Photoluminous Response of Biocomposites Produced with Charcoal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Charcoal
2.2. Characteristics of Charcoal
2.3. Production of Biocomposites
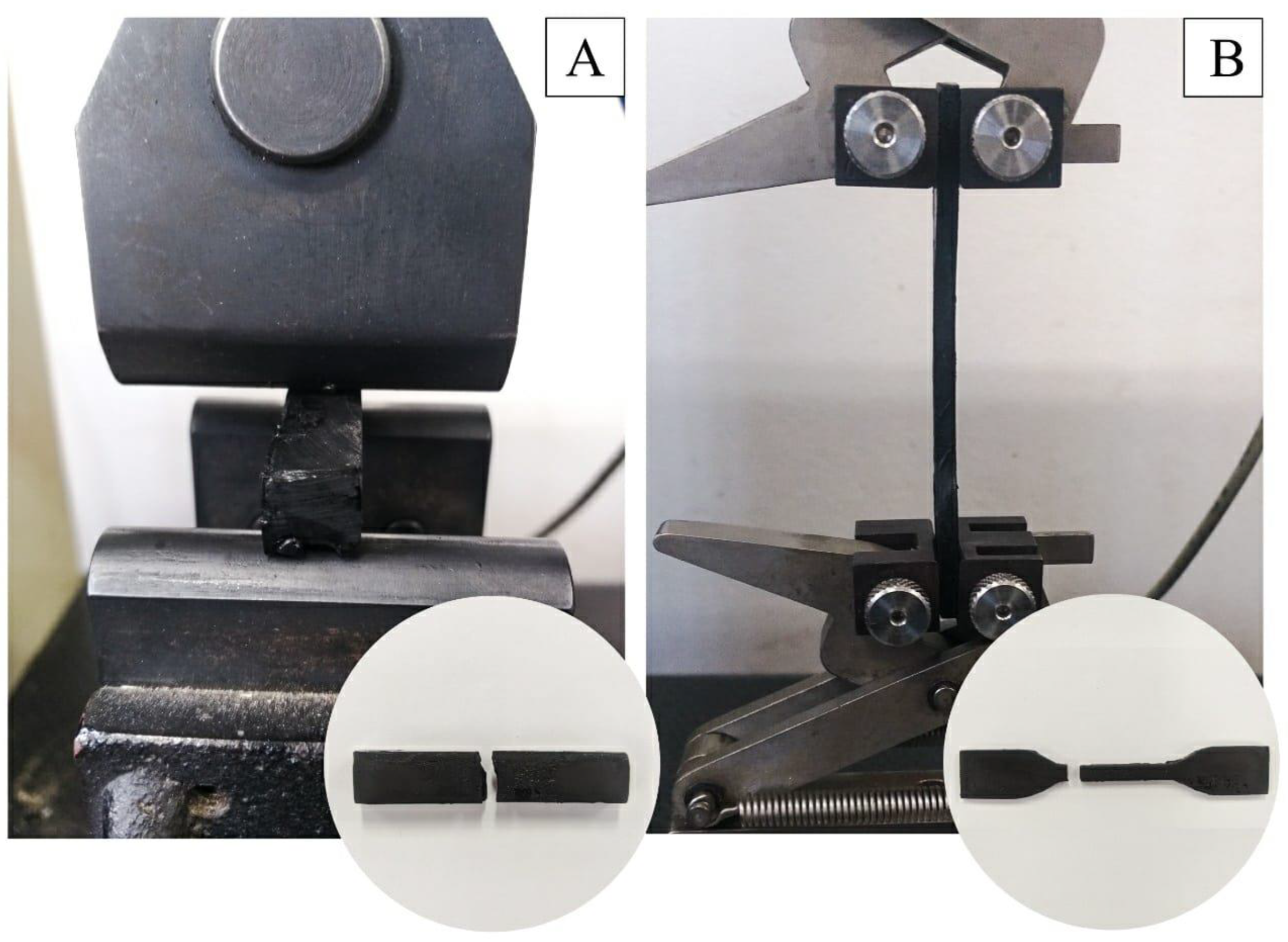
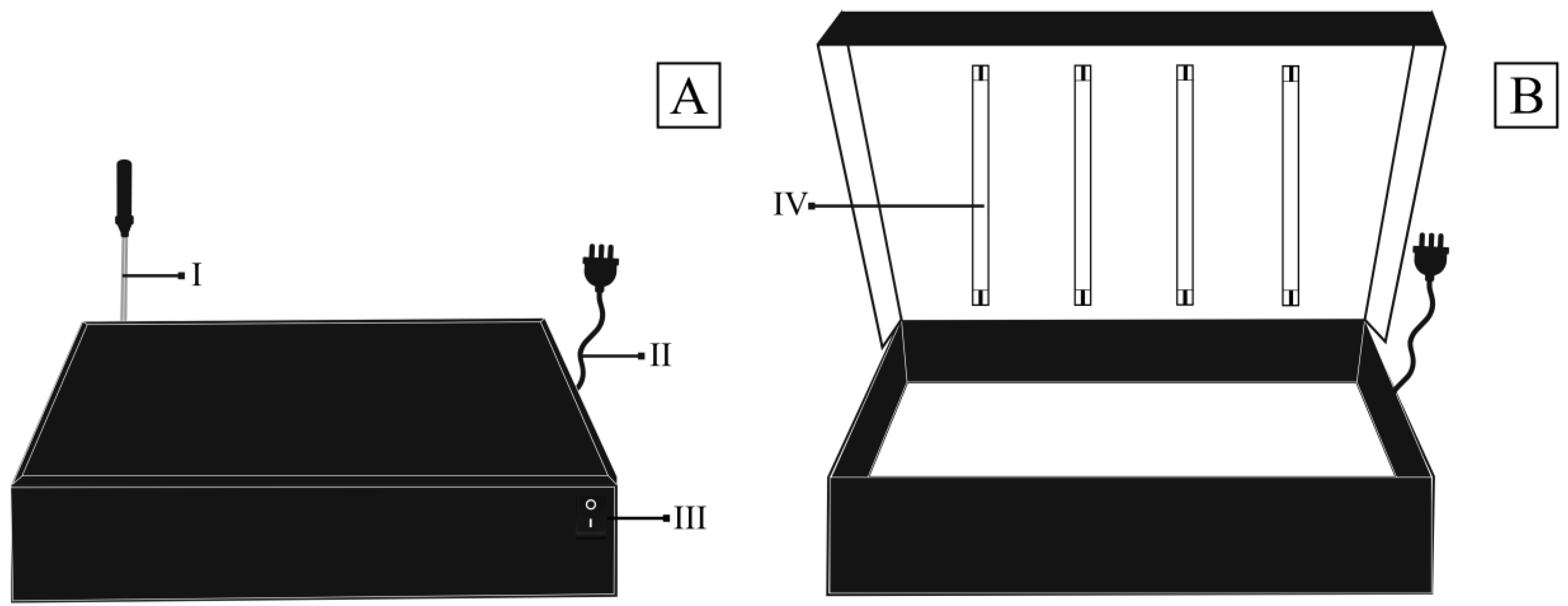
2.4. Photoluminosity Analysis of Biocomposites
2.5. Characterization of Carbonaceous Biocomposites
2.6. Data Analysis
3. Results and Discussion
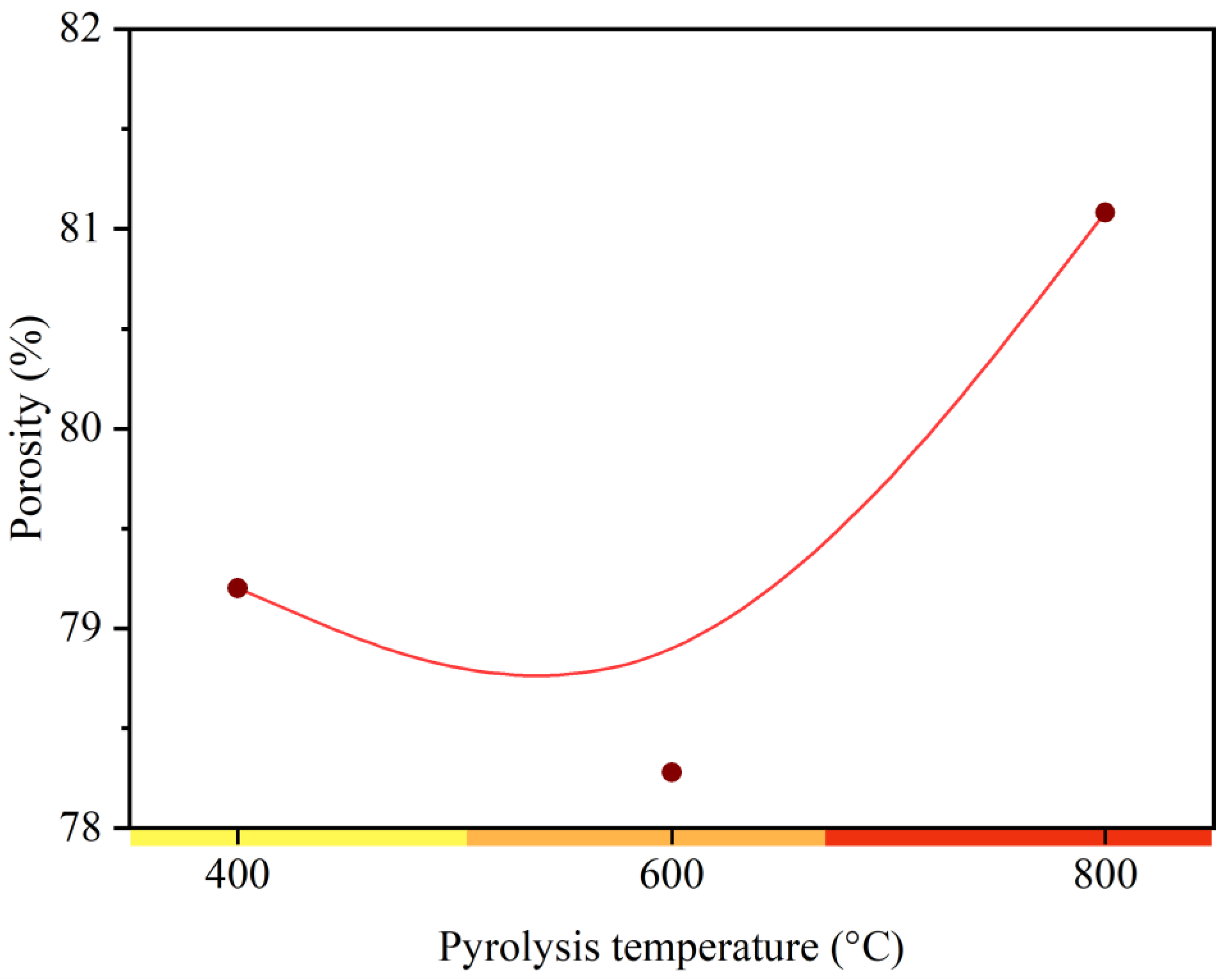
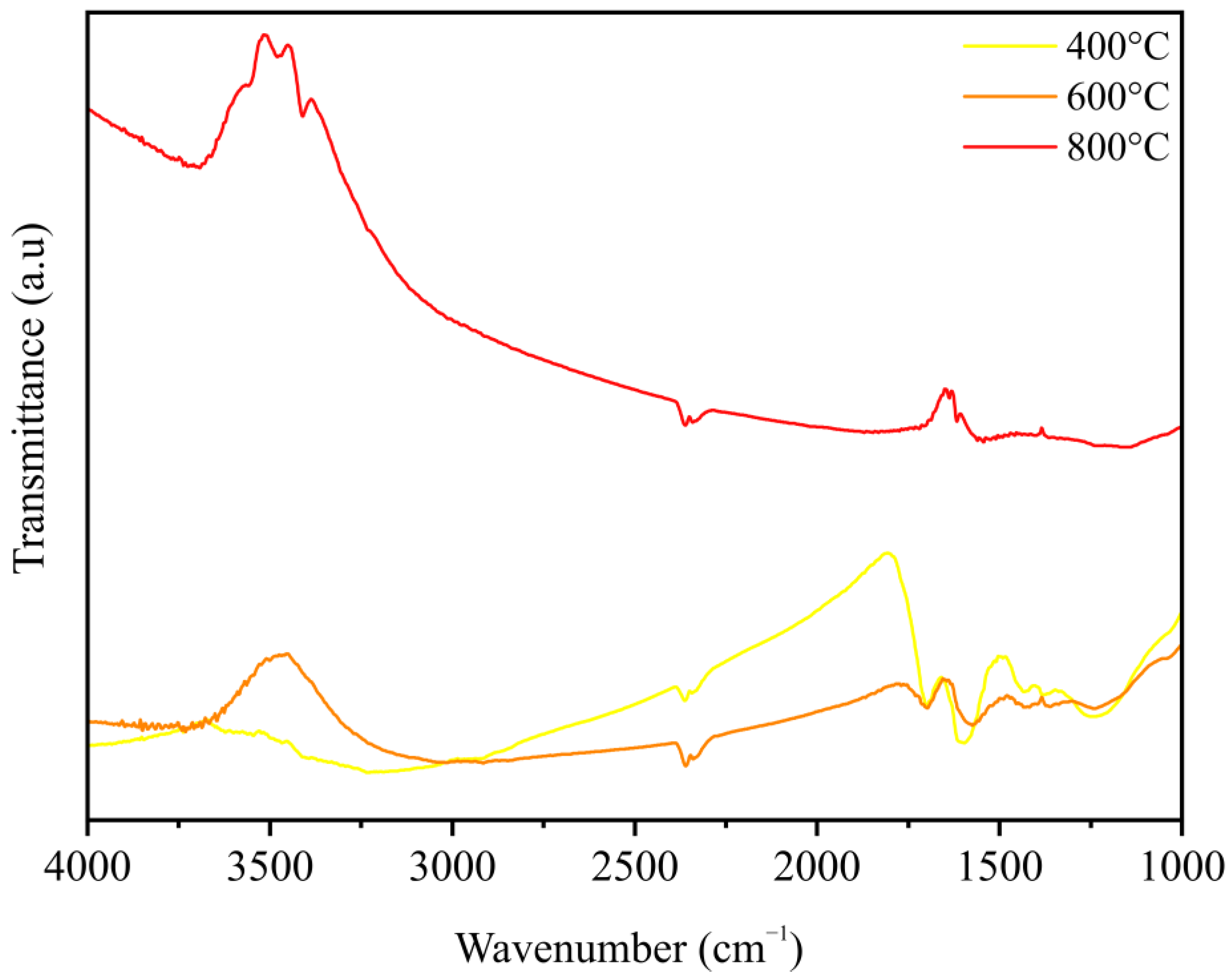
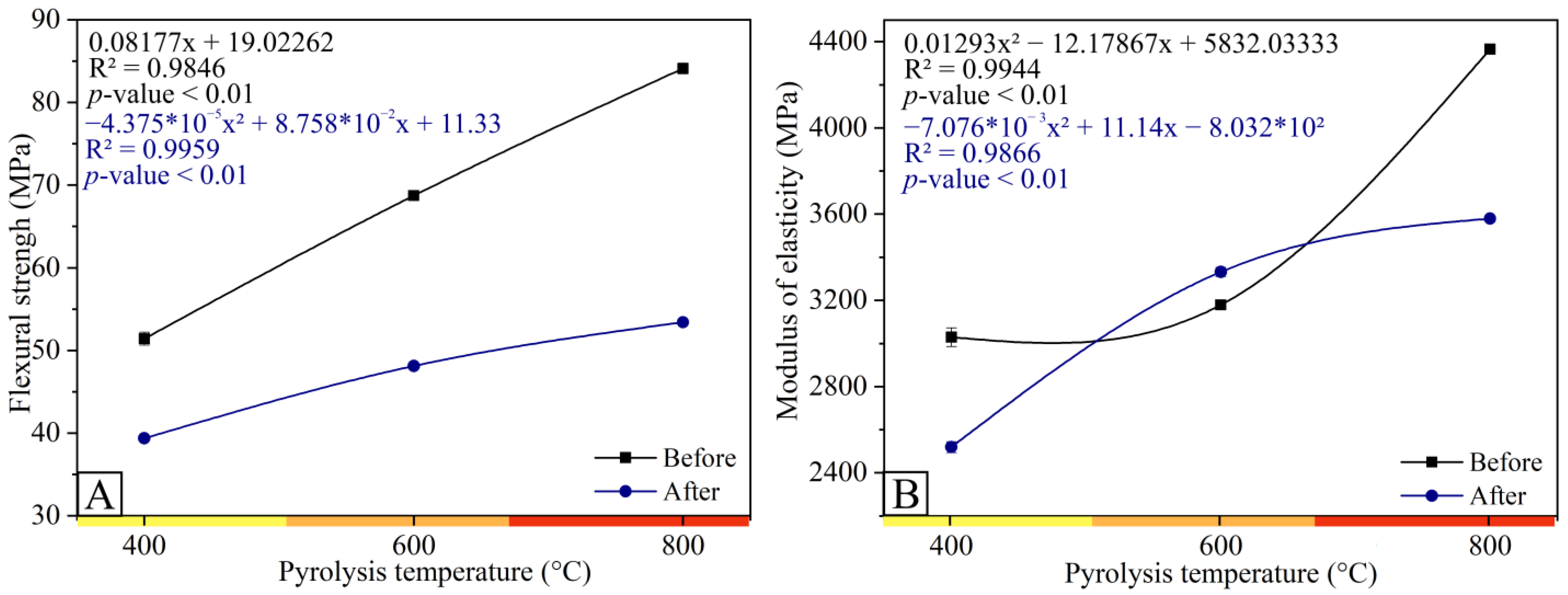
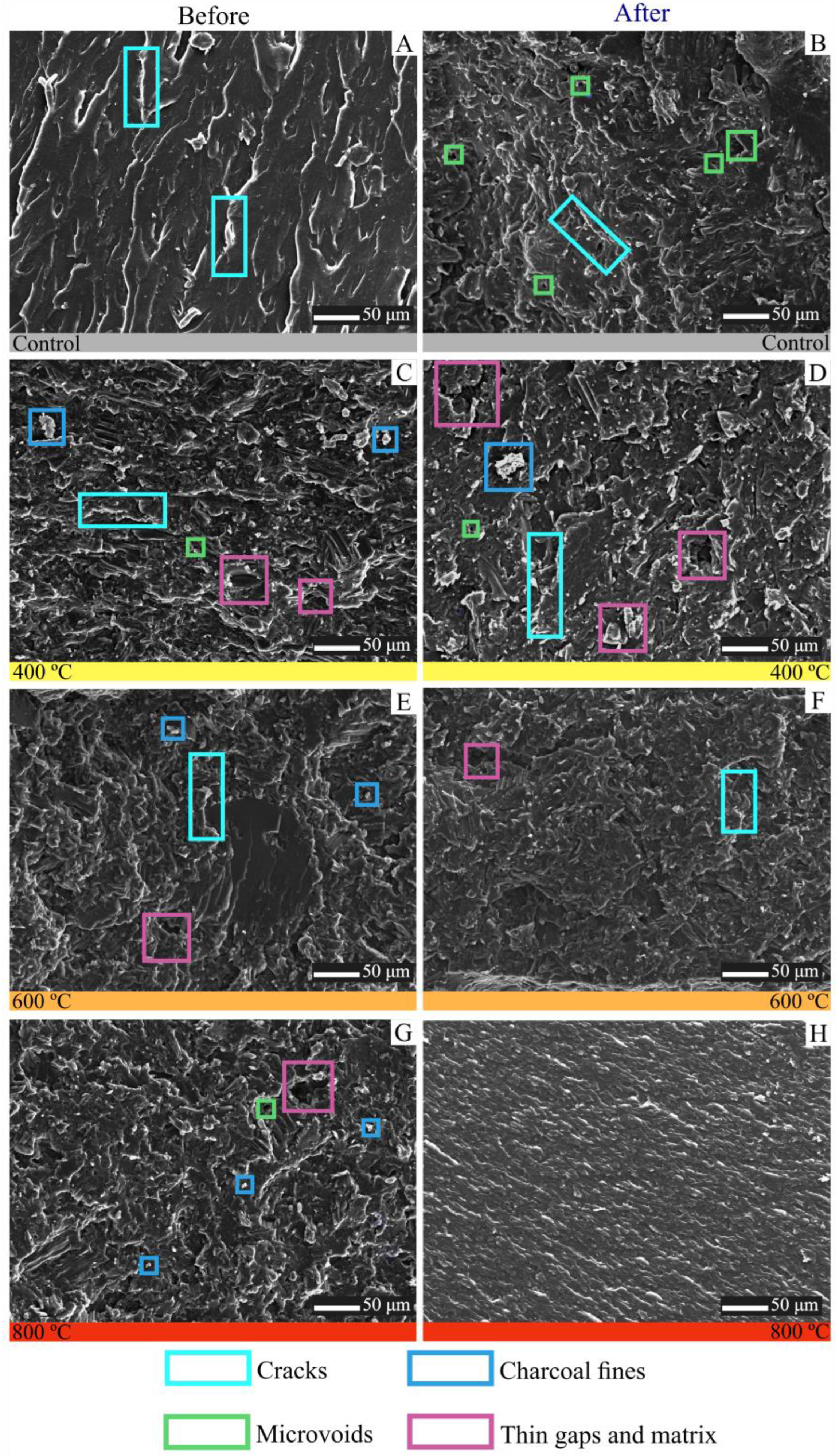
3.1. Characteristics of Charcoal
3.2. Biocomposites from Charcoal
4. Practical Applications and Future Perspective
