Ce-radical Scavenger-Based Perfluorosulfonic Acid Aquivion® Membrane for Pressurised PEM Electrolysers
Abstract
:1. Introduction
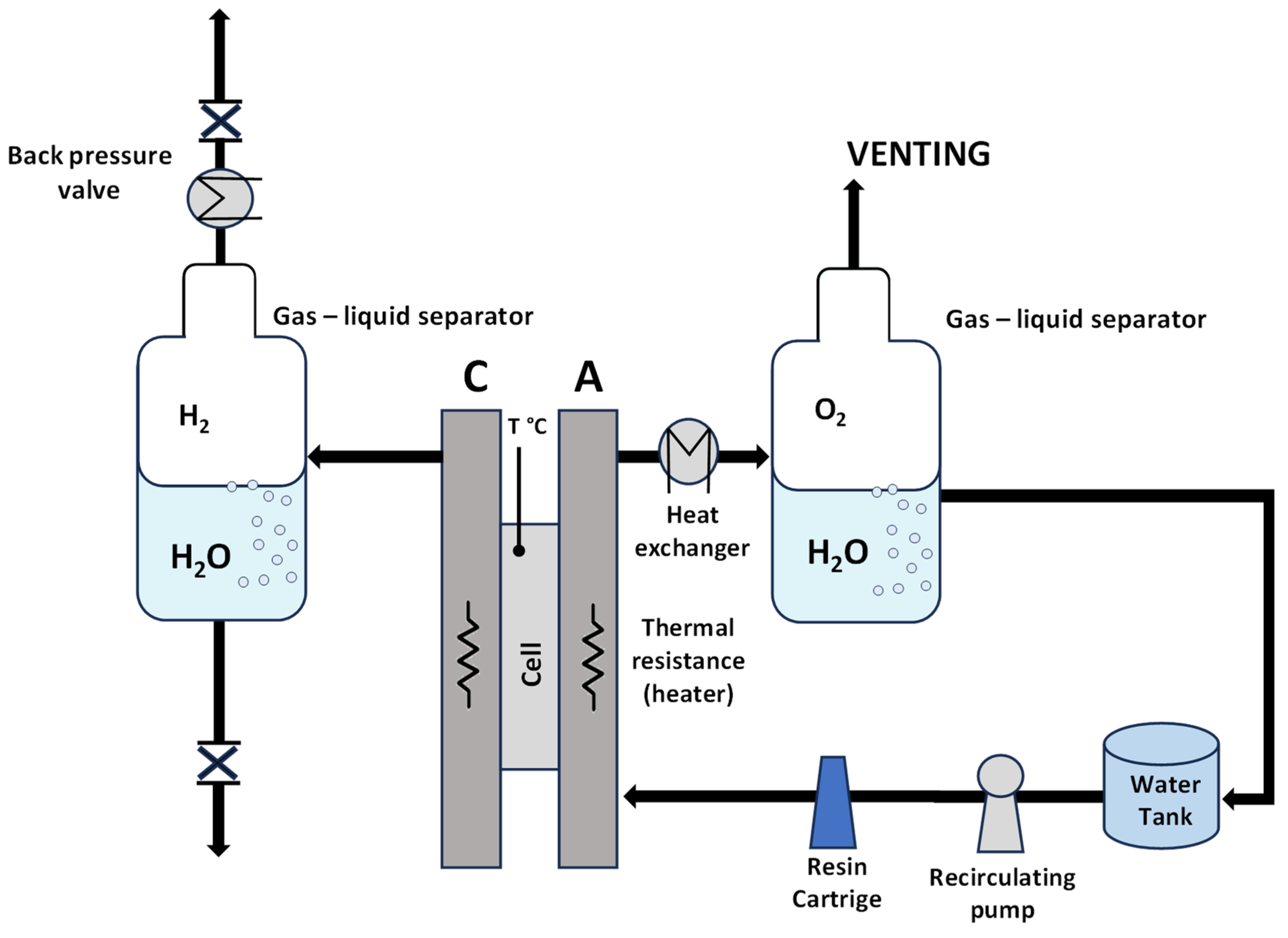
2. Experimental Section
2.1. Membrane-Electrode Assemblies (MEAs) Preparation and Characterization
2.2. Electrochemical Investigations
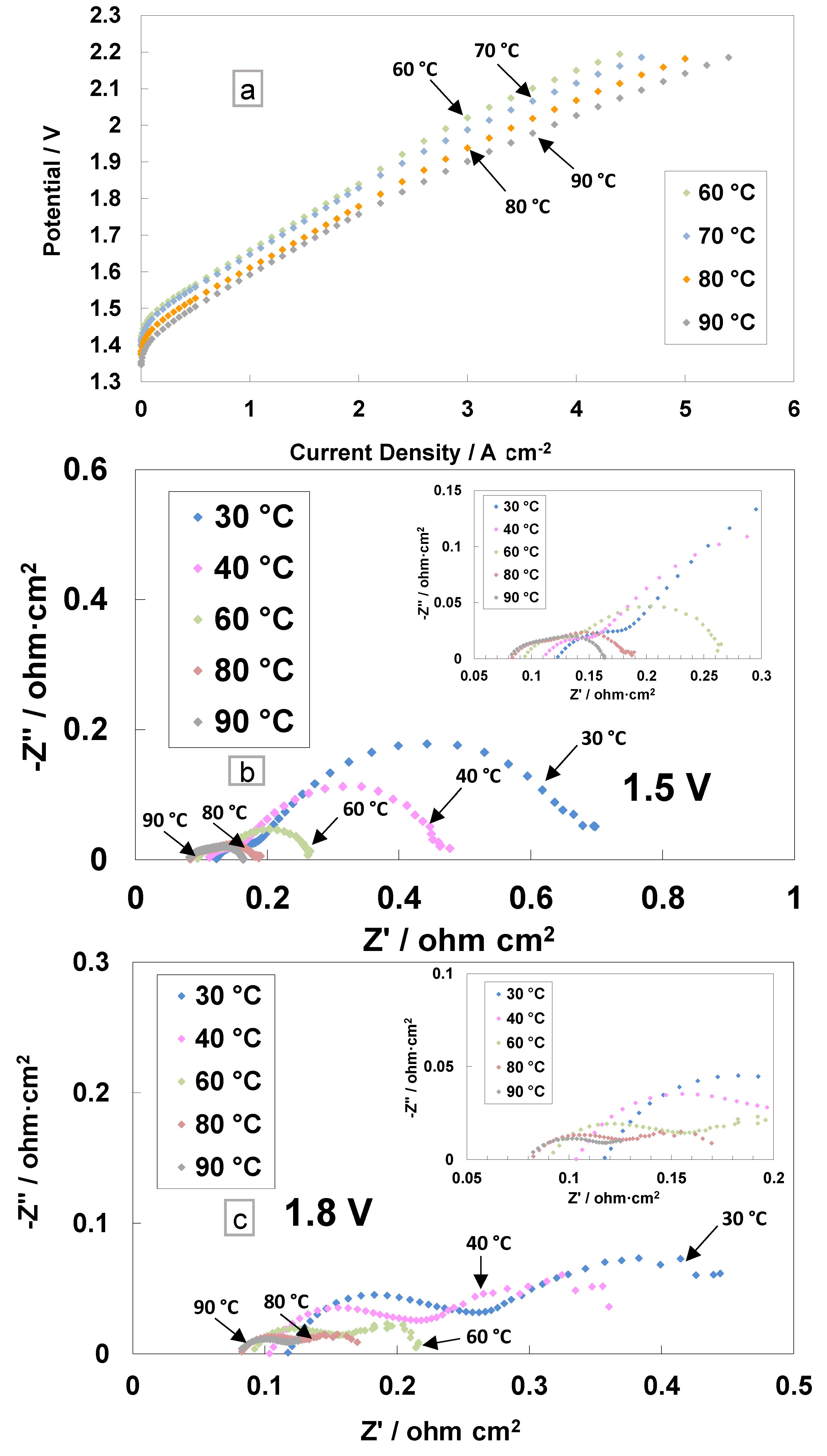
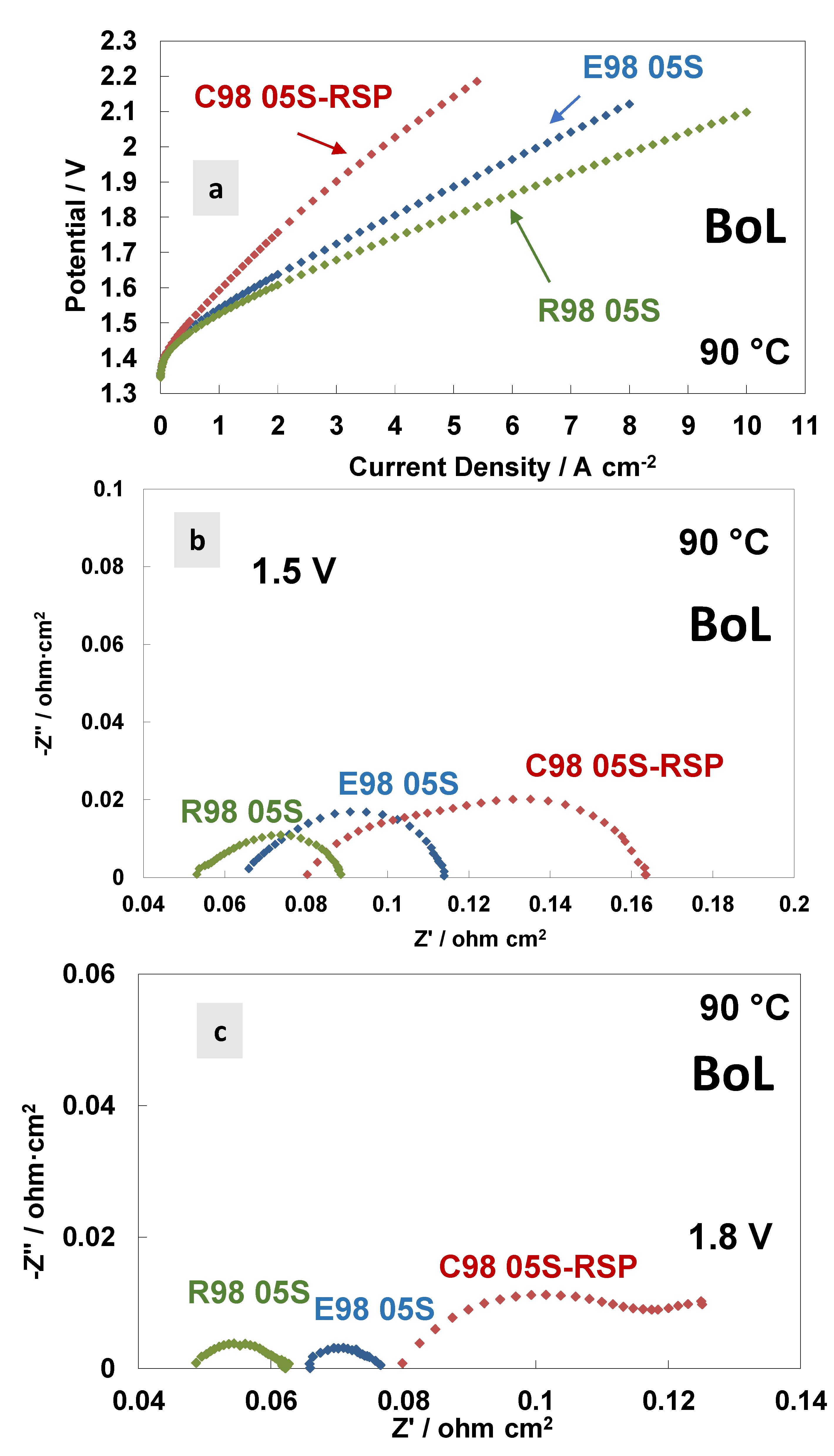
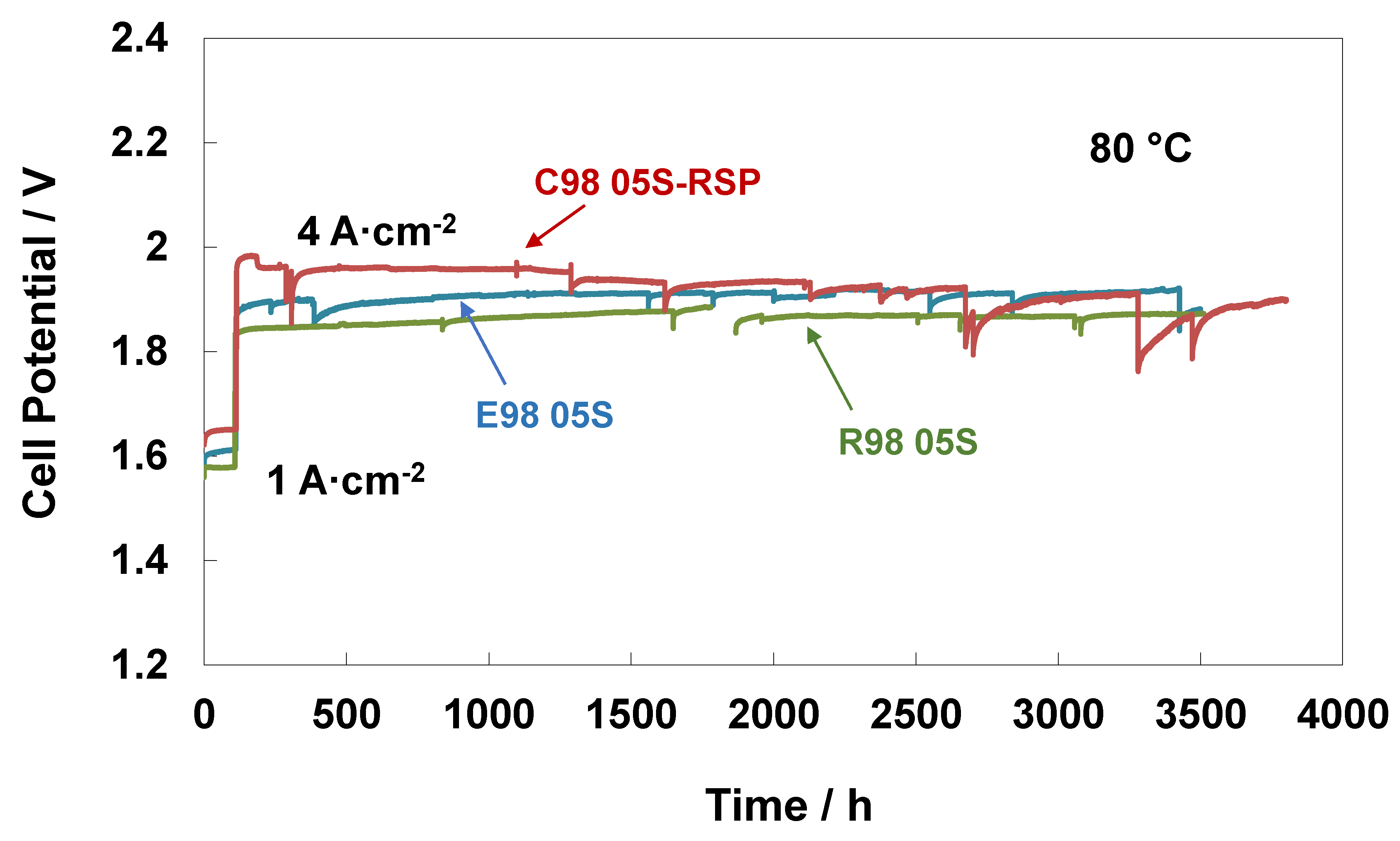
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Yang, Y.; Javanroodi, K.; Nik, V.M. Climate Change and Renewable Energy Generation in Europe—Long-Term Impact Assessment on Solar and Wind Energy Using High-Resolution Future Climate Data and Considering Climate Uncertainties. Energies 2022, 15, 302. [Google Scholar] [CrossRef]
- Pani, A.; Shirkole, S.S.; Mujumdar, A.S. Importance of renewable energy in the fight against global climate change. Dry. Technol. 2022, 40, 2581–2582. [Google Scholar] [CrossRef]
- Ge, C.; Sheng, M.; Yuan, Y.; Shi, F.; Yang, Y.; Zhao, S.; Wang, J.; Wang, Z. Recent advances of the interfacial polymerization process in gas separation membranes fabrication. J. Membr. Sci. 2023, 683, 121854. [Google Scholar] [CrossRef]
- Calnan, S.; Bagacki, R.; Bao, F.; Dorbandt, I.; Kemppainen, E.; Schary, C.; Schlatmann, R.; Leonardi, M.; Lombardo, S.A.; Milazzo, R.G.; et al. Development of Various Photovoltaic-Driven Water Electrolysis Technologies for Green Solar Hydrogen Generation. Sol. RRL 2022, 6, 2100479. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. Performance evaluation of PV panels/wind turbines hybrid system for green hydrogen generation and storage: Energy, exergy, economic, and enviroeconomic. Energy Convers. Manag. 2022, 267, 115870. [Google Scholar] [CrossRef]
