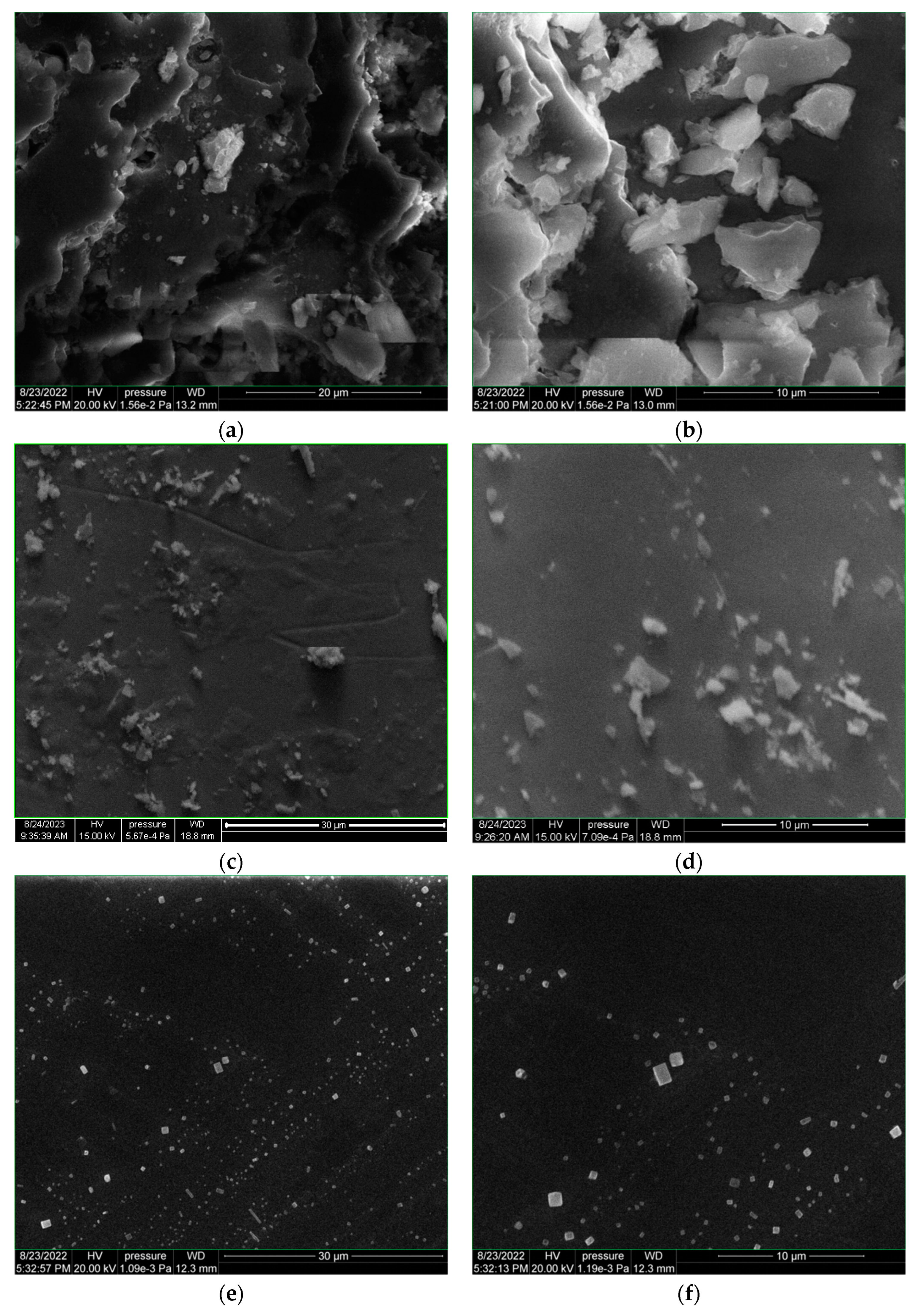
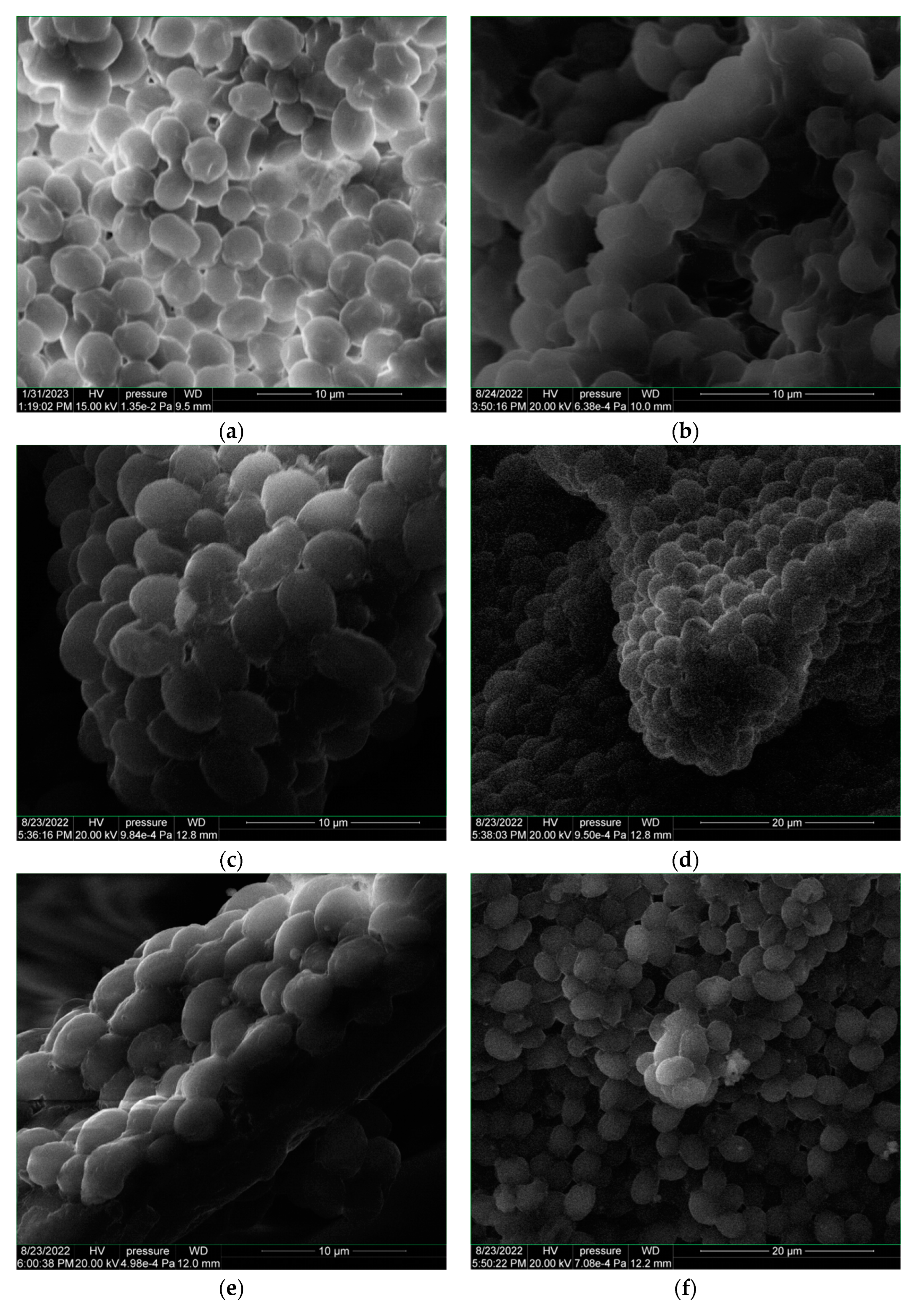
3.2.1. SEM–EDX Morphological and Elemental Composition Analyses
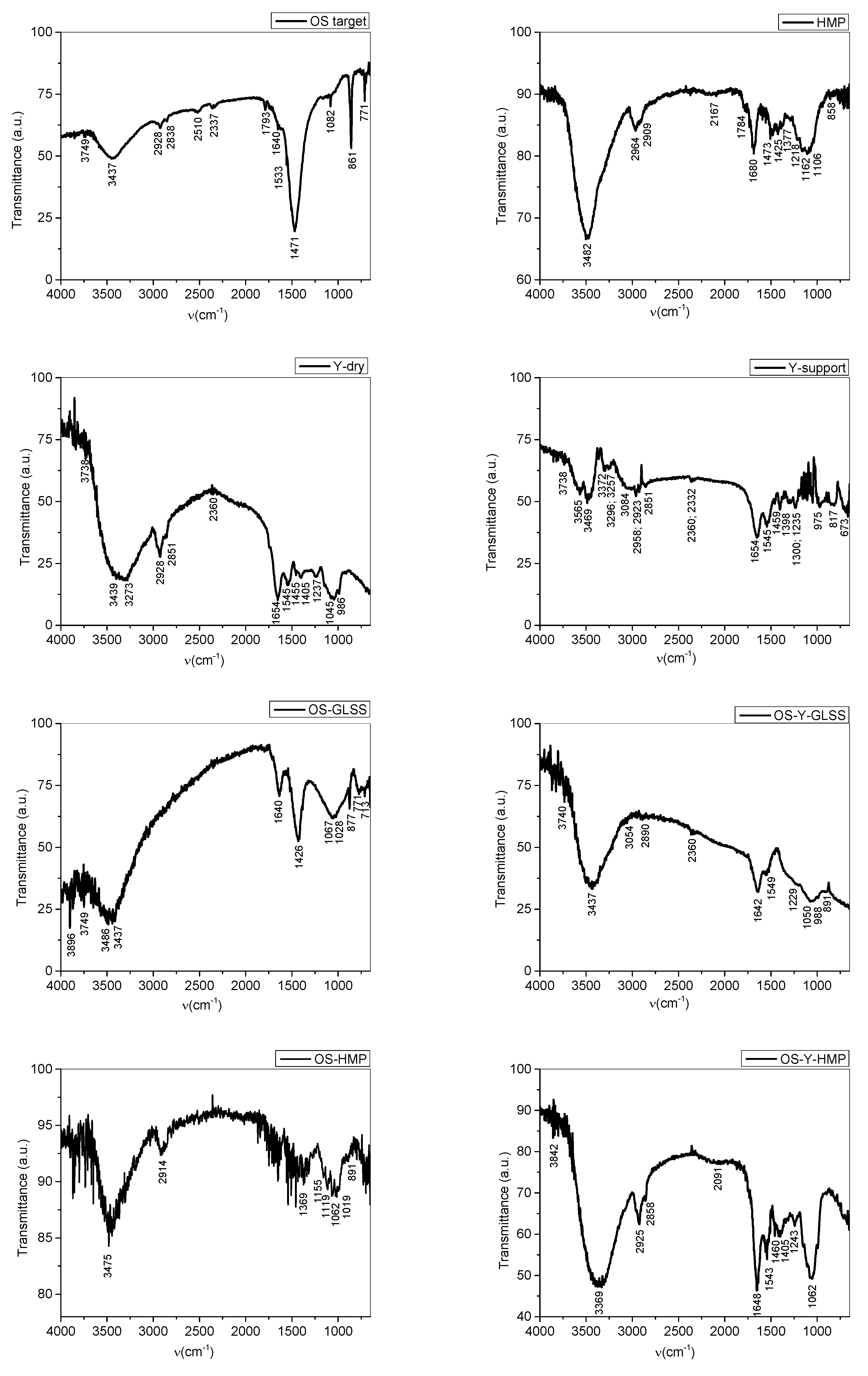
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
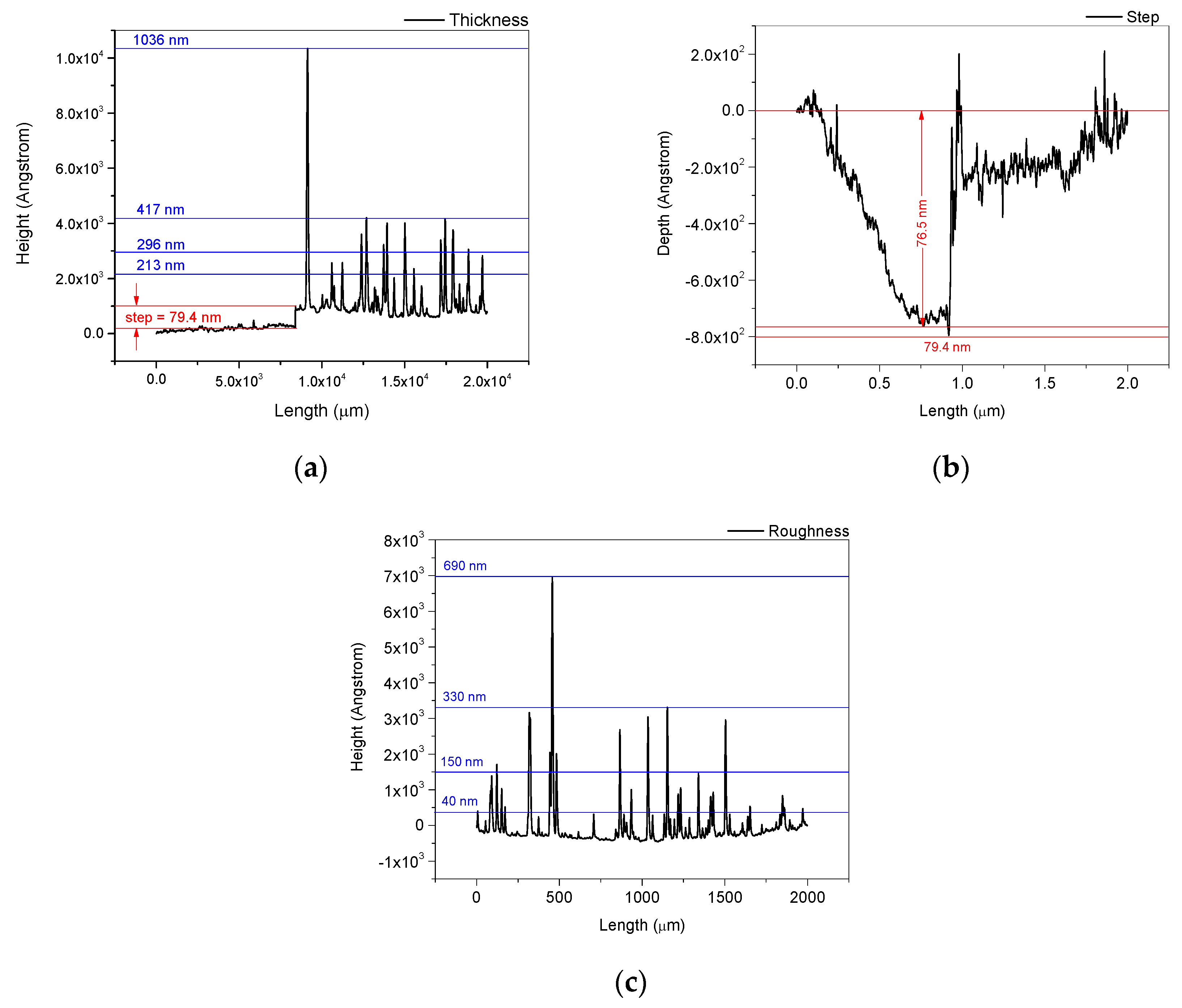
3.2.3. Profilometry of the DPL Thin Film
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cocean, G.; Cocean, A.; Postolachi, C.; Garofalide, S.; Bulai, G.; Munteanu, B.S.; Cimpoesu, N.; Cocean, I.; Gurlui, S. High-Power Laser Deposition of Chitosan Polymers: Medical and Environmental Applications. Polymers 2022, 14, 1537. [Google Scholar] [CrossRef] [PubMed]
- Cocean, I.; Cocean, A.; Postolachi, C.; Pohoata, V.; Cimpoesu, N.; Bulai, G.; Iacomi, F.; Gurlui, S. Alpha keratin amino acids BEHVIOR under high FLUENCE laser interaction. Medical applications. Appl. Surf. Sci. 2019, 488, 418–426. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Cimpoesu, N.; Cocean, G.; Cimpoesu, R.; Postolachi, C.; Popescu, V.; Gurlui, S. Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target. Appl. Sci. 2021, 11, 4030. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, G.; Diaconu, M.; Garofalide, S.; Husanu, F.; Munteanu, B.S.; Cimpoesu, N.; Motrescu, I.; Puiu, I.; Postolachi, C.; et al. Nano-Biocomposite Materials Obtained from Laser Ablation of Hemp Stalks for Medical Applications and Potential Component in New Solar Cells. Int. J. Mol. Sci. 2023, 24, 3892. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Pavlov, G.; Desbrieres, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Hussain, M.R.; Iman, M.; Maji, T.K. Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int. J. Adv. Eng. Appl. 2013, 6, 4–12. [Google Scholar]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Palpandi, C.; Shanmugam, V.; Shanmugam, A. Extraction of chitin and chitosan from shell and operculum of mangrove gastropod Nerita (Dostia) crepidularia Lamarck. Int. J. Med. Med. Sci. 2009, 1, 198–205. [Google Scholar]
