Hydrophobized MFC as Reinforcing Additive in Industrial Silica/SBR Tire Tread Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MFC Modification
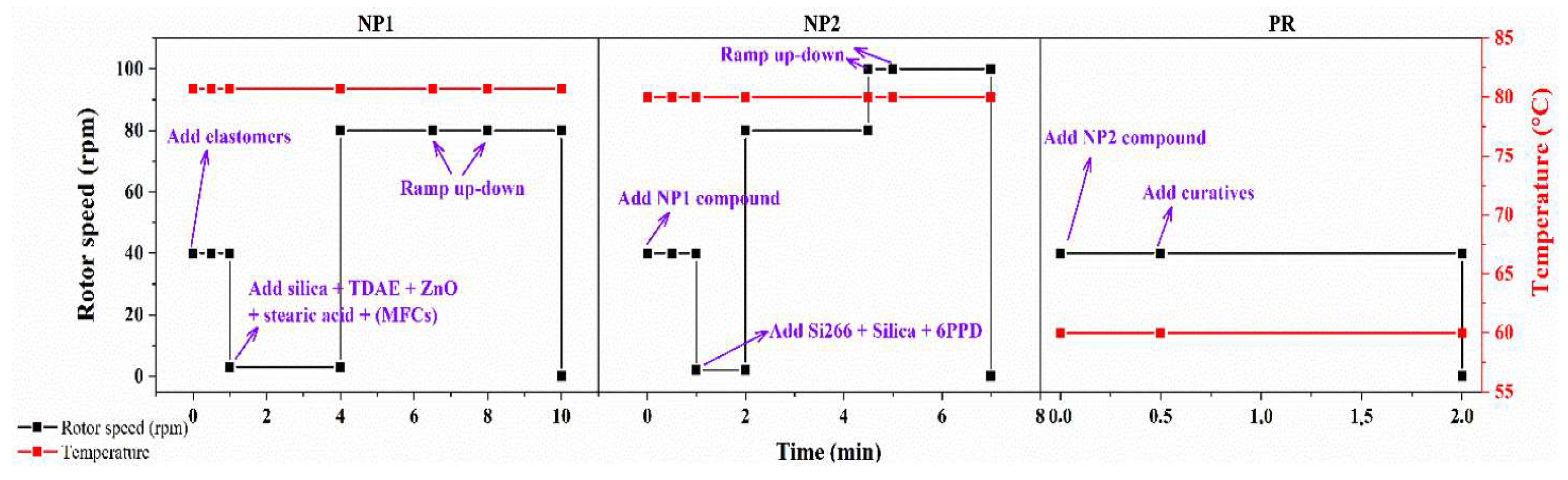
2.3. Composite Preparation
2.4. Characterizations
2.4.1. Chemical Composition Analysis
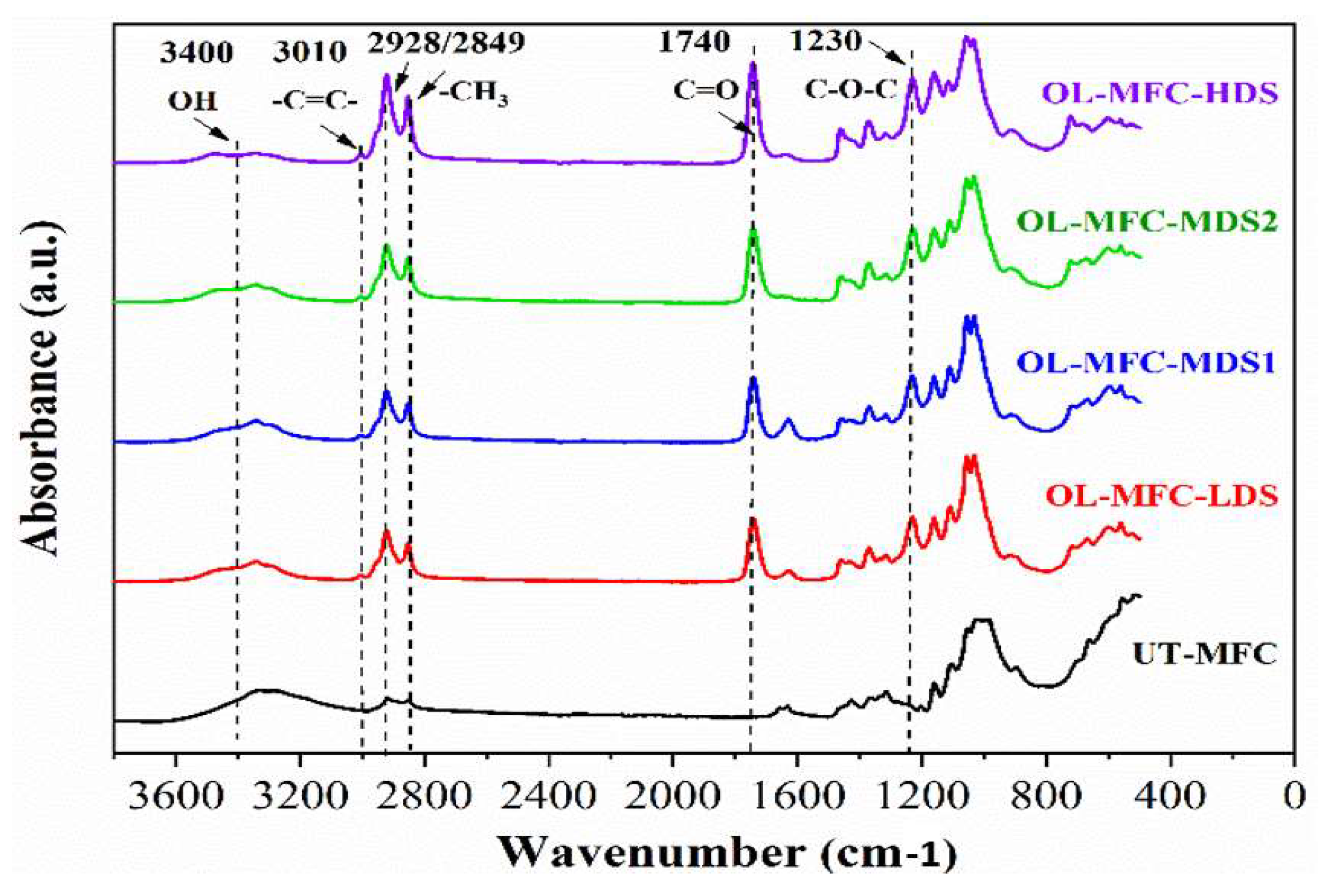
2.4.2. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.3. X-Ray Diffraction (XRD)
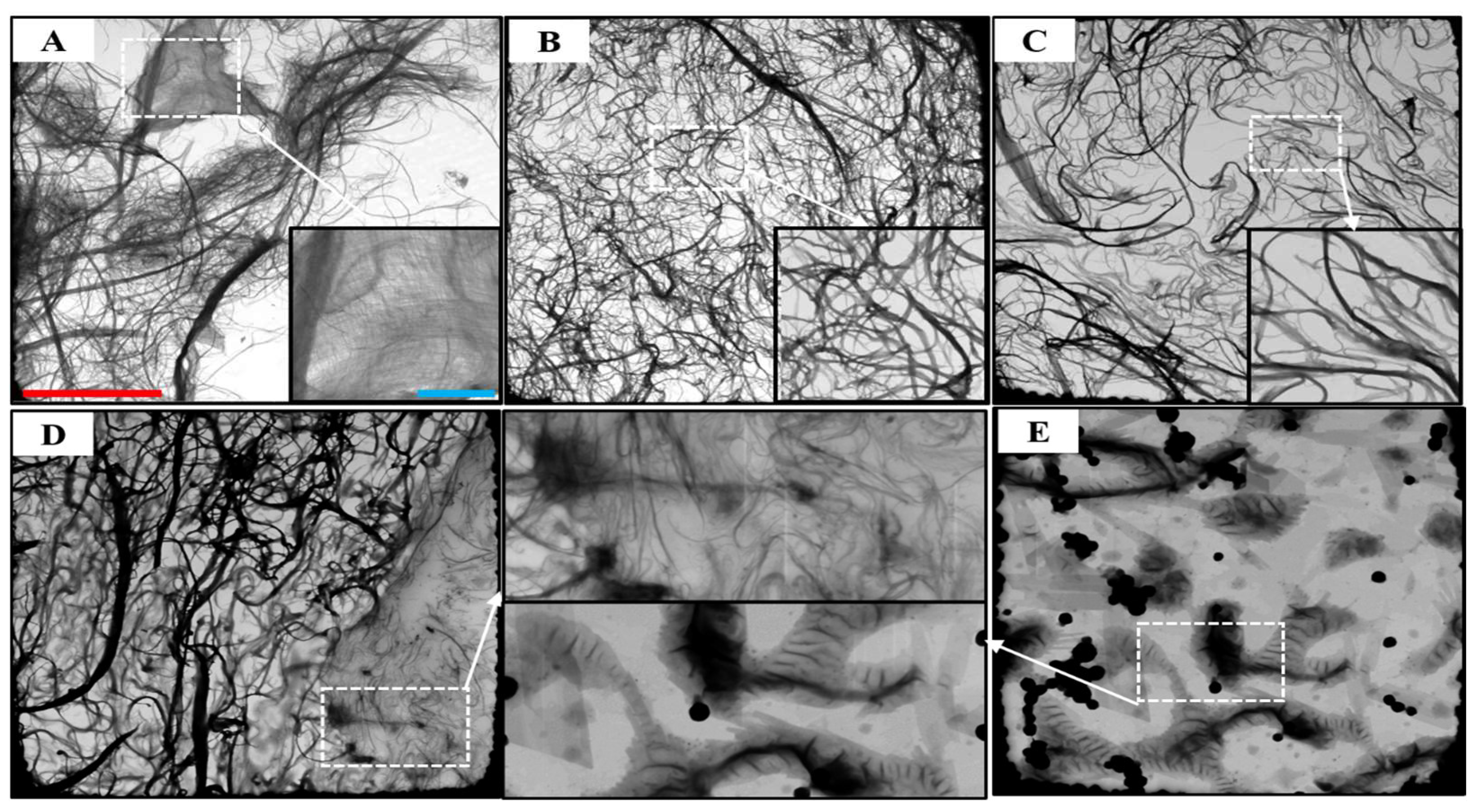
2.4.4. Morphological Characterization
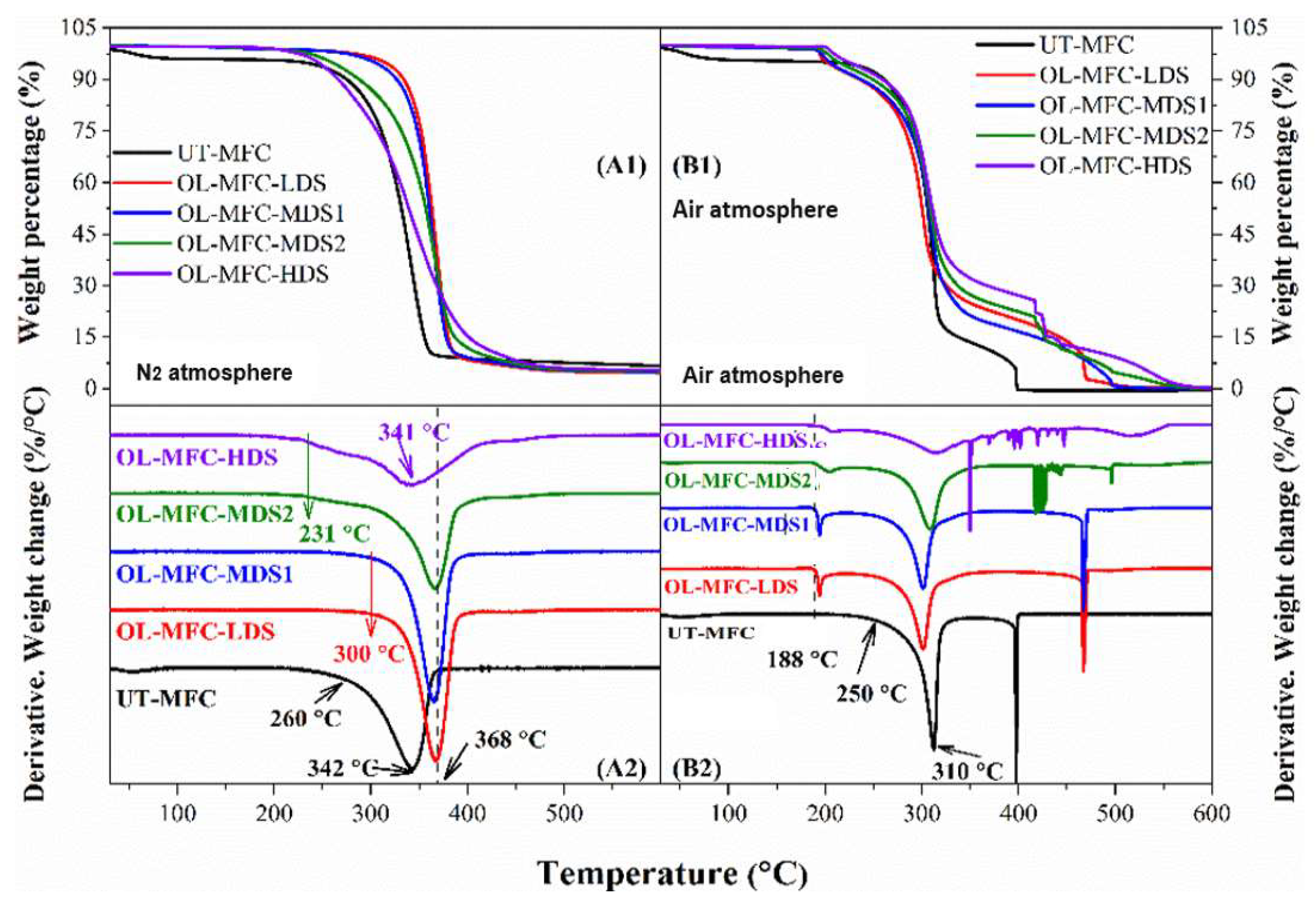
2.4.5. Thermogravimetric Analysis (TGA)
2.4.6. Moving Die Rheometer (MDR)
2.4.7. Dynamic Mechanical Analysis (DMA)
2.4.8. Tensile Test
3. Results and Discussion
3.1. ATR-FTIR Analysis
3.2. XRD Analysis
3.3. STEM Morphology
3.4. Thermal Stability Analysis
3.5. Compounding and Rubber Compound Properties
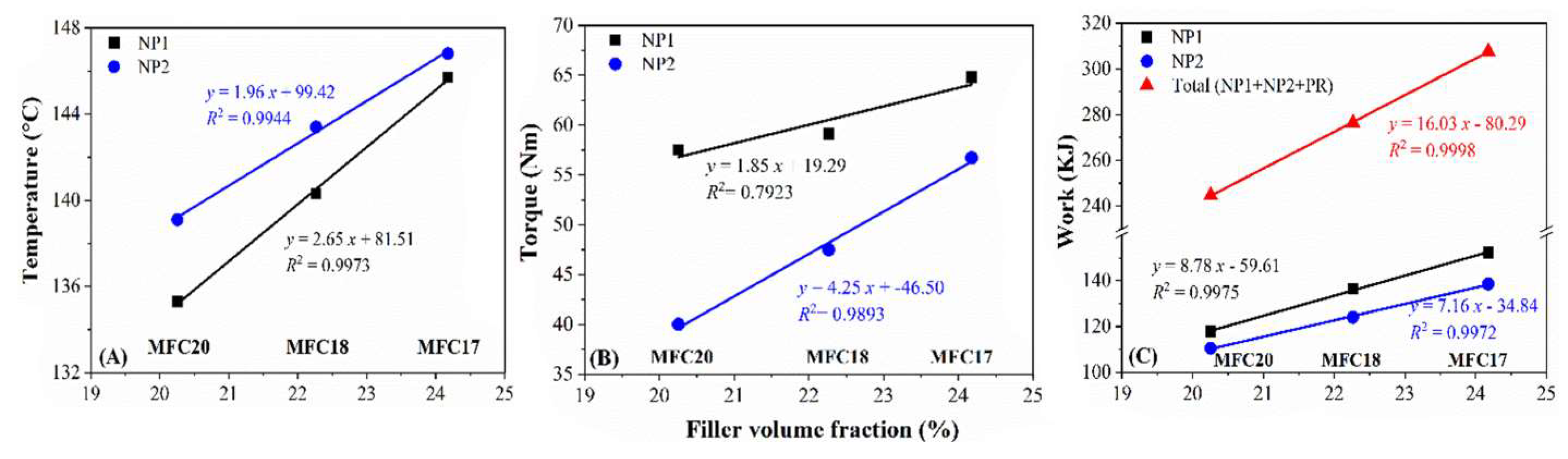
3.5.1. Processability Analysis
3.5.2. MDR Analysis
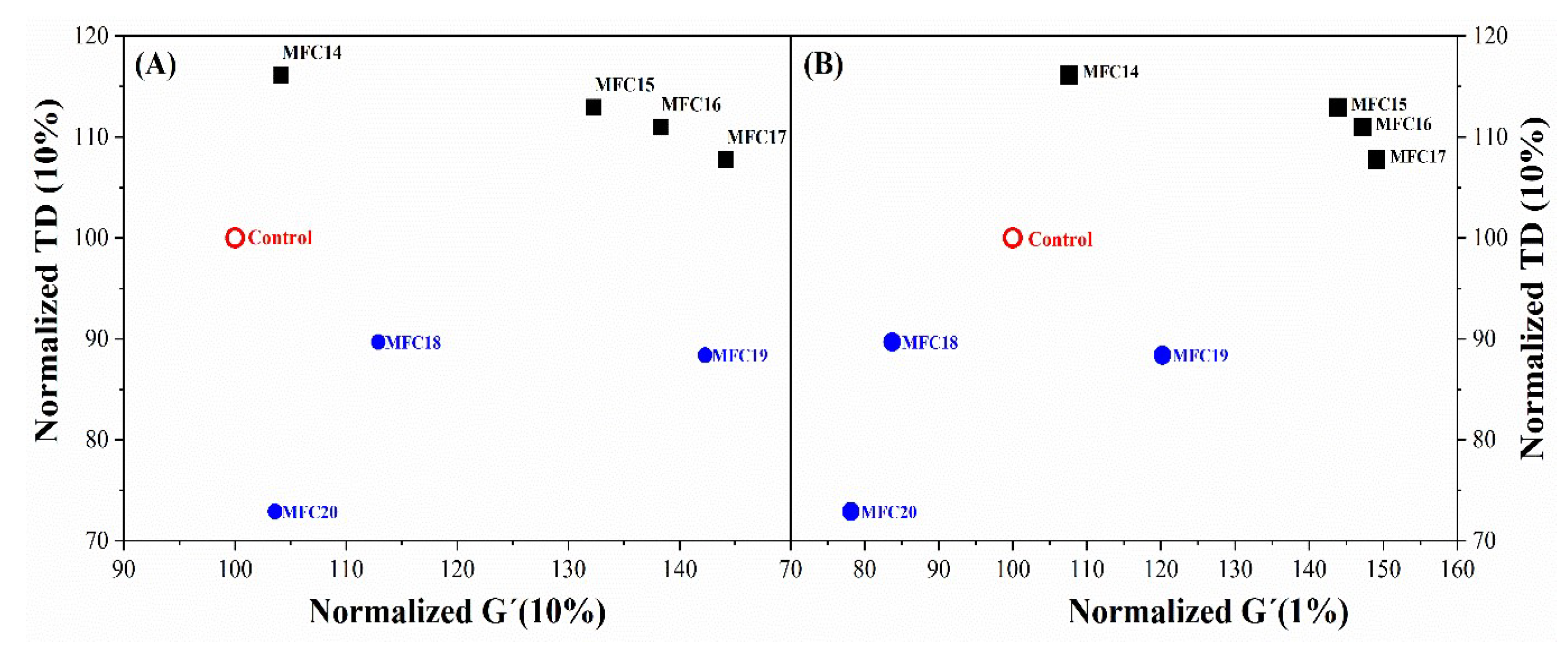
3.5.3. Rubber Process Analysis (RPA) and Dynamic Mechanical Analysis (DMA)
