Porous Thermal Insulation Polyurethane Foam Materials
Abstract
:1. Introduction
2. Structure and Properties of the Porous Thermal Insulation PUF Materials
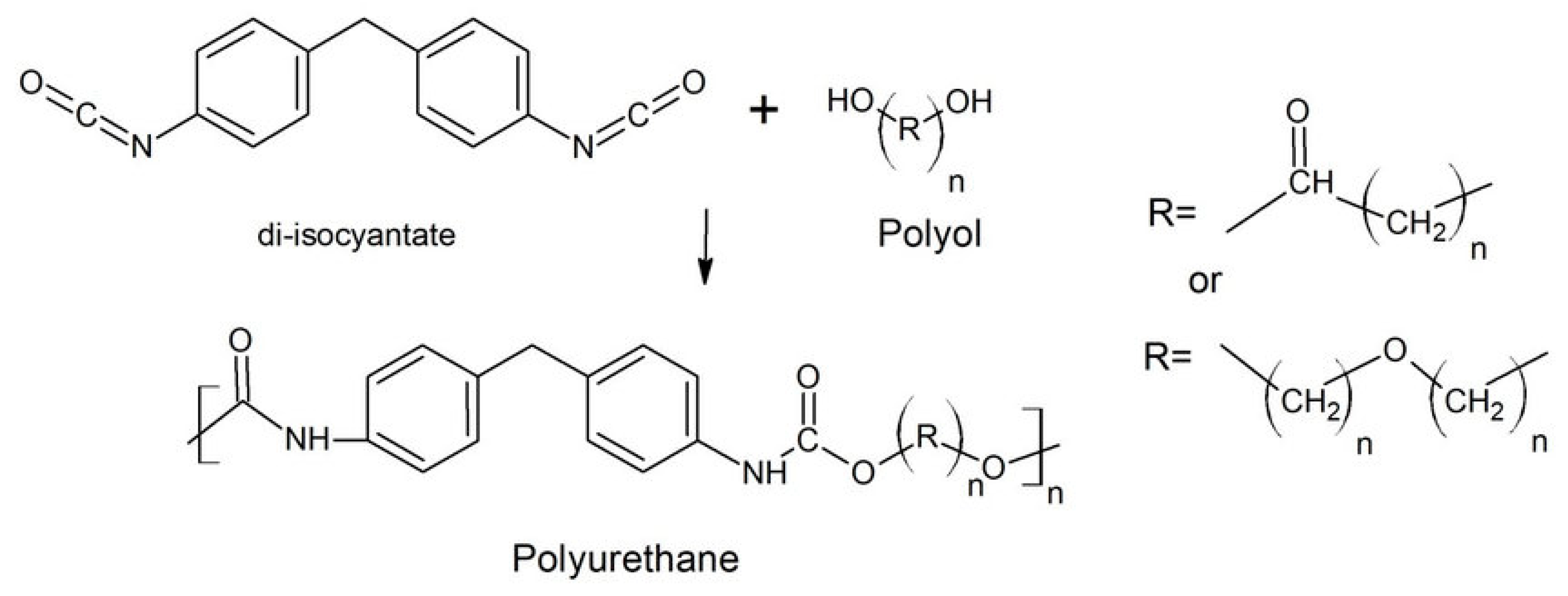
2.1. Structure of the PUF Materials
2.2. Properties of PUF Materials
2.2.1. Physical Properties
2.2.2. Chemical Properties
3. Preparation of Porous Thermal Insulation PUF Materials
3.1. Traditional Foaming Technologies
3.1.1. Solution Casting/Salt Precipitation Method
3.1.2. Phase Separation Method
3.1.3. Melt Molding Method
3.1.4. Gas Foaming Method


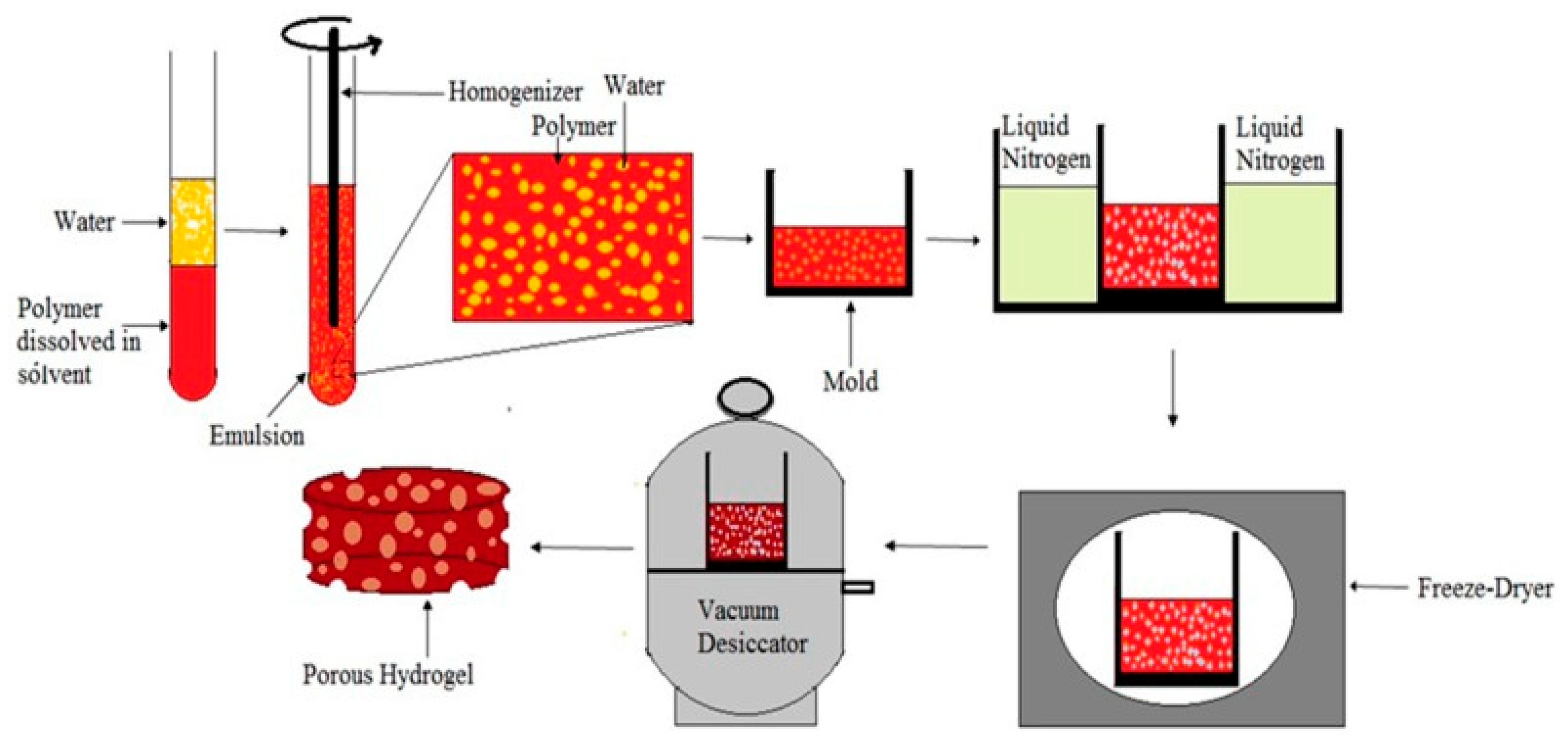
3.1.5. Freeze-Drying Method
3.1.6. Casting Method
3.1.7. Spray-Foaming Method
3.1.8. Reaction Injection Molding Method
3.2. Advanced Foaming Technologies
3.2.1. Electrostatic Spinning Method
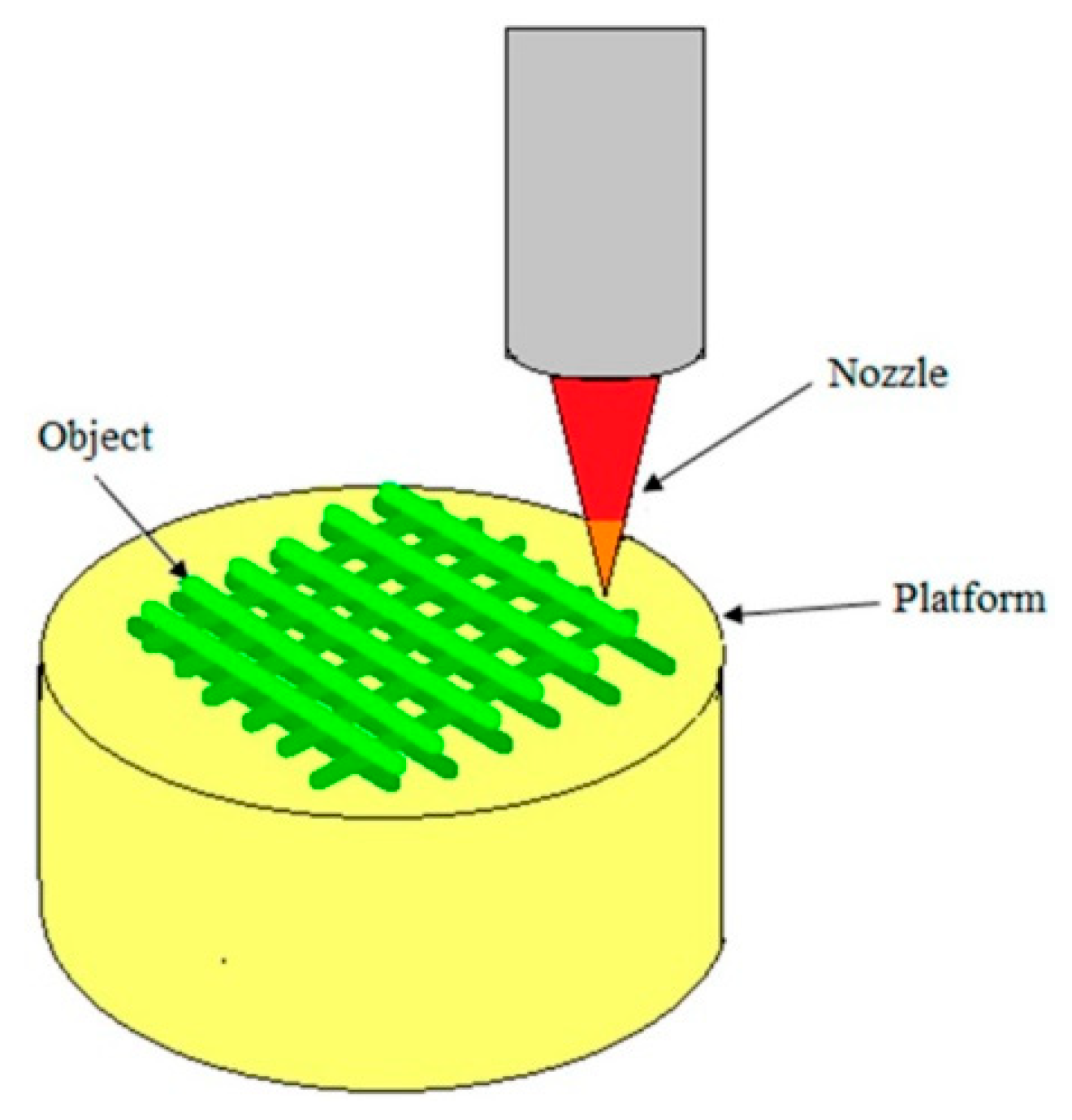
3.2.2. Three-Dimensional Printing Technology
4. Characterization of the Porous Thermal Insulation PUF Materials
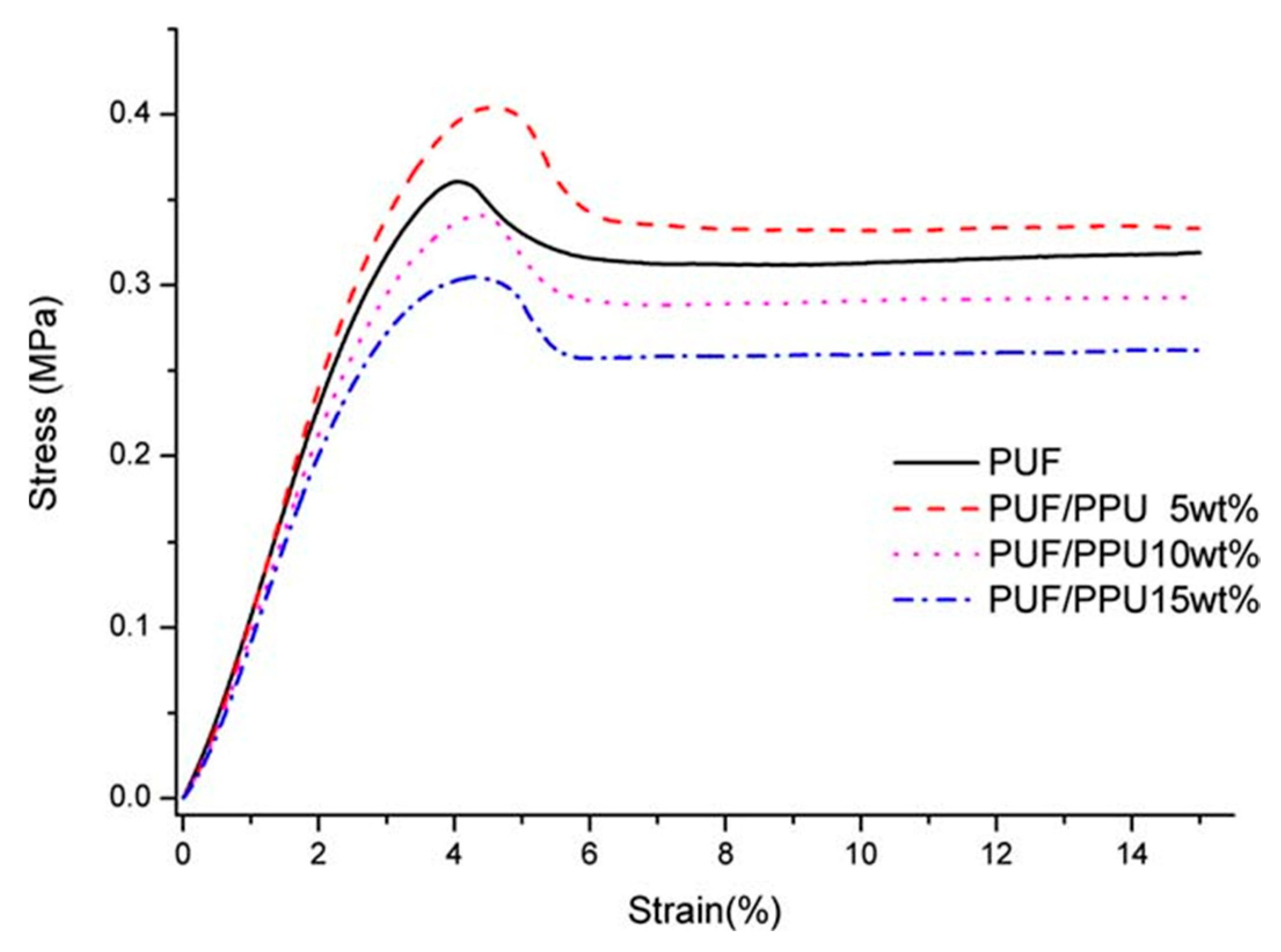
4.1. Characterization of the Mechanical Properties
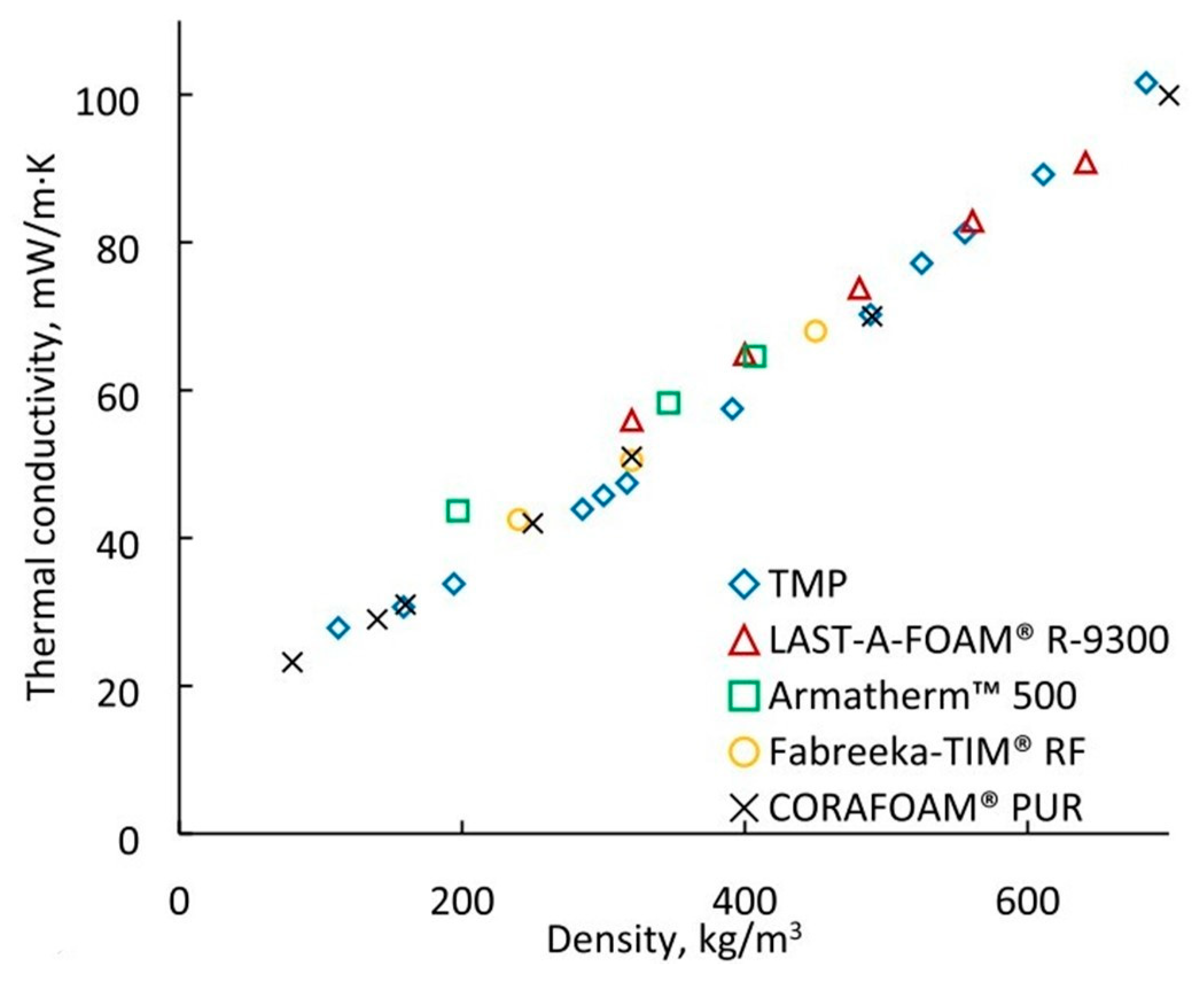
4.2. Characterization of the Thermal Conductivity
4.3. Characterization of the Thermal Stability
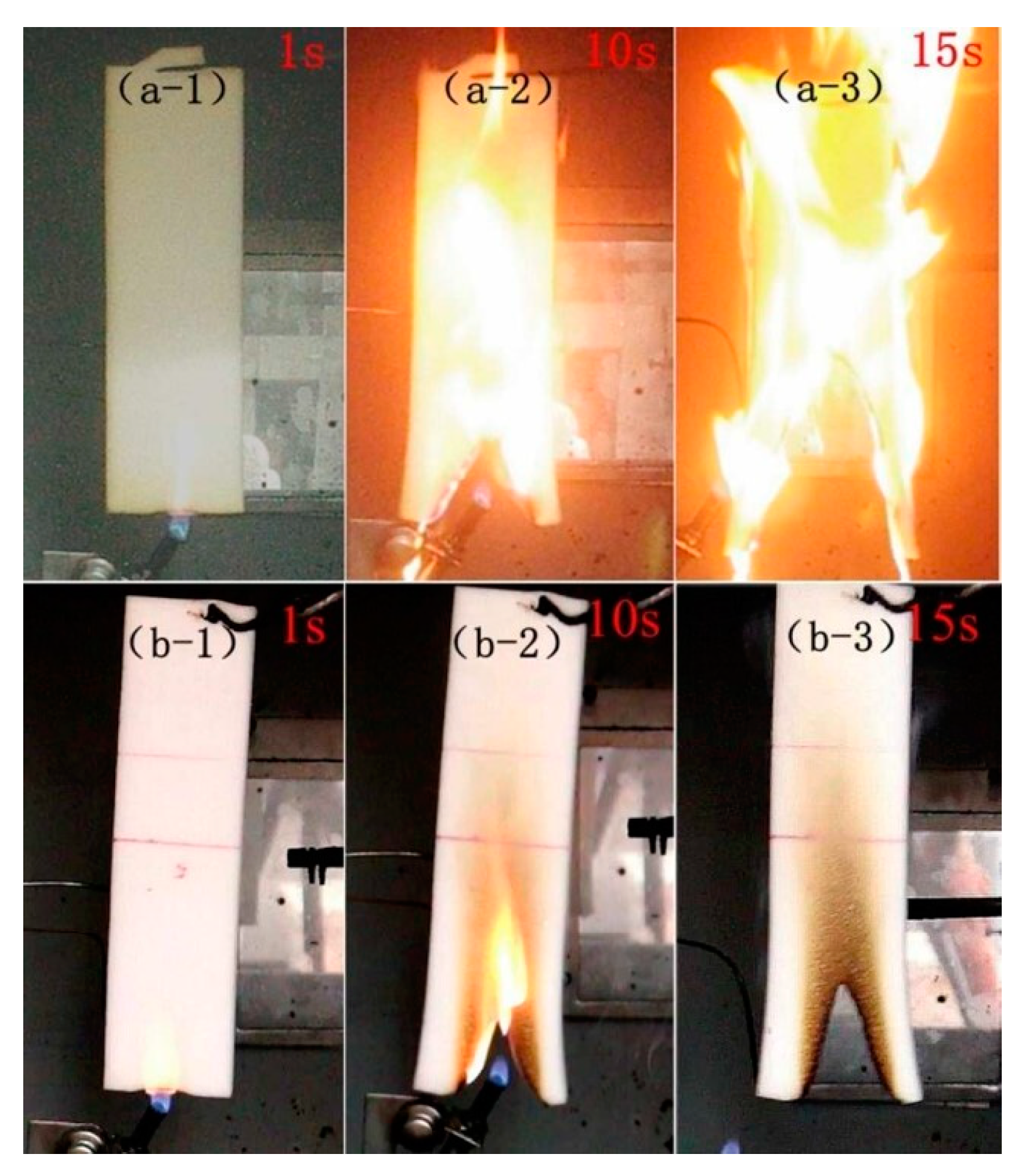
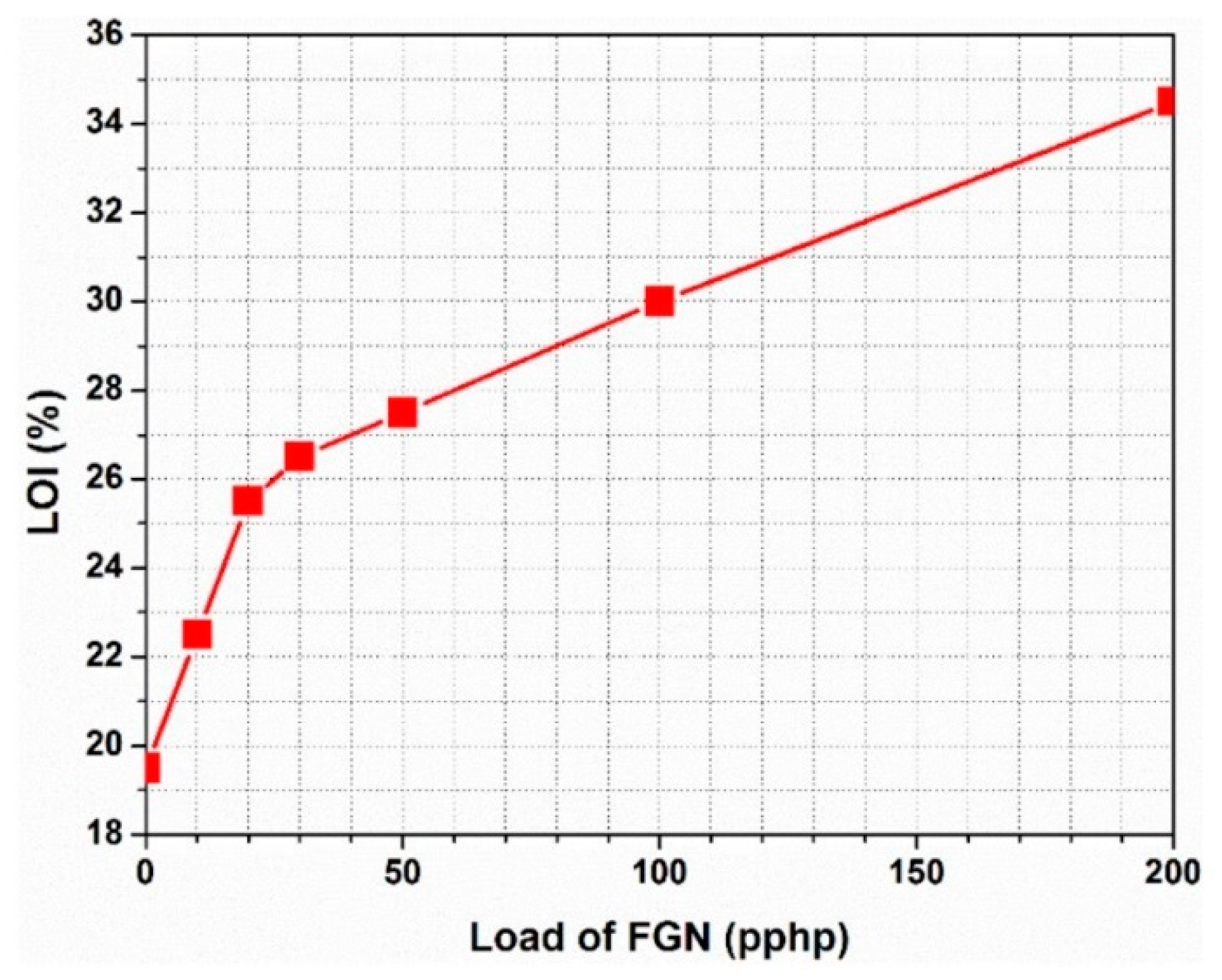
4.4. Characterization of the Flame-Retardant Properties
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Wang, W. China’s energy consumption in construction and building sectors: An outlook to 2100. Energy 2020, 195, 117045–117055. [Google Scholar] [CrossRef]