- Mathaba, M.; Daramola, M.O. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohydr. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, S.; Tan, Y.; Feng, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Biodegradation and Prospect of Polysaccharide from Crustaceans. Mar. Drugs 2022, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, I.; Silva, R.; Meunier, L.; Valente, S.M.; Lago-Lestón, A.; Keller-Costa, T.; Costa, R. Functional metagenomics reveals differential chitin degradation and utilization features across free-living and host-associated marine microbiomes. Microbiome 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Kriangkrai, W. Chitinase-Assisted Bioconversion of Chitinous Waste for Development of Value-Added Chito-Oligosaccharides Products. Biology 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, Z.; Xu, X.; Huang, C.; Yao, Z.; Yang, X.; Zhang, Y.; Wang, D.; Wei, C.; Zhuang, X. Mechano-Enzymatic Degradation of the Chitin from Crustacea Shells for Efficient Production of N-acetylglucosamine (GlcNAc). Molecules 2022, 27, 4720. [Google Scholar] [CrossRef]
- Affes, S.; Aranaz, I.; Hamdi, M.; Acosta, N.; Ghorbel-Bellaaj, O.; Heras, Á.; Nasri, M.; Maalej, H. Preparation of a crude chitosanase from blue crab viscera and its application in the production of biologically active chito-oligosaccharides from chitosan shrimp shells. Int. J. Biol. Macromol. 2019, 139, 558–569. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-wita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Beier, S.; Bertilsson, S. Bacterial chitin degradation—Mechanisms and ecophysiological strategies. Front. Microbiol. 2013, 4, 149. [Google Scholar] [CrossRef]