3.5.4. Tensile Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Affandi, S.; Setyawan, H.; Winardi, S.; Purwanto, A.; Balgis, R. A facile method for production of high-purity silica xerogels from bagasse ash. Adv. Powder Technol. 2009, 20, 468–472. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Santos, R.V.; Santos ECA dos Rocha, T.L.A.C.; Domingues Junior, N.S.; Moraes, C.A.M. Replacement of Commercial Silica by Rice Husk Ash in Epoxy Composites: A Comparative Analysis. Mater. Res. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R. (Eds.) Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.S.; Rezende, C.A.; Cranston, E.D. Fundamentals of cellulose lightweight materials: Bio-based assemblies with tailored properties. Green Chem. 2021, 23, 3542–3568. [Google Scholar] [CrossRef]
- Roy, K.; Pongwisuthiruchte, A.; Chandra Debnath, S.; Potiyaraj, P. Application of cellulose as green filler for the development of sustainable rubber technology. Curr. Res. Green Sustain. Chem. 2021, 4, 100140. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Heiskanen, J.P.; Liimatainen, H. UV-absorbing cellulose nanocrystals as functional reinforcing fillers in polymer nanocomposite films. J. Mater. Chem. A 2016, 4, 6368–6375. [Google Scholar] [CrossRef]
- He, M.; Zhou, J.; Zhang, H.; Luo, Z.; Yao, J. Microcrystalline cellulose as reactive reinforcing fillers for epoxidized soybean oil polymer composites. J. Appl. Polym. Sci. 2015, 132, 42488. [Google Scholar] [CrossRef]
- Staropoli, M.; Rogé, V.; Moretto, E.; Didierjean, J.; Michel, M.; Duez, B.; Steiner, P.; Thielen, G.; Lenoble, D.; Thomann, J.S. Hybrid Silica-Based Fillers in Nanocomposites: Influence of Isotropic/Isotropic and Isotropic/Anisotropic Fillers on Mechanical Properties of Styrene-Butadiene (SBR)-Based Rubber. Polymers 2021, 13, 2413. [Google Scholar] [CrossRef]
- Bai, W.; Li, K. Partial replacement of silica with microcrystalline cellulose in rubber composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1597–1605. [Google Scholar] [CrossRef]
- Xu, S.H.; Gu, J.; Luo, Y.F.; Jia, D.M. Effects of partial replacement of silica with surface modified nanocrystalline cellulose on properties of natural rubber nanocomposites. Express Polym. Lett. 2012, 6, 14–25. [Google Scholar] [CrossRef]
