Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls: A Study on Preparation and Sustained Release Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Temperature-Sensitive Composite Wall Material Preparation
2.3. Preparation of Fragrance Microcapsules with Double Capsule Walls
2.4. Aromatic Fabric Prepared with Fragrance Microcapsules
2.5. Characterizations
2.5.1. Structural Analysis
2.5.2. Thermogravimetric Analysis and Differential Scanning Calorimetry Analysis
2.5.3. Yield and Encapsulation Efficiency of Microencapsulation
2.5.4. Stability Testing
2.5.5. Sustained Release Properties and Mechanism Analysis
3. Results and Discussion
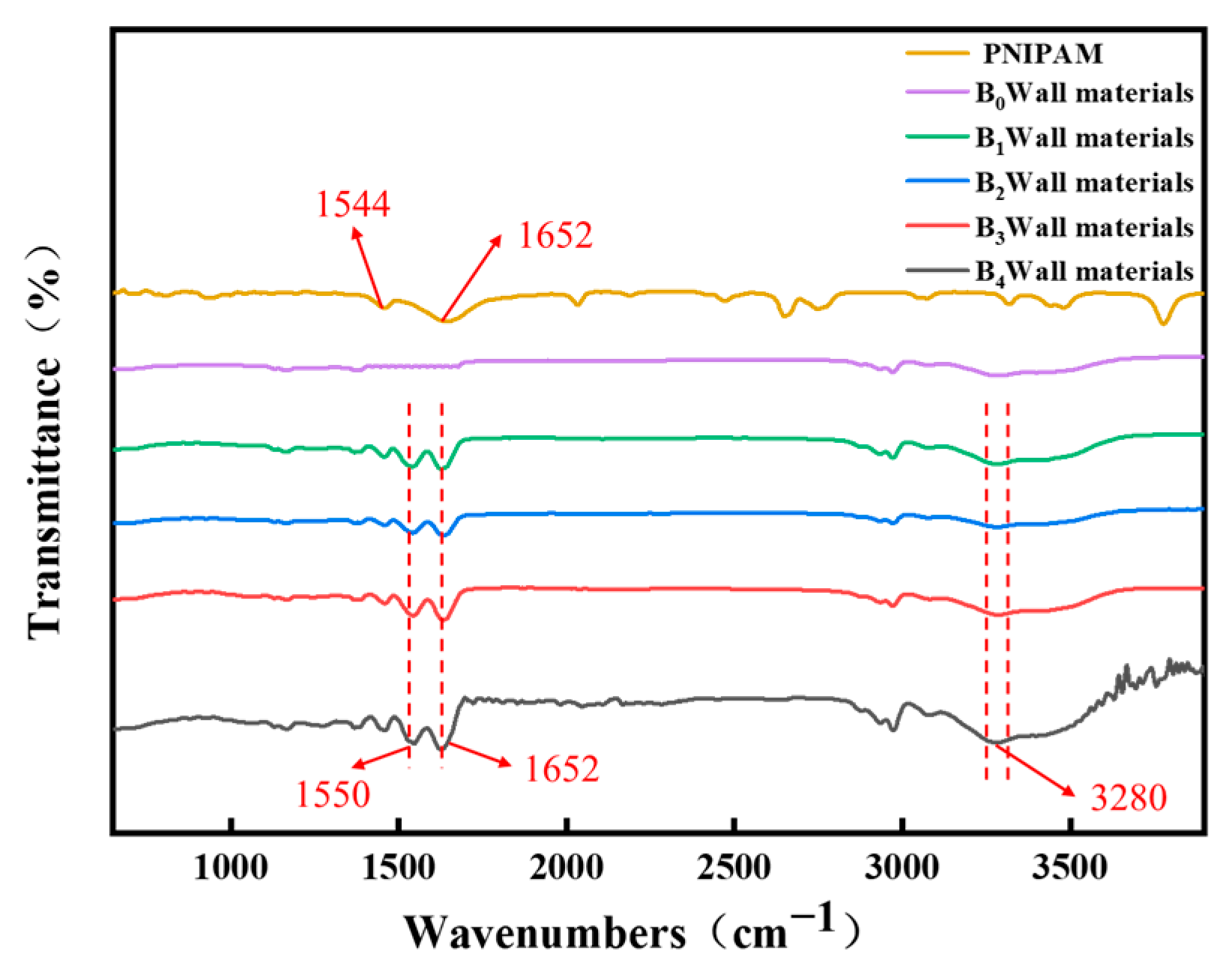