- Mogilevskaya, E.L.; Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. The Crystal Structure of Chitin and Chitosan. Polym. Sci. 2006, 48, 116–123. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Podgorbunskikh, E.; Kuskov, T.; Rychkov, D.; Lomovskii, O.; Bychkov, A. Mechanical Amorphization of Chitosan with Different Molecular Weights. Polymers 2022, 14, 4438. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and Crystal Structure of Hydrated Chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Ogawa, Y.; Naito, P.K.; Nishitama, Y. Hydrogen Bond Network in Anhydrous Chitosan from Neutron Crystallography and Periodic Density Functional Theory Calculations. Carbohydr. Polym. 2019, 207, 211–217. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, R.; Nikolaivits, E.; Zervakis, G.I.; Abdel-Maksoud, G.; Topakas, E.; Christakopoulos, P. The use of chitosan in protecting wooden artifacts from damage by mold fungi. Electron. J. Biotechnol. 2016, 24, 70–78. [Google Scholar] [CrossRef]
- Wu, S.; Yan, K.; Li, J.; Huynh, R.N.; Raub, C.B.; Shen, J.; Shi, X.G.; Payne, F. Electrical cuing of chitosan’s mesoscale organization, Reactive and Functional. Polymers 2020, 148, 104492. [Google Scholar] [CrossRef]
- Vicente, F.A.; Hus, M.; Likozar, B.; Novak, U. Chitin Deacetylation Using Deep Eutectic Solvents: Ab Initio Supported Process Optimization. ACS Sustain. Chem. Eng. 2021, 9, 3874–3886. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rhee, K.Y.; Park, S.J. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef]
- Tommalieh, M.J.; Ibrahium, M.J.; Awwad, N.S.; Menazea, A.A. Gold nanoparticles doped Polyvinyl Alcohol/Chitosan blend via laser ablation for electrical conductivity enhancement. J. Mol. Struct. 2020, 1221, 128814. [Google Scholar] [CrossRef]