- Kazemi, H.; Mighri, F.; Park, K.W.; Frikha, S.; Rodrigue, D. Effect of Cellulose Fiber Surface Treatment to Replace Carbon Black in Natural Rubber Hybrid Composites. Rubber Chem. Technol. 2022, 95, 128–146. [Google Scholar] [CrossRef]
- Lopattananon, N.; Jitkalong, D.; Seadan, M. Hybridized reinforcement of natural rubber with silane-modified short cellulose fibers and silica. J. Appl. Polym. Sci. 2011, 120, 3242–3254. [Google Scholar] [CrossRef]
- Li, K.; Mcgrady, D.; Zhao, X.; Ker, D.; Tekinalp, H.; He, X.; Qu, J.; Aytug, T.; Cakmak, E.; Phipps, J.; et al. Surface-modified and oven-dried microfibrillated cellulose reinforced biocomposites: Cellulose network enabled high performance. Carbohydr. Polym. 2021, 256, 117525. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Berriot, J.; de Gaudemaris, B.; Veyland, A.; Putaux, J.-L.; Molina-Boisseau, S.; Heux, L. Rubber materials from elastomers and nanocellulose powders: Filler dispersion and mechanical reinforcement. Soft Matter 2018, 14, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nanocrystals Using Plant Polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar] [CrossRef]
- Dhuiège, B.; Pecastaings, G.; Sèbe, G. Sustainable Approach for the Direct Functionalization of Cellulose Nanocrystals Dispersed in Water by Transesterification of Vinyl Acetate. ACS Sustain. Chem. Eng. 2019, 7, 187–196. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannia, J.; Pirsa, S.; Zandi, M. Heterogeneous modification of softwoods cellulose nanofibers with oleic acid: Effect of reaction time and oleic acid concentration. Fibers. Polym. 2015, 16, 1715–1722. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass. Biomass Anal Technol. Team Lab Anal Proced. 2004, 1–14. [Google Scholar]
- Liu, M.; Fernando, D.; Daniel, G.; Madsen, B.; Meyer, A.S.; Ale, M.T.; Thygesen, A. Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers. Ind. Crops. Prod. 2015, 69, 29–39. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- DIN 53504; Testing of Rubber—Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test. Deutsches Institut für Normung: Berlin, German, 2009.