- Tychanicz-Kwiecień, M.; Wilk, J.; Gil, P. Review of high-temperature thermal insulation materials. J. Thermophys. Heat Transf. 2019, 33, 271–284. [Google Scholar] [CrossRef]
- Pan, L. A review of research on the performance of building insulation materials. Urban Constr. Theory Res. Electron. Ed. 2015, 1776–1777. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Schiavoni, S.; Bianchi, F.; Asdrubali, F. Insulation materials for the building sector: A review and comparative analysis. Renew. Sustain. Energy Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhao, H.B.; Rao, W.H. Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer 2018, 153, 616–625. [Google Scholar] [CrossRef]
- Šebenik, U.; Krajnc, M. Influence of the soft segment length and content on the synthesis and properties of isocyanate-terminated urethane prepolymers. Int. J. Adhes. Adhes. 2007, 153, 527–535. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Capanidis, D. The influence of hardness of polyurethane on its abrasive wear resistance. Tribologia 2016, 268, 29–39. [Google Scholar] [CrossRef]
- Yarahmadi, N.; Vega, A.; Jakubowicz, I. Accelerated ageing and degradation characteristics of rigid polyurethane foam. Polym. Degrad. Stab. 2017, 138, 192–200. [Google Scholar] [CrossRef]
- Liu, Z.; He, H.; Zhang, J. Experimental investigation and numerical simulation of the combustion of flexible polyurethane foam with larger geometries. Polym. Test. 2020, 81, 106270–106281. [Google Scholar] [CrossRef]