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Ghorpade, C.V.; Shetty, S.C.; Murthy, K. Bioplastics Production and Applications: A Mini Review. Int. J. Sci. Res. Dev. 2022, 10, 229–238. [Google Scholar]
- Wasti, S.; Triggs, E.; Farag, R.; Auad, M.; Adhikari, S.; Bajwa, D.; Li, M.; Ragauskas, A.J. Influence of Plasticizers on Thermal and Mechanical Properties of Biocomposite Filaments Made from Lignin and Polylactic Acid for 3D Printing. Compos. B Eng. 2021, 205, 108483. [Google Scholar] [CrossRef]
- Das, C.; Tamrakar, S.; Kiziltas, A.; Xie, X. Incorporation of Biochar to Improve Mechanical, Thermal and Electrical Properties of Polymer Composites. Polymers 2021, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Sider, I.; Nassar, M.M.A. Chemical Treatment of Bio-Derived Industrial Waste Filled Recycled Low-Density Polyethylene: A Comparative Evaluation. Polymers 2021, 13, 2682. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Bari, E.; Sistani, A.; Morrell, J.J.; Pizzi, A.; Akbari, M.R.; Ribera, J. Current Strategies for the Production of Sustainable Biopolymer Composites. Polymers 2021, 13, 2878. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Kadier, A.; Kalil, M.S.; Ibrahim, R.; Atikah, M.S.N.; Nurazzi, N.M.; Nazrin, A.; Lee, C.H.; Faiz Norrrahim, M.N.; et al. Properties and Characterization of PLA, PHA, and Other Types of Biopolymer Composites. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 111–138. [Google Scholar]