- Qi, P.; Zhanga, T.; Shao, J.; Yang, B.; Fei, T.; Wang, R. A QCM humidity sensor constructed by graphene quantum dots and chitosan composites. Sens. Actuators A Phys. 2019, 287, 93–101. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ramazani, S.A.A.; Mashayekhan, S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, B.; Zhao, J.; Yang, B.; Zheng, X. Benzothiazole derivatives-based supramolecular assemblies as efficient corrosion inhibitors for copper in artificial seawater: Formation, interfacial release and protective mechanisms. Corros. Sci. 2023, 212, 110957. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Cocean, G.; Postolachi, C.; Pricop, D.A.; Munteanu, B.S.; Cimpoesu, N.; Gurlui, S. Study of PhysicoChemical Interactions during the Production of Silver Citrate Nanocomposites with Hemp Fiber. Nanomaterials 2021, 11, 2560. [Google Scholar] [CrossRef] [PubMed]
- Cocean, A.; Pelin, V.; Cazacu, M.M.; Cocean, I.; Sandu, I.; Gurlui, S.; Iacomi, F. Thermal effects induced by laser ablation in non-homogeneous limestone covered by an impurity layer. Appl. Surf. Sci. 2017, 424, 324–329. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Gurlui, S.; Iacomi, F. Study of the pulsed laser deposition phenomena by means of Comsol Multiphysics. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys. 2017, 79, 263–274. [Google Scholar]
- Cocean, A.; Cocean, I.; Gurlui, S. Influence of the Impurities to the Composite Materials in Laser Ablation Phenomena. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys. 2021, 83, 225–238. [Google Scholar]
- Optical Constants of CaCO3 (Calcium Carbonate, Calcite). Available online: http://refractiveindex.info/?shelf=main&book=CaCO3&page= (accessed on 12 August 2022).
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data, 4th ed.; Revised and Enlarged Edition; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93810-1. [Google Scholar]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo1, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef]