- Sin, D.C.; Miao, X.; Liu, G.; Wei, F.; Chadwick, G.; Yan, C. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater. Sci. Eng. C 2010, 30, 78–85. [Google Scholar] [CrossRef]
- Asadpour, S.; Yeganeh, H.; Ai, J.; Kargozar, S.; Rashtbar, M.; Rashtbar, A.; Ghanbari, H. Polyurethane-polycaprolactone blend patches: Scaffold characterization and cardiomyoblast adhesion, proliferation, and function. ACS Biomater. Sci. Eng. 2018, 4, 4299–4310. [Google Scholar] [CrossRef] [PubMed]
- Rarima, R.; Unnikrishnan, G. Poly (lactic acid)/gelatin foams by non-solvent induced phase separation for biomedical applications. Polym. Degrad. Stab. 2020, 177, 109187–109197. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef]
- Yao, X.; Tou, X.L.; Wang, X.G. Preparation of degradable polyurethane type porous scaffold materials for tissue engineering. Adv. Chem. 2009, 21, 1546–1556. [Google Scholar]
- Gorna, K.; Gogolewski, S. Biodegradable porous polyurethane scaffolds for tissue repair and regeneration. J. Biomed. Mater. Res. Part A 2006, 79, 128–138. [Google Scholar] [CrossRef]
- Claeys, B.; Vervaeck, A.; Hillewaere, X. Thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Eur. J. Pharm. Biopharm. 2015, 90, 44–52. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials. 2016, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Turng, L.S. Novel Injection Molding Foaming Approaches Using Gas-Laden Pellets. Ph.D. Thesis, The University of Wisconsin-Madison, Madison, WI, USA, 2014; pp. 899–913. [Google Scholar]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion templating: Porous polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Smith, J.R.; Johnson, E.M. Polyurethane foam preparation techniques: A comprehensive review. J. Mater. Sci. 2018, 45, 2897–2910. [Google Scholar]