- Fuentes Molina, N.; Fragozo Brito, Y.; Polo Benavides, J.M. Recycling of Residual Polymers Reinforced with Natural Fibers as a Sustainable Alternative: A Review. Polymers 2021, 13, 3612. [Google Scholar] [CrossRef]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.P.; Lopresti, F. Use of Biochar as Filler for Biocomposite Blown Films: Structure-Processing-Properties Relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, T.; Das, O.; Tomppo, L. A Review on New Bio-Based Constituents for Natural Fiber-Polymer Composites. J. Clean. Prod. 2017, 149, 582–596. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Sarmah, A.K. Sustainable Eco–Composites Obtained from Waste Derived Biochar: A Consideration in Performance Properties, Production Costs, and Environmental Impact. J. Clean. Prod. 2016, 129, 159–168. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Zaharescu, T.; Dumitriu, R.-P.; Pricope, G.M.; Râpă, M.; Vasilievici, G. Effect of Gamma Irradiation on the PLA-Based Blends and Biocomposites Containing Rosemary Ethanolic Extract and Chitosan. Polymers 2022, 14, 1398. [Google Scholar] [CrossRef]
- Jukka, T.; Mikkola, N. What Is Industrial Symbiosis? 2022. Available online: https://nordregio.org/nordregio-magazine/issues/industrial-symbiosis/what-is-industrial-symbiosis/ (accessed on 20 May 2023).