- Ng, K.H.; Lai, S.Y.; Cheng, C.K.; Cheng, Y.W.; Chong, C.C. Photocatalytic water splitting for solving energy crisis: Myth, Fact or Busted? Chem. Eng. J. 2021, 417, 128847. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Guo, Y.; Li, G.; Zhou, J.; Liu, Y. Comparison between hydrogen production by alkaline water electrolysis and hydrogen production by PEM electrolysis. IOP Conf. Ser. Earth Environ. Sci. 2019, 371, 042022. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Immerz, C.; Schweins, M.; Trinke, P.; Bensmann, B.; Paidar, M.; Bystroň, T.; Bouzek, K.; Hanke-Rauschenbach, R. Experimental characterization of inhomogeneity in current density and temperature distribution along a single-channel PEM water electrolysis cell. Electrochim. Acta 2018, 260, 582–588. [Google Scholar] [CrossRef]
- Chisholm, G.; Cronin, L.; Symes, M.D. Decoupled electrolysis using a silicotungstic acid electron-coupled-proton buffer in a proton exchange membrane cell. Electrochim. Acta 2020, 331, 135255. [Google Scholar] [CrossRef]
- Frensch, S.H.; Olesen, A.C.; Araya, S.S.; Kær, S.K. Model-supported characterization of a PEM water electrolysis cell for the effect of compression. Electrochim. Acta 2018, 263, 228–236. [Google Scholar] [CrossRef]
- Olesen, A.C.; Frensch, S.H.; Kær, S.K. Towards uniformly distributed heat, mass and charge: A flow field design study for high pressure and high current density operation of PEM electrolysis cells. Electrochim. Acta 2019, 293, 476–495. [Google Scholar] [CrossRef]
- Rost, U.; Wirkert, F.J.; Roth, J.; Brodmann, M.; Stiber, S.; Gago, A.S.; Friedrich, K.A. A novel advanced test system for polymer electrolyte membrane water electrolysis based on hydraulic cell compression. Fuel Cells 2022, 22, 284–289. [Google Scholar] [CrossRef]
- Scheepers, F.; Stähler, M.; Stähler, A.; Rauls, E.; Müller, M.; Carmo, M.; Lehnert, W. Temperature optimization for improving polymer electrolyte membrane-water electrolysis system efficiency. Appl. Energy 2021, 283, 116270. [Google Scholar] [CrossRef]