- Brown, A.B.; White, C.D. Casting method for tailored polyurethane foams. Polym. Eng. Sci. 2020, 60, 1800–1812. [Google Scholar]
- Garcia, F.A.; Lee, K.Y. Influence of additives on casting polyurethane foam properties. J. Cell. Plast. 2019, 56, 329–344. [Google Scholar]
- Johnson, A.B.; Smith, C.D. Spray-Foaming techniques for tailored polyurethane foams. J. Appl. Polym. Sci. 2019, 136, 47512. [Google Scholar]
- Garcia, E.F.; Lee, K.Y. Advances in polyurethane spray foaming: From fundamentals to applications. Polym. Rev. 2021, 61, 284–301. [Google Scholar]
- Smith, A.B.; Johnson, C.D. Reaction injection moulding of polyurethane foam: Process advancements. Polym. Process. 2020, 25, 215–230. [Google Scholar]
- Garcia, E.F.; Lee, K.Y. Polyurethane foam via rim: Mechanical and thermal properties. J. Cell. Plast. 2022, 58, 412–425. [Google Scholar]
- Liu, R.; Qu, M.; Qiu, X.; Wang, H.; Fan, M.; Zhang, A.; Chen, Q.; Bin, Y. Poly (ethylene terephthalate) nonwoven fabrics-based membranes modified by electrospinning of thermoplastic polyurethane, nano SiO2 and Ag particles as medical packing materials. Packag. Technol. Sci. 2022, 35, 557–567. [Google Scholar] [CrossRef]