| Samples | ELEMENTS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atomic % (Number of Atoms of a Species out of 100 Atoms of All Detected Species) | ||||||||||||
| C | O | Na | Ca | Mg | Al | Si | Fe | P | K | N | S | |
| OS target area1 | 22.88 | 66.42 | 2.88 | 7.82 | - | - | - | - | - | - | - | - |
| OS target area2 | 24.46 | 63.55 | 3.04 | 8.95 | - | - | - | - | - | - | - | - |
| Average OS target | 23.67 | 64.99 | 2.96 | 8.39 | - | - | - | - | - | - | - | - |
| OS glass area1 | 17.13 | 61.43 | 10.04 | 0.31 | 2.19 | 0.47 | 8.42 | - | - | - | - | - |
| OS glass area2 | 6.17 | 70.50 | 12.63 | 0.14 | 2.44 | - | 8.12 | - | - | - | - | - |
| Average OS glass | 11.65 | 65.97 | 11.34 | 0.23 | 2.32 | 0.24 | 8.27 | - | - | - | - | - |
| OS hemp area1 | 58.13 | 41.75 | - | 0.05 | - | - | - | 0.07 | - | - | - | - |
| OS hemp area2 | 63.23 | 36.57 | - | 0.07 | - | - | - | 0.13 | - | - | - | - |
| Average OS hemp | 60.68 | 39.16 | - | 0.06 | - | - | - | 0.10 | - | - | - | - |
| OSY glass area1 | 59.57 | 39.61 | - | 0.15 | 0.67 | - | - | - | - | - | - | - |
| OSY glass area2 | 59.88 | 39.66 | - | 0.11 | - | - | - | - | 0.19 | 0.16 | - | - |
| Average OSY glass | 59.73 | 39.64 | - | 0.13 | 0.34 | - | - | - | 0.10 | 0.08 | - | - |
| OSY hemp area1 | 51.95 | 35.02 | - | 0.12 | - | - | - | - | 0.08 | 0.07 | 12.76 | 0.02 |
| OSY hemp area2 | 58.87 | 27.97 | 0.64 | 0.14 | 0.20 | - | - | - | 0.23 | 0.19 | 11.70 | 0.06 |
| Average OSY hemp | 58.00 | 36.38 | 0.64 | 0.13 | 0.44 | - | - | - | 0.15 | 0.13 | 12.23 | 0.04 |
| Y-support AREA 1 | 48.83 | 34.49 | - | - | - | 0.14 | - | - | 0.18 | 0.17 | 16.15 | 0.04 |
| Y-support AREA 2 | 50.65 | 32.11 | - | - | - | - | - | - | 0.24 | 0.24 | 16.68 | 0.08 |
| Average Y-support | 49.74 | 33.30 | - | - | - | 0.14 | - | - | 0.21 | 0.21 | 16.42 | 0.06 |
| Functional Groups Vibrations Wavenumber (cm−1) | Observations Based on References [1,23,24,38,39,40] and on the Gaussian 6 Simulated IR Spectra | |||||||