- Siracusano, S.; Pantò, F.; Tonella, S.; Oldani, C.; Aricò, A.S. Reinforced short-side-chain Aquivion® membrane for proton exchange membrane water electrolysis. Int. J. Hydrog. Energy 2022, 47, 15557–15570. [Google Scholar] [CrossRef]
- Martin, A.; Trinke, P.; Stähler, M.; Stähler, A.; Scheepers, F.; Bensmann, B.; Carmo, M.; Lehnert, W.; Hanke-Rauschenbach, R. The Effect of Cell Compression and Cathode Pressure on Hydrogen Crossover in PEM Water Electrolysis. J. Electrochem. Soc. 2022, 169, 014502. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. Hydrogen crossover in proton exchange membrane electrolysers: The effect of current density, pressure, temperature, and compression. Electrochim. Acta 2021, 377, 138085. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Husaini, T.; Goh, J.; Sulong, A.B. High-pressure PEM water electrolyser: A review on challenges and mitigation strategies towards green and low-cost hydrogen production. Energy Convers. Manag. 2022, 268, 115985. [Google Scholar] [CrossRef]
- Hubert, M.A.; King, L.A.; Jaramillo, T.F. Evaluating the Case for Reduced Precious Metal Catalysts in Proton Exchange Membrane Electrolyzers. ACS Energy Lett. 2022, 7, 17–23. [Google Scholar] [CrossRef]
- Rojas, N.; Sánchez-Molina, M.; Sevilla, G.; Amores, E.; Almandoz, E.; Esparza, J.; Cruz Vivas, M.R.; Colominas, C. Coated stainless steels evaluation for bipolar plates in PEM water electrolysis conditions. Int. J. Hydrog. Energy 2021, 46, 25929–25943. [Google Scholar] [CrossRef]
- Lim, T.; Kim, S.-K. Non-precious hydrogen evolution reaction catalysts: Stepping forward to practical polymer electrolyte membrane-based zero-gap water electrolyzers. Chem. Eng. J. 2022, 433, 133681. [Google Scholar] [CrossRef]
- Garbe, S.; Futter, J.; Schmidt, T.J.; Gubler, L. Insight into elevated temperature and thin membrane application for high efficiency in polymer electrolyte water electrolysis. Electrochim. Acta 2021, 377, 138046. [Google Scholar] [CrossRef]