- Rostami, J.; Mathew, A.P.; Edlund, U. Zwitterionic Acetylated Cellulose Nanofibrils. Molecules 2019, 24, 3147. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Khan, G.; Uddin, M.; Haque, M.; Islam, M.; Abdullah-Al-Mamun, M.; Gafur, M.A.; Alam, M.S. Characterization of Micro-fibrillated Cellulose Produced from Sawmill Wastage: Crystallinity and Thermal Properties. Am. Chem. Sci. J. 2015, 9, 1–8. [Google Scholar] [CrossRef]
- Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A. The Crystallinity of Nanocellulose: Dispersion-Induced Disordering of the Grain Boundary in Biologically Structured Cellulose. ACS Appl. Nano Mater. 2018, 1, 5774–5785. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Muñiz GIB de Andrade AS de Simon, L.C.; Nisgoski, S. Morphological, Physical and Thermal Characterization of Microfibrillated CellulosE. Rev. Árvore 2018, 42, e420113. [Google Scholar] [CrossRef]
- Wen, X.; Wang, H.; Wei, Y.; Wang, X.; Liu, C. Preparation and characterization of cellulose laurate ester by catalyzed transesterification. Carbohydr. Polym. 2017, 168, 247–254. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Controlled heterogeneous modification of cellulose fibers with fatty acids: Effect of reaction conditions on the extent of esterification and fiber properties. J. Appl. Polym. Sci. 2006, 100, 1093–1102. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Kargarzadeh, H.; Abdullah, I. Effects of Silane Surface Treatment of Cellulose Nanocrystals on the Tensile Properties of Cellulose-Polyvinyl chloride Nanocomposite. Sains Malays. 2015, 44, 801–810. [Google Scholar] [CrossRef]
- Andresen, M.; Johansson, L.-S.; Tanem, B.S.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Liu, M.; Zhang, C.; Qin, S. Pyrolytic characteristics and kinetics of the marine green tide macroalgae, Enteromorpha prolifera. Chinese J. Oceanol. Limnol. 2011, 29, 996–1001. [Google Scholar] [CrossRef]
- Liu, M.; Baum, A.; Odermatt, J.; Berger, J.; Yu, L.; Zeuner, B.; Thygesen, A.; Holck, J.; Meyer, A.S. Oxidation of lignin in hemp fibres by laccase: Effects on mechanical properties of hemp fibres and unidirectional fibre/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 95, 377–387. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.X.; Zhang, M.M.; Liu, M.; Qin, S. Pyrolysis Characteristics and Kinetics of Enteromorpha Clathrata Biomass: A Potential Way of Converting Ecological Crisis “Green Tide” Bioresource to Bioenergy. Adv. Mater. Res. 2010, 113–116, 170–175. [Google Scholar] [CrossRef]
- Müller-Hagedorn, M.; Bockhorn, H.; Krebs, L.; Müller, U. A comparative kinetic study on the pyrolysis of three different wood species. J. Anal. Appl. Pyrolysis 2003, 68–69, 231–249. [Google Scholar] [CrossRef]
- Francisco-Fernández, M.; Tarrío-Saavedra, J.; Naya, S.; López-Beceiro, J.; Artiaga, R. Classification of wood using differential thermogravimetric analysis. J. Therm. Anal. Calorim. 2015, 120, 541–551. [Google Scholar] [CrossRef]
- Huang, F.-Y. Thermal Properties and Thermal Degradation of Cellulose Tri-Stearate (CTs). Polymers 2012, 4, 1012–1024. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Zhong, L.; Sun, S.; Yang, D.; Zhang, X.; Sun, R. A novel transesterification system to rapidly synthesize cellulose aliphatic esters. Cellulose 2014, 21, 581–594. [Google Scholar] [CrossRef]
- Labafzadeh, S.R.; Kavakka, J.S.; Sievänen, K.; Asikkala, J.; Kilpeläinen, I. Reactive dissolution of cellulose and pulp through acylation in pyridine. Cellulose 2012, 19, 1295–1304. [Google Scholar] [CrossRef]