|---|---|---|---|---|---|---|---|---|
| OS Target | HMP | Y-Dry | Y-Support | OS-GLSS | OS-Y-GLSS | OS-HMP | OS-Y- HMP | |
| - | - | - | - | 3896 | - | - | 3842 | OH free stretching 3941, 3815 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to OH free stretching |
| 3749 | - | 3738 | 3738 | 3749 | 3740 | - | - | OH free stretching Si-OH, Al-OH, H-OH 3716 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to OH free stretching |
| - | - | - | 3565 | - | - | - | - | O-H free and H-bonded stretching, NH free and H-bonded stretching 3518 and 3507 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to OH free stretching |
| 3437 | 3482 | 3439 | 3469 | 3437 | 3437 | 3475 | - | O-H free and H-bonded stretching, NH free and H-bonded stretching |
| - | - | - | 3372 | - | - | - | 3369 | NH free and H-bonded stretching |
| - | - | 3273 | 3296, 3257 | - | - | - | - | O-H free and H-bonded stretching, NH free and H-bonded stretching 3255 and 3205 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to NH free stretching |
| - | - | - | 3084 | - | 3054 | - | - | stretching |
| 2928 | 2964 2909 | 2928, 2923 | 2958, 2923, | - | - | 2914 | 2925 | stretching |
| 2838 | - | 2851 | 2851 | - | 2890 | - | 2858 | stretching, cyclohexanes stretching in amines 2844 and 2719 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to CH alipjhatic stretching |
| 2510 | - | - | - | - | - | - | - | Chelates in CaCO3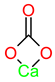 |
| 2337 | - | 2360 | 2360, 2332 | - | - | - | - | Adsorbed gas phase; CO2 molecule adsorbed 2318 cm−1 specific to chitosan as per Gaussian simulation, assigned toN in primary amines |
| - | 2167 | - | - | - | - | - | - | Adsorbed CO (usually on metal oxides or generally metals ionic state) C≡N stretching 2114 cm−1 specific to chitosan as per Gaussian simulation, assigned toN in primary amines |