- Ouimet, R.J.; Yu, H.; Mirshekari, G.; Zeng, Z.; Bonville, L.J.; Bliznakov, S.; Niedzwiecki, A.; Mani, P.; Capuano, C.; Ayers, K.E.; et al. Development of Recombination Layers to Reduce Gas Crossover for Proton Exchange Membrane Water Electrolyzers By Reactive Spray Deposition Technology. ECS Meet. Abstr. 2020, MA2020-02, 2469. [Google Scholar] [CrossRef]
- Wu, X.; Scott, K.; Puthiyapura, V. Polymer electrolyte membrane water electrolyser with Aquivion® short side chain perfluorosulfonic acid ionomer binder in catalyst layers. Int. J. Hydrog. Energy 2012, 37, 13243–13248. [Google Scholar] [CrossRef]
- Shirvanian, P.; van Berkel, F. Novel components in Proton Exchange Membrane (PEM) Water Electrolyzers (PEMWE): Status, challenges and future needs. A mini review. Electrochem. Commun. 2020, 114, 106704. [Google Scholar] [CrossRef]
- Ghielmi, A.; Vaccarono, P.; Troglia, C.; Arcella, V. Proton exchange membranes based on the short-side-chain perfluorinated ionomer. J. Power Sources 2005, 145, 108–115. [Google Scholar] [CrossRef]
- Briguglio, N.; Pantò, F.; Siracusano, S.; Aricò, A.S. Enhanced performance of a PtCo recombination catalyst for reducing the H2 concentration in the O2 stream of a PEM electrolysis cell in the presence of a thin membrane and a high differential pressure. Electrochim. Acta 2020, 344, 136153. [Google Scholar] [CrossRef]
- Giancola, S.; Zatoń, M.; Reyes-Carmona, Á.; Dupont, M.; Donnadio, A.; Cavaliere, S.; Rozière, J.; Jones, D.J. Composite short side chain PFSA membranes for PEM water electrolysis. J. Membr. Sci. 2019, 570–571, 69–76. [Google Scholar] [CrossRef]
- Shin, S.-H.; Nur, P.J.; Kodir, A.; Kwak, D.-H.; Lee, H.; Shin, D.; Bae, B. Improving the Mechanical Durability of Short-Side-Chain Perfluorinated Polymer Electrolyte Membranes by Annealing and Physical Reinforcement. ACS Omega 2019, 4, 19153–19163. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.-J.; Kim, K.-Y.; Choi, M.-J.; Yang, E.; Kim, I.S.; Ren, X.; Lee, M. Sulfonated polyether ether ketone (SPEEK)-based composite proton exchange membrane reinforced with nanofibers for microbial electrolysis cells. Chem. Eng. J. 2014, 254, 393–398. [Google Scholar] [CrossRef]