- Andrews, K.D.; Hunt, J.A. Up regulation of matrix and adhesion molecules induced bycon-trolled topography. J. Mater. Sci. Mater. Med. 2008, 19, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Du, J.; Li, Q.; Wang, Y.; Lu, Y.; Fan, J.; Yu, S.; Yang, Y. Preparation, Properties, and Application of Graphene-Based Materials in Tissue Engineering Scaffolds. Tissue Eng. Part B Rev. 2022, 28, 1121–1136. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, G.; Xu, J.; Liu, Y.; Chen, R.; Yan, H. Modification of diatomite with melamine coated zeolitic imidazolate framework-8 as an effective flame retardant to enhance flame retardancy and smoke suppression of rigid polyurethane foam. J. Hazard. Mater. 2019, 379, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Przybytek, A.; Gubańska, I.; Kucińska-Lipka, J.; Janik, H. Polyurethanes as a potential medical-grade filament for use in fused deposition modeling 3D printers-a brief review. Fibres Text. East. Eur. 2018, 26, 120–125. [Google Scholar] [CrossRef]
- Bates, S.R.G.; Farrow, I.R.; Trask, R. 3D printed polyurethane honeycombs for repeated tailored energy absorption. Mater. Des. 2016, 112, 172–183. [Google Scholar] [CrossRef]
- Sakkadech, L.; Adekunle, O.; Kriskrai, S.; Yin, X.; Cheng, Y. 3D-printed cellular structures for bone biomimetic implants. Addit. Manuf. 2017, 15, 93–101. [Google Scholar]
- Adriana, P.R.; Ataúlfo, M.T.; Castro, M.; Talavera, J.; Susana, V.; Víctor, M.; Maykel, G.T. Novel poly(3-hydroxybu-tyrate-g-vinyl alcohol) polyurethane scaffold for tissue engineering. Sci. Rep. 2016, 6, 31140–31151. [Google Scholar]