- Ferjan, Š.; Jovičić, M.; Lardiés Miazza, N.; Ligthart, T.; Harvey, C.; Fita, S.; Mehta, R.; Samani, P. Sustainability Assessment of the End-of-Life Technologies for Biocomposite Waste in the Aviation Industry. Polymers 2023, 15, 2689. [Google Scholar] [CrossRef]
- Rusu, L.; Suceveanu, E.-M.; Blaga, A.-C.; Nedeff, F.M.; Șuteu, D. Insights into Recent Advances of Biomaterials Based on Microbial Biomass and Natural Polymers for Sustainable Removal of Pharmaceuticals Residues. Polymers 2023, 15, 2923. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Podkościelna, B.; Goliszek, M.; Kubiak, A.; Młynarczyk, K.; Jesionowski, T. Synthesis, Characterization and Aging Tests of Functional Rigid Polymeric Biocomposites with Kraft Lignin. Int. J. Biol. Macromol. 2021, 178, 344–353. [Google Scholar] [CrossRef]
- Yatigala, N.S.; Bajwa, D.S.; Bajwa, S.G. Compatibilization Improves Physico-Mechanical Properties of Biodegradable Biobased Polymer Composites. Compos. Part A Appl. Sci. Manuf. 2018, 107, 315–325. [Google Scholar] [CrossRef]
- Ares-Elejoste, P.; Seoane-Rivero, R.; Gandarias, I.; Iturmendi, A.; Gondra, K. Sustainable Alternatives for the Development of Thermoset Composites with Low Environmental Impact. Polymers 2023, 15, 2939. [Google Scholar] [CrossRef] [PubMed]
- Gañán, P.; Barajas, J.; Zuluaga, R.; Castro, C.; Marín, D.; Tercjak, A.; Builes, D.H. The Evolution and Future Trends of Unsaturated Polyester Biocomposites: A Bibliometric Analysis. Polymers 2023, 15, 2970. [Google Scholar] [CrossRef]
- Campos, A.; Marconcini, J.M.; Martins-Franchetti, S.M.; Mattoso, L.H.C. The Influence of UV-C Irradiation on the Properties of Thermoplastic Starch and Polycaprolactone Biocomposite with Sisal Bleached Fibers. Polym. Degrad. Stab. 2012, 97, 1948–1955. [Google Scholar] [CrossRef]
- Walters, R.C.; Fini, E.H.; Abu-Lebdeh, T. enhancing asphalt rheological behavior and aging susceptibility using bio-char and nano-clay. Am. J. Eng. Appl. Sci. 2014, 7, 66–76. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, B.; Shu, X.; Ye, P. Laboratory Investigation of Biochar-Modified Asphalt Mixture. Transp. Res. Rec. J. Transp. Res. Board. 2014, 2445, 56–63. [Google Scholar] [CrossRef]
- Vašíček, A.; Lenfeld, P.; Běhálek, L. Degradation of Polylactic Acid Polymer and Biocomposites Exposed to Controlled Climatic Ageing: Mechanical and Thermal Properties and Structure. Polymers 2023, 15, 2977. [Google Scholar] [CrossRef] [PubMed]
- Dias Junior, A.F.; Esteves, R.P.; da Silva, Á.M.; Sousa Júnior, A.D.; Oliveira, M.P.; Brito, J.O.; Napoli, A.; Braga, B.M. Investigating the Pyrolysis Temperature to Define the Use of Charcoal. Eur. J. Wood Wood Prod. 2020, 78, 193–204. [Google Scholar] [CrossRef]
- ABNT NBR; NBR 9165 Carvão Vegetal: Determinação Da Densidade Relativa Aparente, Relativa Verdadeira e Porosidade. Brazilian Standard: Aveiro, Brazil, 1985.
- ASTM D 7264M-21; Standard Test Method for Flexural Properties of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2021; p. 11.
- ASTM D 3039M-17; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017; p. 13.