- Raba, D.N.; Chambre, D.R.; Copolovici, D.-M.; Moldovan, C.; Copolovici, L.O. The influence of high-temperature heating on composition and thermo-oxidative stability of the oil extracted from Arabica coffee beans. PLoS ONE 2018, 13, e0200314. [Google Scholar] [CrossRef]
- Faria, E.A.D.; Leles, M.I.G.; Ionashiro, M.; Zuppa, T.D.O.; Antoniosi Filho, N.R. Estudo da estabilidade térmica de óleos e gorduras vegetais por TG/DTG e DTA. Eclética Química 2002, 27, 47–56. [Google Scholar] [CrossRef]
- Klie, B.; Teich, S.; Haberstroh, E.; Giese, U. Newmethod for evaluating rubber mixing Quality by means of alternative representation of the Fingerprint chart. KGK Kautsch. Gummi Kunstst. 2015, 68, 31–38. [Google Scholar]
- Chigondo, F.; Shoko, P.; Nyamunda, B.; Guyo, U.; Moyo, M. Maize stalk as reinforcement in natural rubber composites. Int. J. Sci. Technol. Res. 2013, 2, 263–271. [Google Scholar]
- Ramier, J.; Gauthier, C.; Chazeau, L.; Stelandre, L.; Guy, L. Payne effect in silica-filled styrene–butadiene rubber: Influence of surface treatment. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 286–298. [Google Scholar] [CrossRef]
- Bokobza, L.; Gaulliard, V.; Ladouce, L. Silica Reinforcement of StyreneButadiene Rubbers. Elastomers and Plastics. 2001, 54, 177–180. [Google Scholar]
- Cichomski, E.M.; Dierkes, W.K.; Tolpekina, T.V.; Schultz, S. Influence of physical and chemical polymer-filler bonds on tire wet-traction performance indicators for passenger car tire tread materials. KGK Kautsch. Gummi Kunstst. 2014, 67, 50–57. [Google Scholar]
- Kawahara, S. Controlling Performance of Filled Rubbers. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 453–460. [Google Scholar] [CrossRef]
- Kuta, A.; Hrdlicka, Z.; Voldanova, J.; Brejcha, J.; Pokorny, J.; Plitz, J. Dynamic Mechanical Properties of Rubbers with Standard Oils and Oils with Low Content of Polycyclic Aromatic Hydrocarbons. Test Meas. 2010, 63, 120–122. [Google Scholar]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted pre-treatment of hemp bast fibres for optimal performance in biocomposite materials: A review. Ind. Crops. Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Liu, M.; Meyer, A.S.; Fernando, D.; Silva, D.A.S.; Daniel, G.; Thygesen, A. Effect of pectin and hemicellulose removal from hemp fibres on the mechanical properties of unidirectional hemp/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 724–735. [Google Scholar] [CrossRef]
- Abraham, E.; Thomas, M.S.; John, C.; Pothen, L.A.; Shoseyov, O.; Thomas, S. Green nanocomposites of natural rubber/nanocellulose: Membrane transport, rheological and thermal degradation characterisations. Ind. Crops. Prod. 2013, 51, 415–424. [Google Scholar] [CrossRef]
- Jarnthong, M.; Wang, F.; Wei, X.Y.; Wang, R.; Li, J.H. Preparation and Properties of Biocomposite Based on Natural Rubber and Bagasse Nanocellulose. MATEC Web Conf. 2015, 26, 01005. [Google Scholar] [CrossRef]
- Kato, H.; Nakatsubo, F.; Abe, K.; Yano, H. Crosslinking via sulfur vulcanization of natural rubber and cellulose nanofibers incorporating unsaturated fatty acids. RSC Adv. 2015, 5, 29814–29819. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; John, M.; Narine, S.S.; Thomas, S.; Anandjiwala, R. Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Cellulose 2013, 20, 417–427. [Google Scholar] [CrossRef]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef] [PubMed]





| Stage | Composition | Amount (phr) |
|---|---|---|
| NP1 | Polystyrene butadiene | 80 |
| Polybutadiene | 20 | |
| TDAE oil | 3.75–25 | |
| Zinc oxide | 0.5 | |
| Stearic acid | 3 | |
| Silica | 50–80 | |
| MFCs | 10 | |
| NP2 | 6PPD | 2.5 |
| TESPD silane | 8 | |
| Silica | 15 | |
| PR | Zinc oxide | 2 |
| Sulfur | 1.1 | |
| MBT | 0.3 | |
| DPG | 3.2 | |
| CBS | 2.3 |