- Lei, J.; Liu, X.; Chen, X.; Guan, P.; Feng, W.; Zhang, J.; Luo, H.; Liu, F.; Zhang, Y. Multi-length-scale heterogeneous structured ion exchange membranes for cost-effective electrolysis and hydrogen production. Chem. Eng. J. 2022, 431, 133994. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Pintauro, P.N. Preparation of nanofiber composite proton-exchange membranes from dual fiber electrospun mats. J. Membr. Sci. 2013, 442, 187–195. [Google Scholar] [CrossRef]
- Lee, P.-C.; Han, T.-H.; Kim, D.O.; Lee, J.-H.; Kang, S.-J.; Chung, C.-H.; Lee, Y.; Cho, S.M.; Choi, H.-G.; Kim, T.; et al. In situ formation of platinum nanoparticles in Nafion recast film for catalyst-incorporated ion-exchange membrane in fuel cell applications. J. Membr. Sci. 2008, 322, 441–445. [Google Scholar] [CrossRef]
- Donnadio, A.; D’Amato, R.; Marmottini, F.; Panzetta, G.; Pica, M.; Battocchio, C.; Capitani, D.; Ziarelli, F.; Casciola, M. On the evolution of proton conductivity of Aquivion membranes loaded with CeO2 based nanofillers: Effect of temperature and relative humidity. J. Membr. Sci. 2019, 574, 17–23. [Google Scholar] [CrossRef]
- Weissbach, T.; Peckham, T.J.; Holdcroft, S. CeO2, ZrO2 and YSZ as mitigating additives against degradation of proton exchange membranes by free radicals. J. Membr. Sci. 2016, 498, 94–104. [Google Scholar] [CrossRef]
- Radice, S.; Oldani, C.; Merlo, L.; Rocchia, M. Aquivion® PerfluoroSulfonic Acid ionomer membranes: A micro-Raman spectroscopic study of ageing. Polym. Degrad. Stab. 2013, 98, 1138–1143. [Google Scholar] [CrossRef]
- Danilczuk, M.; Schlick, S.; Coms, F.D. Cerium(III) as a Stabilizer of Perfluorinated Membranes Used in Fuel Cells: In Situ Detection of Early Events in the ESR Resonator. Macromolecules 2009, 42, 8943–8949. [Google Scholar] [CrossRef]