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New Approaches to Measuring Biochar Density and Porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Gao, X.; Driver, L.E.; Kasin, I.; Masiello, C.A.; Pyle, L.A.; Dugan, B.; Ohlson, M. Effect of Environmental Exposure on Charcoal Density and Porosity in a Boreal Forest. Sci. Total Environ. 2017, 592, 316–325. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Qin, Y.; Wei, J.; Liu, H. Effect of Torrefaction on the Properties of Rice Straw High Temperature Pyrolysis Char: Pore Structure, Aromaticity and Gasification Activity. Bioresour. Technol. 2017, 228, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Ashek-E-Khoda, S.; Sayeed, M.A.; Suruzzaman; Paul, D.; Dhar, S.A.; Grammatikos, S.A. On the Use of Wood Charcoal Filler to Improve the Properties of Natural Fiber Reinforced Polymer Composites. Mater. Today Proc. 2021, 44, 926–929. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Lu, W.; Khan, M.U.; Xu, H.; Yi, W.; Lei, H.; Huo, E.; Qian, M.; Zhao, Y.; et al. Production of High-Density Polyethylene Biocomposites from Rice Husk Biochar: Effects of Varying Pyrolysis Temperature. Sci. Total Environ. 2020, 738, 139910. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G. Microencapsulation of Ammonium Polyphosphate with Melamine-Formaldehyde-Tris(2-Hydroxyethyl)Isocyanurate Resin and Its Flame Retardancy in Polypropylene. RSC Adv. 2015, 5, 88445–88455. [Google Scholar] [CrossRef]
- Li, D.-C.; Jiang, H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.; Hu, W.; Lian, J.; Hu, Y. Influence of Ammonium Polyphosphate Microencapsulation on Flame Retardancy, Thermal Degradation and Crystal Structure of Polypropylene Composite. Compos. Sci. Technol. 2013, 81, 17–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, J.; Feng, Y.; Feng, W. Assembly of Graphene-Aligned Polymer Composites for Thermal Conductive Applications. Compos. Commun. 2018, 9, 33–41. [Google Scholar] [CrossRef]
- Lila, M.K.; Shukla, K.; Komal, U.K.; Singh, I. Accelerated Thermal Ageing Behaviour of Bagasse Fibers Reinforced Poly (Lactic Acid) Based Biocomposites. Compos. B Eng. 2019, 156, 121–127. [Google Scholar] [CrossRef]
- González-López, M.E.; Martín del Campo, A.S.; Robledo-Ortíz, J.R.; Arellano, M.; Pérez-Fonseca, A.A. Accelerated Weathering of Poly(Lactic Acid) and Its Biocomposites: A Review. Polym. Degrad. Stab. 2020, 179, 109290. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Tanaka, T.; Hayakawa, Y. Nucleating and Plasticization Effects in Drawn Poly(Lactic Acid) Fiber during Accelerated Weathering Degradation. Polymers 2018, 10, 365. [Google Scholar] [CrossRef]
- Zheng, G.; Kang, X.; Ye, H.; Fan, W.; Sonne, C.; Lam, S.S.; Liew, R.K.; Xia, C.; Shi, Y.; Ge, S. Recent Advances in Functional Utilisation of Environmentally Friendly and Recyclable High-Performance Green Biocomposites: A Review. Chin. Chem. Lett. 2023, 108817. [Google Scholar] [CrossRef]
- Naveen, J.; Jawaid, M.; Zainudin, E.S.; Sultan, M.T.H.; Yahaya, R.; Abdul Majid, M.S. Thermal Degradation and Viscoelastic Properties of Kevlar/Cocos Nucifera Sheath Reinforced Epoxy Hybrid Composites. Compos. Struct. 2019, 219, 194–202. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Ma, C.; Chang, P.; Yao, F.; Zhu, Y.; Zhang, C.; Wan, Y. Mechanical and Thermo-Mechanical Behaviors of Sizing-Treated Corn Fiber/Polylactide Composites. Polym. Test. 2014, 39, 45–52. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Luchesi, B.R.; Dantas, A.P.S.; Vanderlei, R.M.; Claro, P.C.; Neto, A.R.d.S.; Mattoso, L.H.C.; Martins, M.A. Thermo-Physical and Mechanical Characteristics of Composites Based on High-Density Polyethylene (HDPE) e Spent Coffee Grounds (SCG). J. Polym. Env. 2021, 29, 2888–2900. [Google Scholar] [CrossRef]
- Akderya, T.; Özmen, U.; Baba, B.O. Investigation of Long-Term Ageing Effect on the Thermal Properties of Chicken Feather Fibre/Poly(Lactic Acid) Biocomposites. J. Polym. Res. 2020, 27, 162. [Google Scholar] [CrossRef]