| Type of MFC | Degree of Substitution (DS) | Amount (phr) | |||
|---|---|---|---|---|---|
| Silica | MFCs | TDAE | |||
| Control | / | / | 80 | 0 | 25 |
| MFC14 | LDS | 0.24 | 70 | 10 | 21 |
| MFC15 | MDS1 | 0.49 | 70 | 10 | 17 |
| MFC16 | MDS2 | 0.67 | 70 | 10 | 14 |
| MFC17 | HDS | 0.91 | 70 | 10 | 10 |
| MFC18 | HDS | 0.91 | 60 | 10 | 10 |
| MFC19 | HDS | 0.91 | 60 | 10 | 3.75 |
| MFC20 | HDS | 0.91 | 50 | 10 | 10 |
| Amount (%) | |||||
|---|---|---|---|---|---|
| Arabinan | Galactan | Glucan | Xylan | Mannan | Klason Lignin |
| n.d. | n.d. | 94.5 (2.8) | 2.1 (0.2) | 1.2 (0.1) | 0.3 (0.2) |
| Sample | NP1 | NP2 | PR | Total Work W (KJ) | |||
|---|---|---|---|---|---|---|---|
| Tm (°C) | W (KJ) | Tm (°C) | W (KJ) | Tm (°C) | W (KJ) | ||
| Control | 147.9 | 133.8 | 138.1 | 117.5 | 77.6 | 14.6 | 265.9 |
| MFC14 | 141.1 | 128 | 139.6 | 112.9 | 79 | 16.3 | 257.2 |
| MFC15 | 141.4 | 142.2 | 144.2 | 126.6 | 80.8 | 17.7 | 286.5 |
| MFC16 | 141.5 | 143.9 | 144.4 | 126.7 | 80.5 | 19.3 | 289.9 |
| MFC17 | 145.7 | 152.2 | 146.8 | 138.6 | 81.7 | 16.8 | 307.6 |
| MFC18 | 140.3 | 136.5 | 143.4 | 124 | 77.4 | 15.9 | 276.4 |
| MFC19 | 143 | 152.5 | 147.7 | 141.7 | 83.6 | 19.8 | 314 |
| MFC20 | 135.3 | 117.8 | 139.1 | 110.5 | 78.1 | 16.4 | 244.7 |
| Sample | Min Torque (Tmin) (Nm) | Max Torque (Tmax) (Nm) | 25% Cure T25 (min) | Optimum Cure T90 (min) |
|---|---|---|---|---|
| Control | 2.9 | 23.5 | 4.6 | 20.5 |
| MFC14 | 2.9 | 22.3 | 6.1 | 22.5 |
| MFC15 | 3.7 | 27.3 | 5.5 | 22.3 |
| MFC16 | 4.0 | 28.2 | 5.1 | 22.5 |
| MFC17 | 4.2 | 29.0 | 6.0 | 24.3 |
| MFC18 | 3.3 | 25.5 | 5.3 | 23.0 |
| MFC19 | 4.5 | 29.6 | 4.7 | 22.0 |
| MFC20 | 2.9 | 23.5 | 4.6 | 20.5 |
| Sample | Tg (°C) | TD (0 °C) | TD (60 °C) | E′ (MPa, 30 °C) |
|---|---|---|---|---|
| Control | −21.8 | 0.306 | 0.081 | 12.6 |
| MFC14 | −24.0 | 0.258 | 0.095 | 20.5 |
| MFC15 | −24.2 | 0.228 | 0.107 | 36.2 |
| MFC16 | −24.8 | 0.205 | 0.111 | 56.3 |
| MFC17 | −23.6 | 0.241 | 0.097 | 29.7 |
| MFC18 | −23.1 | 0.249 | 0.084 | 21.5 |
| MFC19 | −23.1 | 0.236 | 0.087 | 28.8 |
| MFC20 | −23.3 | 0.237 | 0.080 | 17.6 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Strain (%) |
|---|---|---|---|
| Control | 13.1 (0.5) | 3.8 (0.0) | 277.1 (6.9) |
| MFC14 | 14.2 (0.4) | 3.4 (0.1) | 343.2 (5.7) |
| MFC15 | 13.4 (1.2) | 4.7 (0.3) | 245.2 (13.3) |
| MFC16 | 15.0 (0.7) | 4.7 (0.1) | 243.9 (10.0) |
| MFC17 | 14.8 (1.2) | 5.1 (0.1) | 251.8 (24.4) |
| MFC18 | 12.4 (0.6) | 4.2 (0.1) | 233.3 (11.0) |
| MFC19 | 13.8 (0.8) | 5.8 (0.1) | 198.5 (12.0) |
| MFC20 | 11.5 (0.6) | 3.7 (0.1) | 238.4 (15.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Imiete, I.E.; Staropoli, M.; Steiner, P.; Duez, B.; Lenoble, D.; Scolan, E.; Thomann, J.-S. Hydrophobized MFC as Reinforcing Additive in Industrial Silica/SBR Tire Tread Compound. Polymers 2023, 15, 3937. https://doi.org/10.3390/polym15193937
Liu M, Imiete IE, Staropoli M, Steiner P, Duez B, Lenoble D, Scolan E, Thomann J-S. Hydrophobized MFC as Reinforcing Additive in Industrial Silica/SBR Tire Tread Compound. Polymers. 2023; 15(19):3937. https://doi.org/10.3390/polym15193937
Chicago/Turabian StyleLiu, Ming, Iikpoemugh Elo Imiete, Mariapaola Staropoli, Pascal Steiner, Benoît Duez, Damien Lenoble, Emmanuel Scolan, and Jean-Sébastien Thomann. 2023. "Hydrophobized MFC as Reinforcing Additive in Industrial Silica/SBR Tire Tread Compound" Polymers 15, no. 19: 3937. https://doi.org/10.3390/polym15193937
APA StyleLiu, M., Imiete, I. E., Staropoli, M., Steiner, P., Duez, B., Lenoble, D., Scolan, E., & Thomann, J.-S. (2023). Hydrophobized MFC as Reinforcing Additive in Industrial Silica/SBR Tire Tread Compound. Polymers, 15(19), 3937. https://doi.org/10.3390/polym15193937