| - | - | - | - | - | - | - | 2091 | C≡C stretching monosubstituted CH aromatic bending |
| 1793 | 1784 | - | - | - | - | - | - | C=O stretching, also in calcium carbonate CaCO3 1750 and 1759 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to NH bending |
| 1640 | 1680 | 1654 | 1654 | 1640 | 1642 | - | 1648 | C=ONHC=O NH bending in primary amines C=C stretching CH aromatic bending 1667 cm−1C and NH bending |
| 1533 | - | 1545 | 1545 | - | 1549 | - | 1543 | NH bending vibration in amines 1533 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to NH bending |
| 1471 | 1473 | 1455 | 1459 | 1426 | - | - | 1460; 1405 | (carboxyl) (carboxylate) bending vibrations Lattice vibration CH3 asymmetric; acetyl group 1450 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to OH bending |
| - | 1377 | - | - | - | - | 1369 | - | OH alcoholic bending Corresponds to: 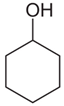 1394 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to OH bending |
| - | - | 1237 | - | - | 1229 | - | - | 1290 cm−1 specific to chitin and chitosan as per Gaussian simulation, assigned to skeletal vibrations |
| - | 1162; 1106 | - | - | - | - | 1155 | - | stretching symmetric vibrations 1100–1108 cm−1 specific to chitin and chitosan as per Gaussian sim-ulation, assigned to the bridge symmetric vibrations |
| 1082 | 1045 | - | - | 1067; 1028; | 1050 | 1062 | 1062 | C-O stretching OH; CH-OH stretching C stretching asymmetric C-O-O stretching symmetric stretching stretching; 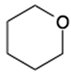 oxane (oxacyclohexane) in glucosamine ring oxane (oxacyclohexane) in glucosamine ring |
| - | - | 986 | 975 | - | 988 | - | - | (carboxyl) (carboxylate) bending vibrations; ~925 acetates (acetates and acetic acid resulted from chitin deacetylation) 994 cm−1 specific to chitin and chi-tosan as per Gaussian simulation, assigned to the bridge asymmetric vibrations |