- Prabhakaran, V.; Arges, C.G.; Ramani, V. Investigation of polymer electrolyte membrane chemical degradation and degradation mitigation using in situ fluorescence spectroscopy. Proc. Natl. Acad. Sci. USA 2012, 109, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Pei, S.; Zhang, W.; Han, Z.; Liu, G.; Xu, X.; Ma, J.; Zhang, Y.; Liu, F.; Zhang, Y.; et al. Chemical stability of proton exchange membranes synergistically promoted by organic antioxidant and inorganic radical scavengers. J. Membr. Sci. 2022, 655, 120594. [Google Scholar] [CrossRef]
- Gubler, L.; Koppenol, W.H. Kinetic Simulation of the Chemical Stabilization Mechanism in Fuel Cell Membranes Using Cerium and Manganese Redox Couples. J. Electrochem. Soc. 2011, 159, B211. [Google Scholar] [CrossRef]
- Siracusano, S.; Oldani, C.; Navarra, M.A.; Tonella, S.; Mazzapioda, L.; Briguglio, N.; Aricò, A.S. Chemically stabilised extruded and recast short side chain Aquivion® proton exchange membranes for high current density operation in water electrolysis. J. Membr. Sci. 2019, 578, 136–148. [Google Scholar] [CrossRef]
- Grot, W. Fluorinated Ionomers, 1st ed.; William Andrew: Norwich, NY, USA, 2007. [Google Scholar]
- D’Urso, C.; Oldani, C.; Baglio, V.; Merlo, L.; Aricò, A.S. Towards fuel cell membranes with improved lifetime: Aquivion® Perfluorosulfonic Acid membranes containing immobilized radical scavengers. J. Power Sources 2014, 272, 753–758. [Google Scholar] [CrossRef]
- Pantò, F.; Siracusano, S.; Briguglio, N.; Aricò, A.S. Durability of a recombination catalyst-based membrane-electrode assembly for electrolysis operation at high current density. Appl. Energy 2020, 279, 115809. [Google Scholar] [CrossRef]
- Siracusano, S.; Van Dijk, N.; Payne-Johnson, E.; Baglio, V.; Aricò, A.S. Nanosized IrOx and IrRuOx electrocatalysts for the O2 evolution reaction in PEM water electrolysers. Appl. Catal. B Environ. 2015, 164, 488–495. [Google Scholar] [CrossRef]