- Hassan, M.M.; Koyama, K. Thermomechanical and Viscoelastic Properties of Green Composites of PLA Using Chitin Micro-Particles as Fillers. J. Polym. Res. 2020, 27, 27. [Google Scholar] [CrossRef]
- Wu, C.-S.; Tsou, C.-H. Fabrication, Characterization, and Application of Biocomposites from Poly(Lactic Acid) with Renewable Rice Husk as Reinforcement. J. Polym. Res. 2019, 26, 44. [Google Scholar] [CrossRef]
- Varley, D.; Yousaf, S.; Youseffi, M.; Mozafari, M.; Khurshid, Z.; Sefat, F. Fiber-Reinforced Composites. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 301–315. [Google Scholar]
- Anand, P.; Rajesh, D.; Senthil Kumar, M.; Saran Raj, I. Investigations on the Performances of Treated Jute/Kenaf Hybrid Natural Fiber Reinforced Epoxy Composite. J. Polym. Res. 2018, 25, 94. [Google Scholar] [CrossRef]
- Delatorre, F.M.; Cupertino, G.F.M.; Junior, A.J.d.S.; Silva, Á.M.d.; Júnior, A.F.D.; Silveira, M.P.R. Insights Acerca Do Uso de Finos de Carvão Vegetal Para Geração de Bioenergia. Agropecuária Científica No Semiárido 2020, 16, 138. [Google Scholar] [CrossRef]
- Prusek, J.; Boruvka, M.; Lenfeld, P. Natural Aerobic Degradation of Polylactic Acid (Composites) with Natural Fiber Additives. Mater. Sci. Forum 2018, 919, 167–174. [Google Scholar] [CrossRef]
- Batista, M.A.J.; Moraes, R.P.; Barbosa, J.C.S.; Oliveira, P.C.; Santos, A.M. Effect of the Polyester Chemical Structure on the Stability of Polyester–Melamine Coatings When Exposed to Accelerated Weathering. Prog. Org. Coat. 2011, 71, 265–273. [Google Scholar] [CrossRef]
- Al-Kadhemy, M.F.H.; Rasheed, Z.S.; Salim, S.R. Fourier Transform Infrared Spectroscopy for Irradiation Coumarin Doped Polystyrene Polymer Films by Alpha Ray. J. Radiat. Res. Appl. Sci. 2016, 9, 321–331. [Google Scholar] [CrossRef]
- Sullalti, S.; Totaro, G.; Askanian, H.; Celli, A.; Marchese, P.; Verney, V.; Commereuc, S. Photodegradation of TiO2 Composites Based on Polyesters. J. Photochem. Photobiol. A Chem. 2016, 321, 275–283. [Google Scholar] [CrossRef]
- Hermán, V.; Takacs, H.; Duclairoir, F.; Renault, O.; Tortai, J.H.; Viala, B. Core Double–Shell Cobalt/Graphene/Polystyrene Magnetic Nanocomposites Synthesized by in Situ Sonochemical Polymerization. RSC Adv. 2015, 5, 51371–51381. [Google Scholar] [CrossRef]
- Ali, F.; Ishfaq, N.; Said, A.; Nawaz, Z.; Ali, Z.; Ali, N.; Afzal, A.; Bilal, M. Fabrication, Characterization, Morphological and Thermal Investigations of Functionalized Multi-Walled Carbon Nanotubes Reinforced Epoxy Nanocomposites. Prog. Org. Coat. 2021, 150, 105962. [Google Scholar] [CrossRef]
- Koto, N.; Soegijono, B. Effect of Rice Husk Ash Filler of Resistance Against of High-Speed Projectile Impact on Polyester-Fiberglass Double Panel Composites. J. Phys. Conf. Ser. 2019, 1191, 012058. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delatorre, F.M.; Cupertino, G.F.M.; Pereira, A.K.S.; de Souza, E.C.; da Silva, Á.M.; Ucella Filho, J.G.M.; Saloni, D.; Profeti, L.P.R.; Profeti, D.; Dias Júnior, A.F. Photoluminous Response of Biocomposites Produced with Charcoal. Polymers 2023, 15, 3788. https://doi.org/10.3390/polym15183788
Delatorre FM, Cupertino GFM, Pereira AKS, de Souza EC, da Silva ÁM, Ucella Filho JGM, Saloni D, Profeti LPR, Profeti D, Dias Júnior AF. Photoluminous Response of Biocomposites Produced with Charcoal. Polymers. 2023; 15(18):3788. https://doi.org/10.3390/polym15183788
Chicago/Turabian StyleDelatorre, Fabíola Martins, Gabriela Fontes Mayrinck Cupertino, Allana Katiussya Silva Pereira, Elias Costa de Souza, Álison Moreira da Silva, João Gilberto Meza Ucella Filho, Daniel Saloni, Luciene Paula Roberto Profeti, Demetrius Profeti, and Ananias Francisco Dias Júnior. 2023. "Photoluminous Response of Biocomposites Produced with Charcoal" Polymers 15, no. 18: 3788. https://doi.org/10.3390/polym15183788
APA StyleDelatorre, F. M., Cupertino, G. F. M., Pereira, A. K. S., de Souza, E. C., da Silva, Á. M., Ucella Filho, J. G. M., Saloni, D., Profeti, L. P. R., Profeti, D., & Dias Júnior, A. F. (2023). Photoluminous Response of Biocomposites Produced with Charcoal. Polymers, 15(18), 3788. https://doi.org/10.3390/polym15183788