- Saha, M.C.; Kabir, M.E.; Jeelani, S. Enhancement in thermal and mechanical properties of polyurethane foam infused with nanoparticles. Mater. Sci. Eng. A 2008, 479, 213–222. [Google Scholar] [CrossRef]
- Laureto, J.; Pearce, J. Anisotropic mechanical property variance between ASTM D638-14 type i and type iv fused filament fabricated specimens. Polym. Test. 2018, 68, 294–301. [Google Scholar] [CrossRef]
- Theobald, C. Analysis of ASTM D790-72 and Its Application to Reinforced Plastic Structural Members; The University of Mississippi: University, MS, USA, 2001. [Google Scholar]
- Yin, Z.; Lu, J.; Hong, N.; Cheng, W.; Jia, P.; Wang, H.; Hu, W.; Wang, B.; Song, L.; Hu, Y. Functionalizing Ti3C2Tx for enhancing fire resistance and reducing toxic gases of flexible polyurethane foam composites with reinforced mechanical properties. J. Colloid Interface Sci. 2022, 607, 1300–1312. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, F.; Xu, Z. The improvement of fire safety performance of flexible polyurethane foam by Highly-efficient PNS elemental hybrid synergistic flame retardant. J. Colloid Interface Sci. 2022, 606, 768–783. [Google Scholar] [CrossRef]
- Chao, S.; Zou, F.; Wan, F.; Dong, X.; Wang, Y.; Wang, Y.; Guan, Q.; Wang, G.; Li, W. Nitrogen-doped carbon derived from ZIF-8 as a high-performance metal-free catalyst for acetylene hydrochlorination. Sci. Rep. 2017, 7, 39789–39799. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Yu, M.; Xiu, L.; Qiu, J. Stabilizing the MXenes by carbon nanoplating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv. Mater. 2017, 29, 1607017–1607027. [Google Scholar] [CrossRef]