- Siracusano, S.; Stassi, A.; Baglio, V.; Aricò, A.S.; Capitanio, F.; Tavares, A.C. Investigation of carbon-supported Pt and PtCo catalysts for oxygen reduction in direct methanol fuel cells. Electrochim. Acta 2009, 54, 4844–4850. [Google Scholar] [CrossRef]
- Skulimowska, A.; Dupont, M.; Zaton, M.; Sunde, S.; Merlo, L.; Jones, D.J.; Rozière, J. Proton exchange membrane water electrolysis with short-side-chain Aquivion® membrane and IrO2 anode catalyst. Int. J. Hydrog. Energy 2014, 39, 6307–6316. [Google Scholar] [CrossRef]
- Barsoukov, E.; Ross Macdonald, J. (Eds.) Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Aricò, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer electrolyte membrane water electrolysis: Status of technologies and potential applications in combination with renewable power sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Proost, J. State-of-the art CAPEX data for water electrolysers, and their impact on renewable hydrogen price settings. Int. J. Hydrog. Energy 2019, 44, 4406–4413. [Google Scholar] [CrossRef]
- Briguglio, N.; Siracusano, S.; Bonura, G.; Sebastián, D.; Aricò, A.S. Flammability reduction in a pressurised water electrolyser based on a thin polymer electrolyte membrane through a Pt-alloy catalytic approach. Appl. Catal. B Environ. 2019, 246, 254–265. [Google Scholar] [CrossRef]
- Cheikhravat, H.; Chaumeix, N.; Bentaib, A.; Paillard, C.E. Flammability Limits of Hydrogen-Air Mixtures. Nucl. Technol. 2012, 178, 5–16. [Google Scholar] [CrossRef]

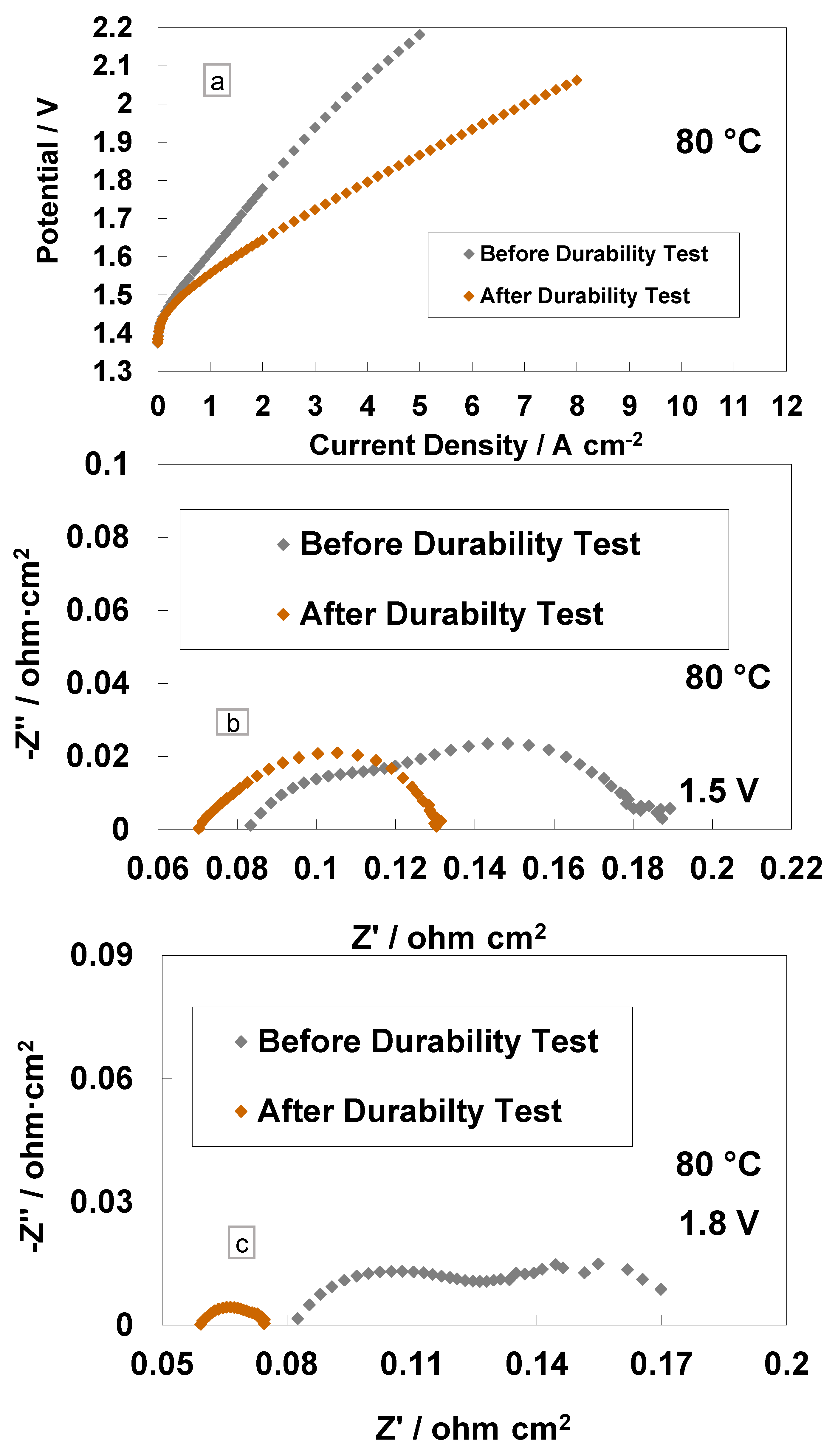
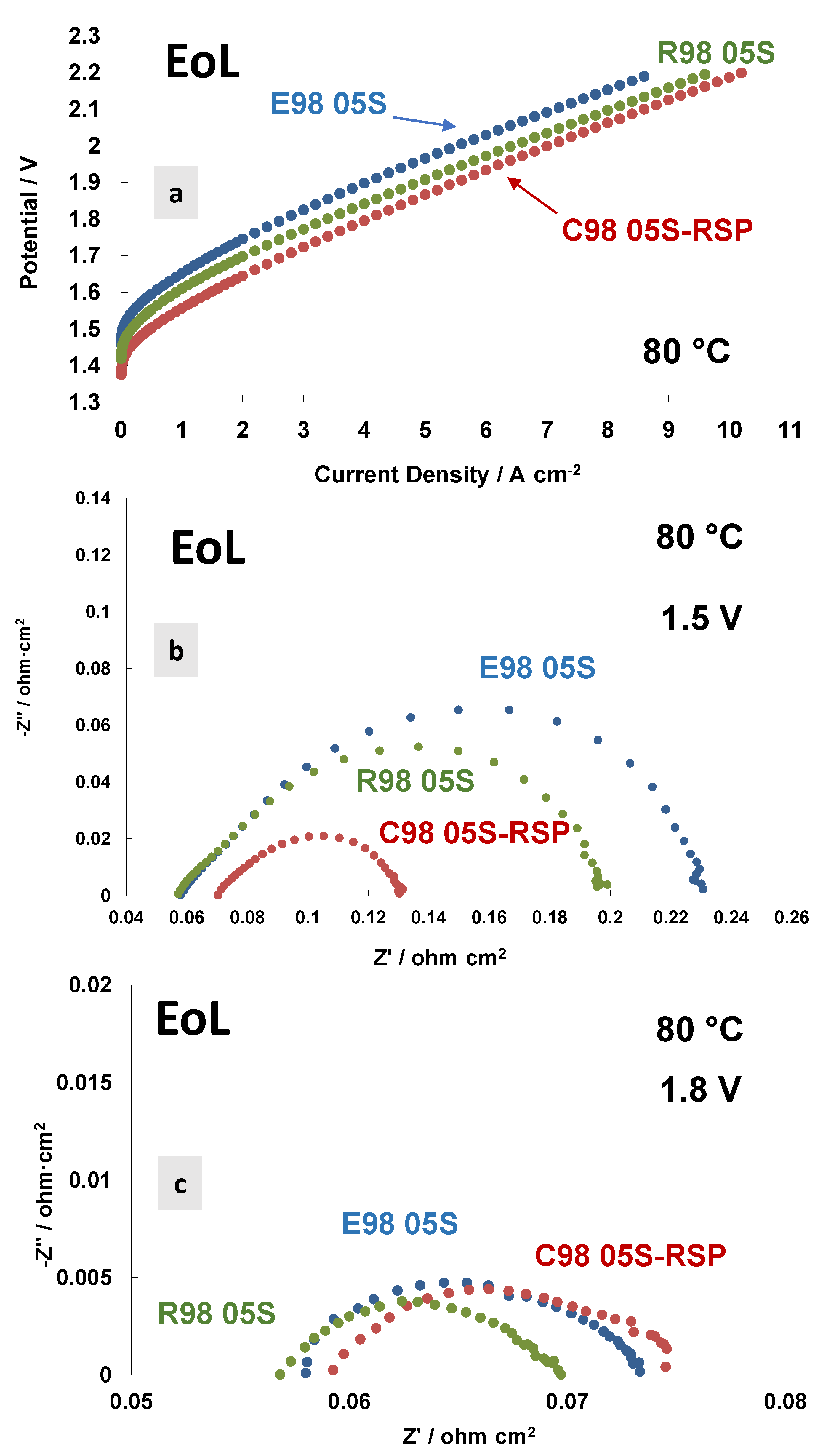
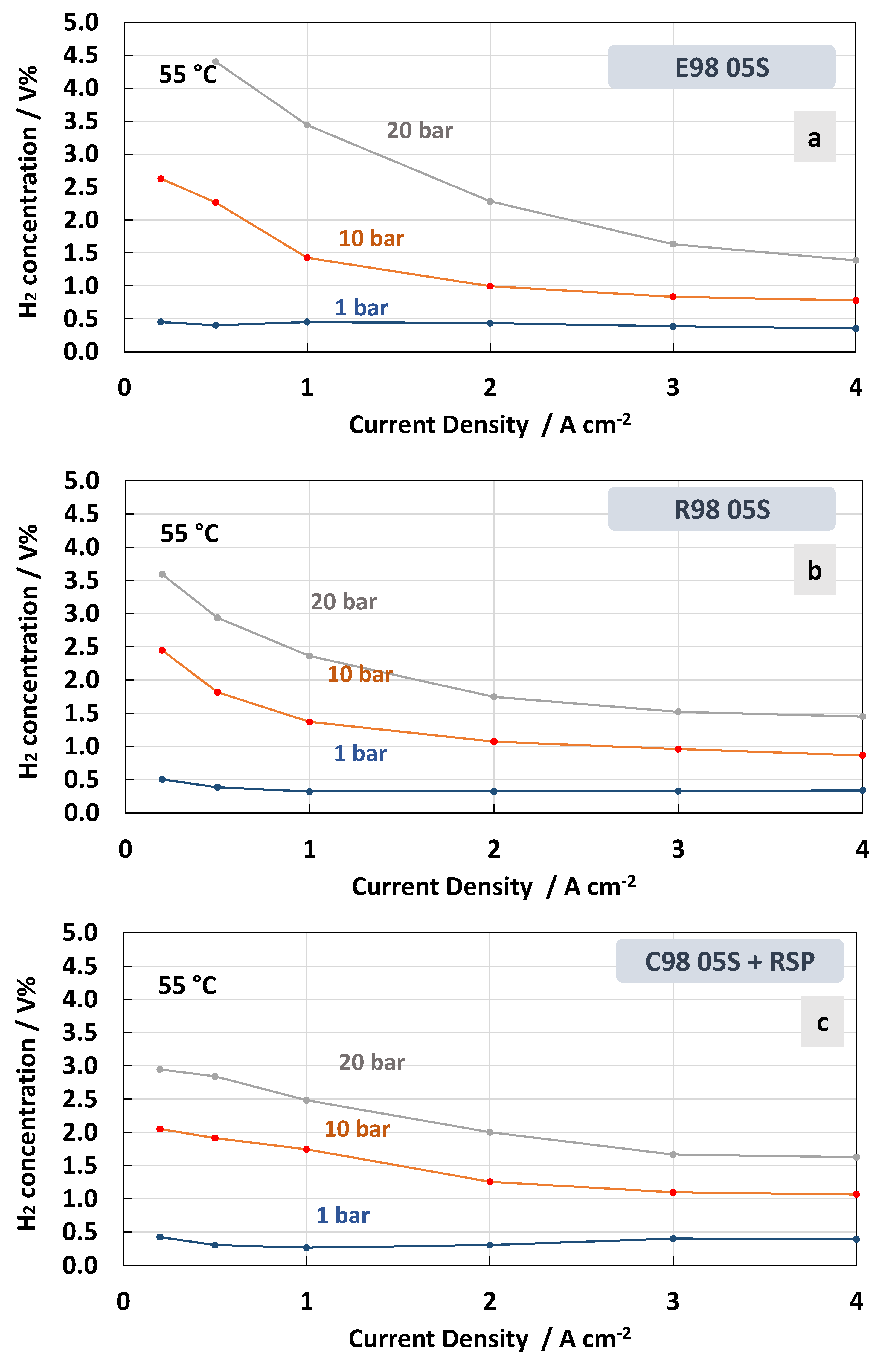
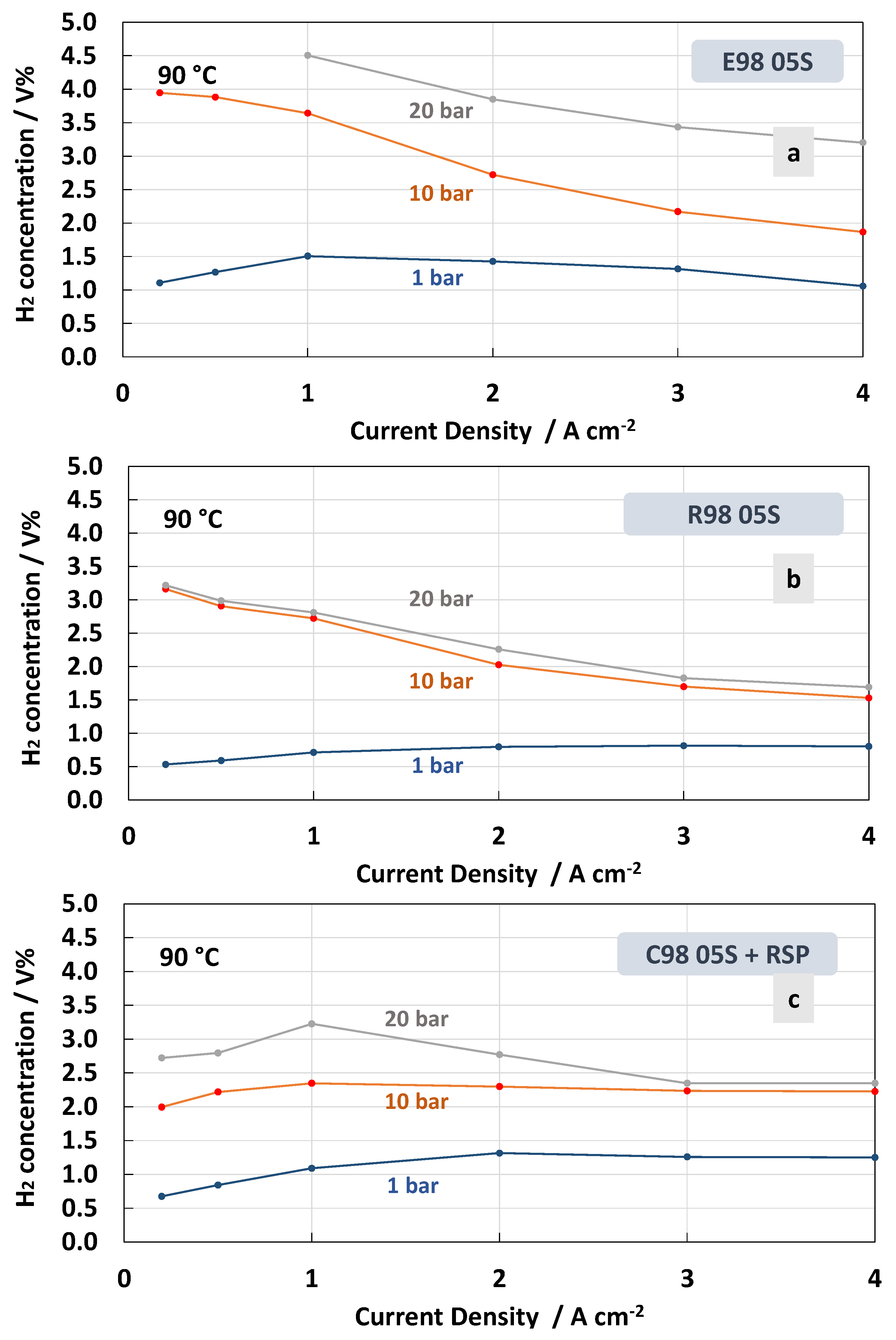
| Membrane | Equivalent Weight (g/mol) | Thickness (µm) | Filler | Glass Transition Temperature Tg/°C | Conductivity at 80 °C and 100% RH (mS/cm) |
|---|---|---|---|---|---|
| E 98 05S | 980 | 50 | none | 140 | 140.4 |
| C98 05S-RSP | 980 | 50 | Ce-scavenger | 140 | 131.3 |