| 861 | 858 | - | 817 | 877 | 891 | 891 | - | out-of-plane bending modes 937 cm−1 specific to calcium car-bonate, chitin and chitosan as per Gaussian simulation, assigned to the bridge bending vibrations |
| 771 | - | - | - | 771 | - | - | - | CH2 bending 771 cm−1 specific to calcium carbonate as per Gaussian simulation, assigned to the C=O bending vibrations |
| - | - | - | - | 713 | - | - | - | C=O out-of-plane bending modes 713 cm−1 specific tas per Gaussian simulation, assigned to C=O out-of-plane bendings in carbonate ions |
| - | - | - | 673 | - | - | - | - | CO2 molecule adsorbed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocean, G.; Cocean, A.; Garofalide, S.; Pelin, V.; Munteanu, B.S.; Pricop, D.A.; Motrescu, I.; Dimitriu, D.G.; Cocean, I.; Gurlui, S. Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae. Polymers 2023, 15, 3953. https://doi.org/10.3390/polym15193953
Cocean G, Cocean A, Garofalide S, Pelin V, Munteanu BS, Pricop DA, Motrescu I, Dimitriu DG, Cocean I, Gurlui S. Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae. Polymers. 2023; 15(19):3953. https://doi.org/10.3390/polym15193953
Chicago/Turabian StyleCocean, Georgiana, Alexandru Cocean, Silvia Garofalide, Vasile Pelin, Bogdanel Silvestru Munteanu, Daniela Angelica Pricop, Iuliana Motrescu, Dan Gheorghe Dimitriu, Iuliana Cocean, and Silviu Gurlui. 2023. "Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae" Polymers 15, no. 19: 3953. https://doi.org/10.3390/polym15193953
APA StyleCocean, G., Cocean, A., Garofalide, S., Pelin, V., Munteanu, B. S., Pricop, D. A., Motrescu, I., Dimitriu, D. G., Cocean, I., & Gurlui, S. (2023). Dual-Pulsed Laser Ablation of Oyster Shell Producing Novel Thin Layers Deposed to Saccharomyces cerevisiae. Polymers, 15(19), 3953. https://doi.org/10.3390/polym15193953