- Yang, C.; Zhuang, Z.H.; Yang, Z.G. Pulverized polyurethane foam particles reinforced rigid polyurethane foam and phenolic foam. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Zhuang, Z.; He, B.; Yang, Z. Preparation and characterisation of phenolic foam/HTAB-ATP nanocomposites. Plast. Rubber Compos. 2010, 39, 460–464. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non-Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Li, M.E.; Wang, S.X.; Han, L.X. Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef]
- Jiang, L.; Kato, K.; Mayumi, K. One-pot synthesis and characterization of polyrotaxane-silica hybrid aerogel. ACS Macro Lett. 2017, 6, 281–286. [Google Scholar] [CrossRef]
- Mandal, J.; Fu, Y.; Overvig, A.C. Hierarchically porous polymer coatings for highly efficient passive daytime radiative cooling. Science 2018, 362, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Petkova-Slipets, R.; Zlateva, P. Thermal insulating properties of straw-filled environmentally friendly building materials. Civ. Environ. Eng. 2017, 13, 52–57. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Andersons, J.; Kirpluks, M.; Cabulis, P.; Kalnins, K.; Cabulis, U. Bio-based rigid high-density polyurethane foams as a structural thermal break material. Constr. Build. Mater. 2020, 260, 120471–120482. [Google Scholar] [CrossRef]
- ASTM E1461-07; Standard Test Method for Thermal Diffusivity by the Flash Method. ASTM International: West Conshohocken, PA, USA, 2013.