| R98 05S | 980 | 50 | ePTFE fibres | 140 | 131.6 |
| MEA | Anode | Membrane | Cathode | Ref. | ||
|---|---|---|---|---|---|---|
| IrRuOx Catalyst Loading (mgIr+Ru/cm2) | Aquivion® D98-06AS Ionomer (wt.%) | Aquivion® | Pt/C Catalyst Loading (mgPt/cm2) | Aquivion® D98-06AS Ionomer (wt.%) | ||
| Cast-RSP | 0.37 | 15 | C98 05S-RSP | 0.1 | 28 | This work |
| Extruded | 0.37 | 15 | E98 05S | 0.1 | 28 | [18] |
| Reinforced | 0.37 | 15 | R98 05S | 0.1 | 28 | [18] |
| 1.5 V@90 °C | Rs/mOhm cm2 | Rp/mOhm cm2 | Rt/mOhm cm2 | j/A cm−2 |
|---|---|---|---|---|
| R98 05S | 52 | 35 | 87 | 0.75 |
| E98 05S | 66 | 47 | 113 | 0.65 |
| C98 05S-RSP | 80 | 83 | 163 | 0.47 |
| 1.8 V@90 °C | Rs/mOhm cm2 | Rp/mOhm cm2 | Rt/mOhm cm2 | j/A cm−2 |
|---|---|---|---|---|
| R98 05S | 49 | 13 | 62 | 4.9 |
| E98 05S | 66 | 10 | 76 | 3.9 |
| C98 05S-RSP | 80 | 40 | 120 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusano, S.; Giacobello, F.; Tonella, S.; Oldani, C.; Aricò, A.S. Ce-radical Scavenger-Based Perfluorosulfonic Acid Aquivion® Membrane for Pressurised PEM Electrolysers. Polymers 2023, 15, 3906. https://doi.org/10.3390/polym15193906
Siracusano S, Giacobello F, Tonella S, Oldani C, Aricò AS. Ce-radical Scavenger-Based Perfluorosulfonic Acid Aquivion® Membrane for Pressurised PEM Electrolysers. Polymers. 2023; 15(19):3906. https://doi.org/10.3390/polym15193906
Chicago/Turabian StyleSiracusano, Stefania, Fausta Giacobello, Stefano Tonella, Claudio Oldani, and Antonino S. Aricò. 2023. "Ce-radical Scavenger-Based Perfluorosulfonic Acid Aquivion® Membrane for Pressurised PEM Electrolysers" Polymers 15, no. 19: 3906. https://doi.org/10.3390/polym15193906
APA StyleSiracusano, S., Giacobello, F., Tonella, S., Oldani, C., & Aricò, A. S. (2023). Ce-radical Scavenger-Based Perfluorosulfonic Acid Aquivion® Membrane for Pressurised PEM Electrolysers. Polymers, 15(19), 3906. https://doi.org/10.3390/polym15193906