- Kurańska, M.; Pinto, J.; Salach, K.; Barreiro, M.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crops Prod. 2020, 143, 111882–111895. [Google Scholar] [CrossRef]
- Smith, J.R. Sustainable polyols for polyurethane production: A review. ACS Sustain. Chem. Eng. 2019, 7, 16004–16021. [Google Scholar]
- Johnson, M.; Brown, L. Biobased Polyols for Polyurethane Foams. In Biobased Polyols for Polyurethanes; Springer: Cham, Switzerland, 2020; Volume 55, pp. 79–115. [Google Scholar]
- Verdolotti, L.; Oliviero, M.; Lavorgna, M.; Santillo, C.; Tallia, F.; Iannace, S.; Chen, S.; Jones, J. “Aerogel-like” polysiloxane-polyurethane hybrid foams with enhanced mechanical and thermal-insulating properties. Compos. Sci. Technol. 2021, 213, 108917–108928. [Google Scholar] [CrossRef]
- Burgaz, E.; Kendirlioglu, C. Thermomechanical behavior and thermal stability of polyurethane rigid nanocomposite foams containing binary nanoparticle mixtures. Polym. Test. 2019, 77, 105930–105942. [Google Scholar] [CrossRef]
- Pan, H.; Shen, Q.; Zhang, Z.; Yu, B.; Lu, Y. MoS2-filled coating on flexible polyurethane foam via layer-by-layer assembly technique: Flame-retardant and smoke suppression properties. J. Mater. Sci. 2018, 53, 9340–9349. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2020, 137, 47611–47623. [Google Scholar] [CrossRef]
- Rao, W.H.; Hu, Z.Y.; Xu, H.X.; Xu, Y.J.; Qi, M.; Liao, W.; Xu, S.; Wang, Y.Z. Flame-retardant flexible polyurethane foams with highly efficient melamine salt. Ind. Eng. Chem. Res. 2017, 56, 7112–7119. [Google Scholar] [CrossRef]
- Cao, Z.J.; Liao, W.; Wang, S.X.; Zhao, H.B.; Wang, Y.Z. Polyurethane foams with functionalized graphene towards high fire-resistance, low smoke release, superior thermal insulation. Chem. Eng. J. 2019, 361, 1245–1254. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Lu, H.; Song, L.; Hu, Y. The effect of metal oxide decorated graphene hybrids on the improved thermal stability and the reduced smoke toxicity in epoxy resins. Chem. Eng. J. 2014, 250, 214–221. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, C.R.; Tan, Y.; Huang, J.Q.; Wang, X.L.; Chen, L.; Wang, Y.Z. Inherently flame-retardant flexible polyurethane foam with low content of phosphorus-containing cross-linking agent. Ind. Eng. Chem. Res. 2014, 53, 1160–1171. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wang, L.P.; Ning, B.; Xue, C.; Su, T.D. Research progress of halogen-free flame retardant technology for polyurethane foam. Chem. Prog. 2012, 31, 1521–1527. [Google Scholar]
- Zhang, J.C.; Wu, S.H.; Zheng, Q.; Tian, S.H. Preparation of nitrogen-containing reactive flame retardants and the effect on flame retardant properties of polyurethane foam. Plast. Sci. Technol. 2019, 47, 31–36. [Google Scholar]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 46224–46235. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, M.; Mispreuve, H.; Naescher, R.; Gaan, S. Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds. Polym. Degrad. Stab. 2012, 97, 2428–2440. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, X.S. Application of new DOPO-based reactive flame retardants in polyurethane. Plastics 2013, 42, 82–85. [Google Scholar]





| Silica Aerogel (wt%) | Blowing Agent | |||
|---|---|---|---|---|
| CFC–11 | Cyclopentane | Normal Pentane | Normal Hexane | |
| 0% | 0.0209 ± 0.0003 | 0.0314 ± 0.0001 | 0.0301 ± 0.0001 | 0.0345 ± 0.0002 |
| 1% | 0.0189 ± 0.0002 | 0.0295 ± 0.0003 | 0.2879 ± 0.0002 | 0.0328 ± 0.0001 |
| 3% | 0.0178 + 0.0001 | 0.0277 ± 0.0002 | 0.0266 ± 0.0001 | 0.0308 ± 0.0002 |
| 5% | 0.0171 ± 0.0002 | 0.0268 ± 0.0001 | 0.0257 ± 0.0001 | 0.0299 ± 0.0001 |
| Sample | Density (kg/m3) | Weight Gain (wt%) | Compressive Strength (kPa) | Thermal Conductivity mW/(m·K) |
|---|---|---|---|---|
| Pure PUF | 28.5 ± 0.1 | / | 221 ± 13 | 30.9 |
| SiO2/PUF–1 | 32.2 ± 0.8 | 13.0 | 335 ± 26 | 29.6 |
| SiO2/PUF–3 | 34.7 ± 0.6 | 21.8 | 418 ± 22 | 29.0 |
| SiO2/PUF–5 | 37.9 ± 1.2 | 33.0 | 486 ± 37 | 28.2 |
| Sample | Tmax1 | Tmax2 | Tmax3 | Residue at 1000 °C, wt% SiO2 | Tg (°C) | λ (W/mK) |
|---|---|---|---|---|---|---|
| Pristine PUR | 316 | 330 | 560 | 0 | 24.5 | 0.04 |
| HPURwca | 273 | 314 | 561 | 17 | 40 | 0.032 |
| HPURca1 | 215 | 335 | 570 | 17.8 | 49.1 | 0.028 |
| HPURca2 | 267 | 340 | 620 | 20 | 59.2 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, C.; Gao, Y.; Li, Z.; Shang, Y.; Li, H. Porous Thermal Insulation Polyurethane Foam Materials. Polymers 2023, 15, 3818. https://doi.org/10.3390/polym15183818
Wang Z, Wang C, Gao Y, Li Z, Shang Y, Li H. Porous Thermal Insulation Polyurethane Foam Materials. Polymers. 2023; 15(18):3818. https://doi.org/10.3390/polym15183818
Chicago/Turabian StyleWang, Zhiguo, Chengzhu Wang, Yuebin Gao, Zhao Li, Yu Shang, and Haifu Li. 2023. "Porous Thermal Insulation Polyurethane Foam Materials" Polymers 15, no. 18: 3818. https://doi.org/10.3390/polym15183818
APA StyleWang, Z., Wang, C., Gao, Y., Li, Z., Shang, Y., & Li, H. (2023). Porous Thermal Insulation Polyurethane Foam Materials. Polymers, 15(18), 3818. https://doi.org/10.3390/polym15183818