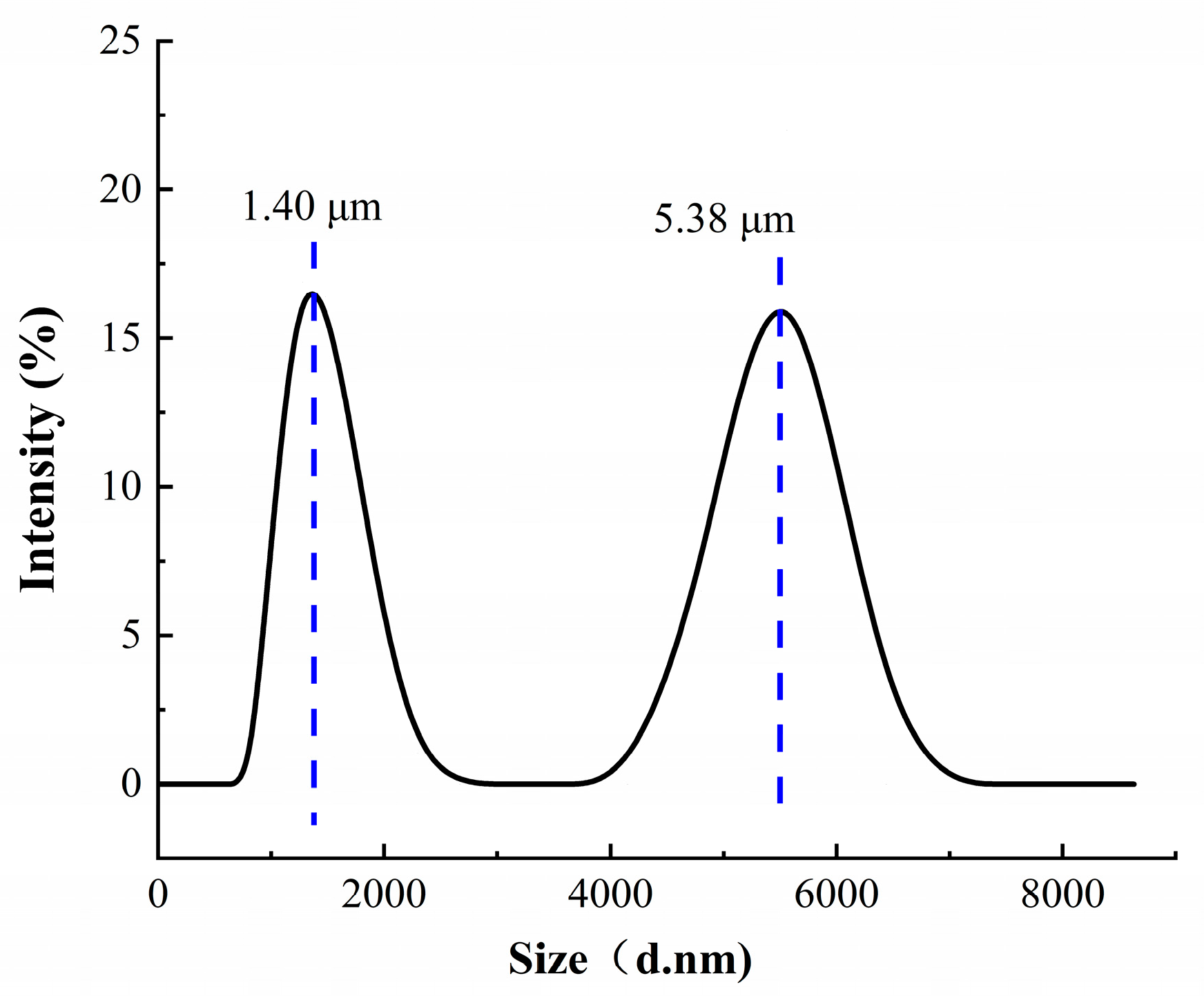
3.1. Structure and Characteristics of Temperature-Sensitive Composite Materials
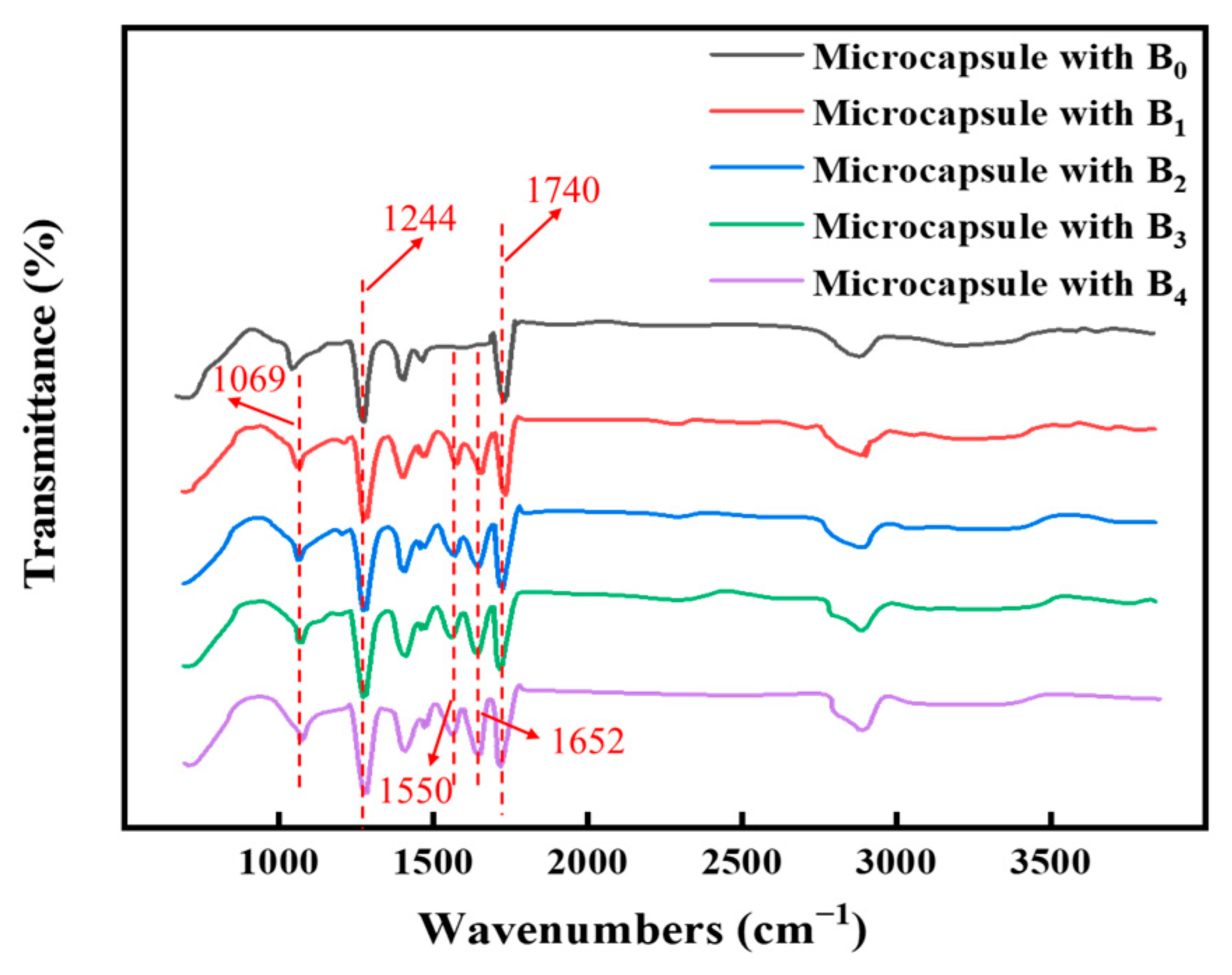
3.2. Structure, Yield and Encapsulation Efficiency of Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls
3.3. Thermal Performance of Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls
3.4. Stability of Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls
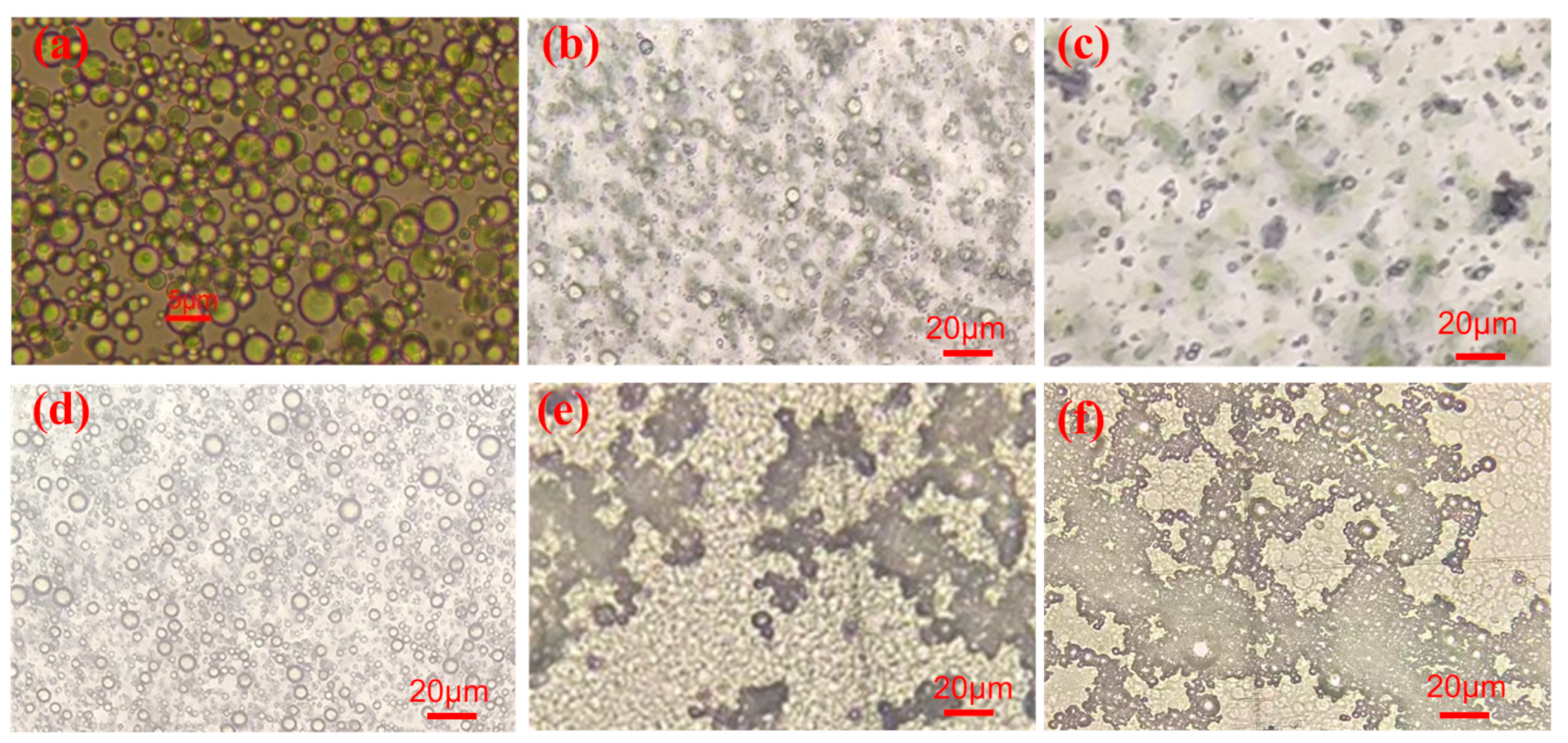
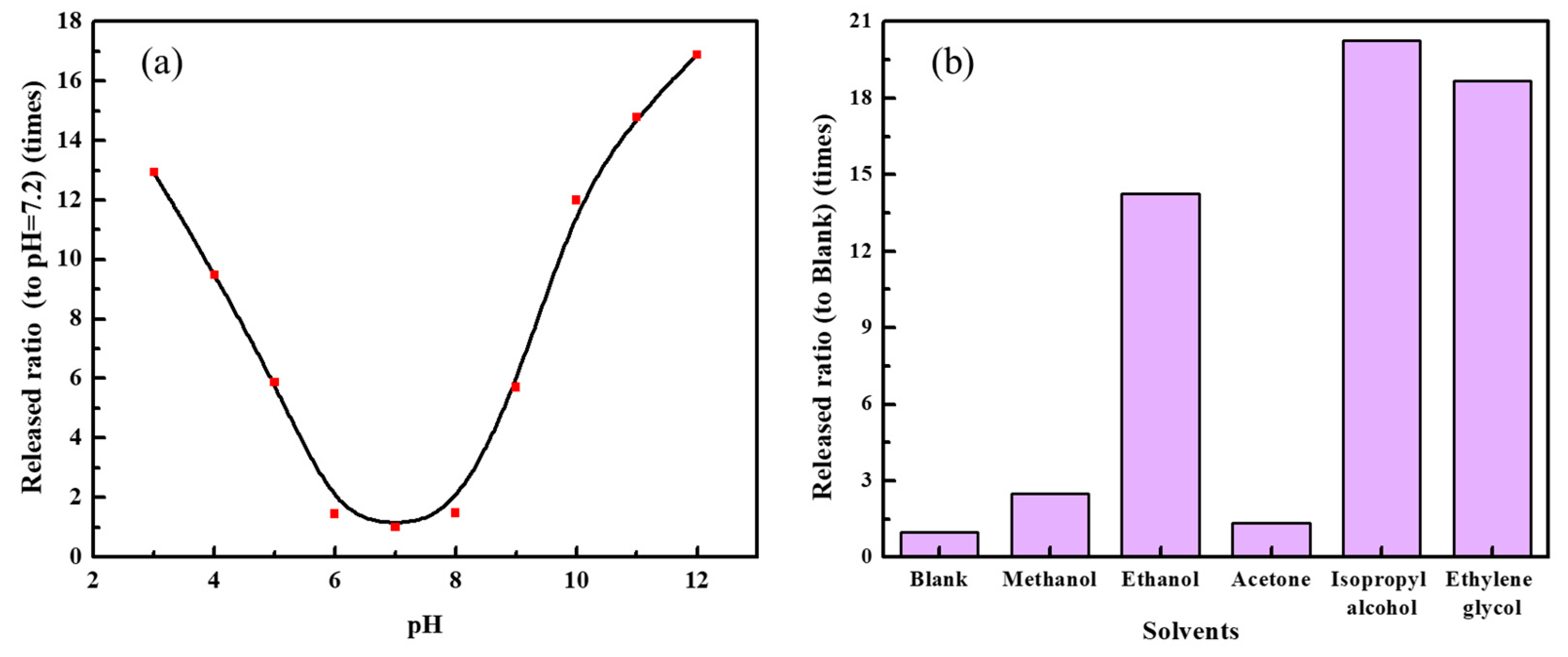
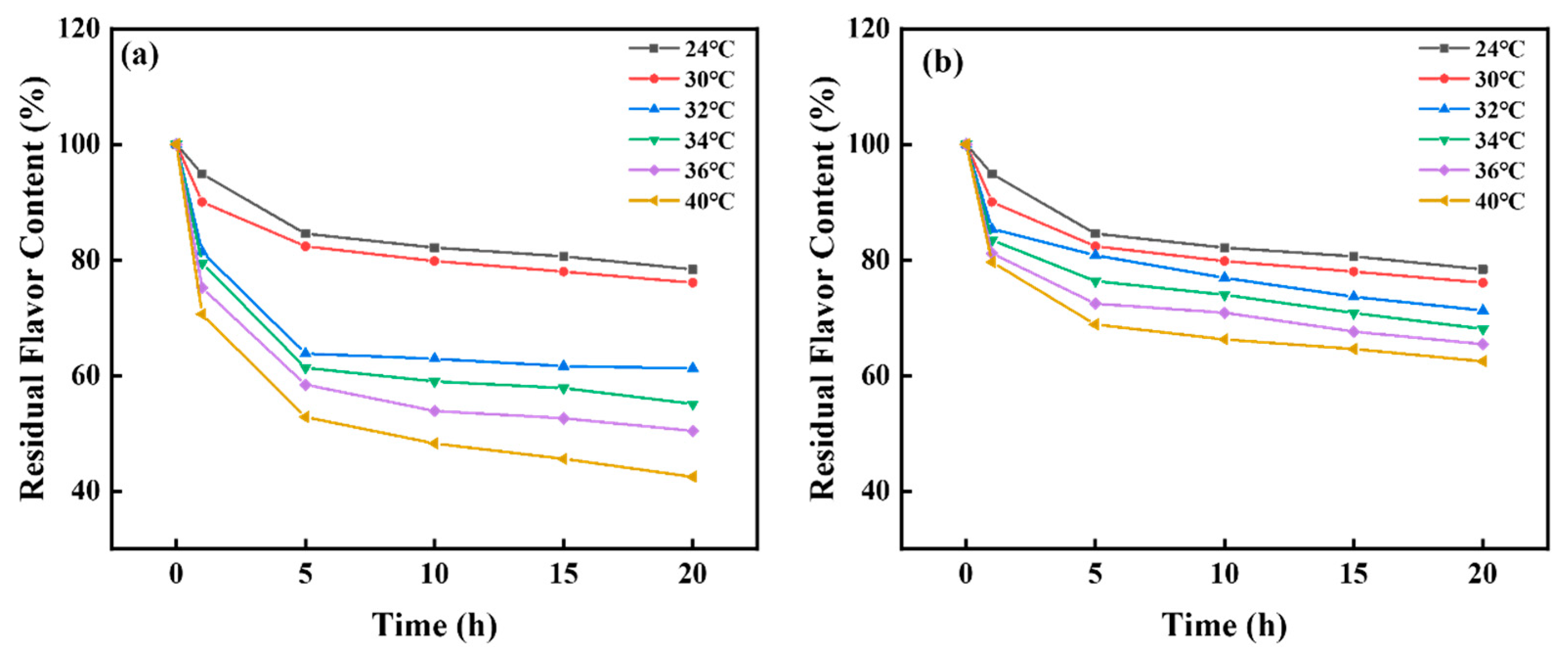
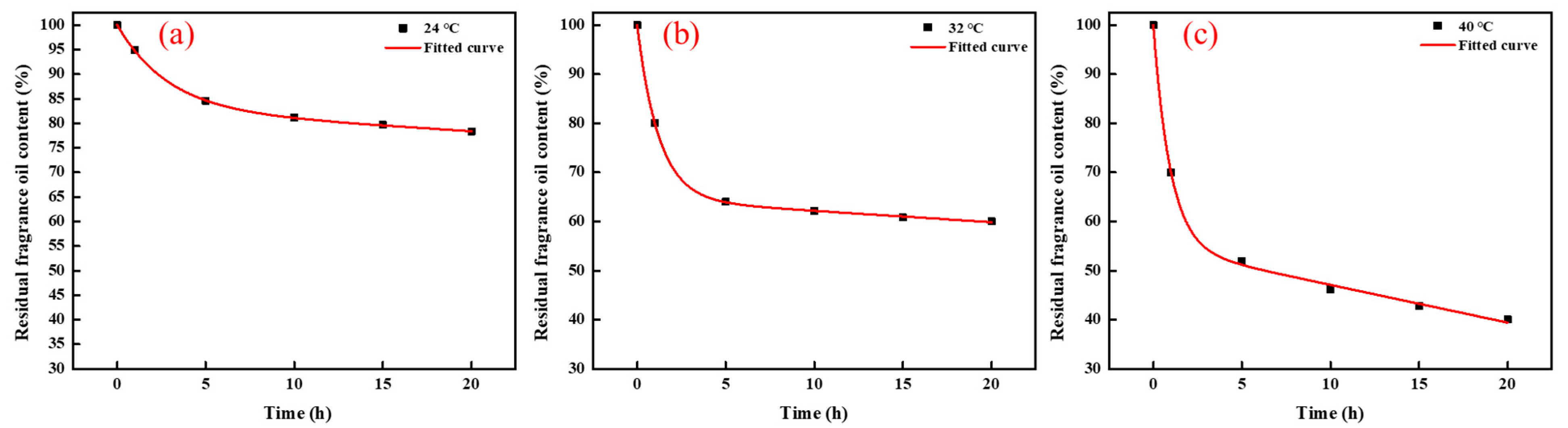
3.5. Microstructure, Sustained Release Property and Mechanism Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, R. Microencapsulation technology and applications. Defence Sci. J. 2009, 59, 82–95. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=e205cf57fda2024156582660d59d6614cc936055 (accessed on 1 January 2009).
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends. Food. Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Poshadri, A.; Aparna, K. Microencapsulation technology: A review. J. Res. Angrau 2010, 38, 86–102. Available online: http://oar.icrisat.org/id/eprint/6375 (accessed on 1 June 2018).
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Specos, M.M.M.; Escobar, G.; Marino, P.; Puggia, C.; Tesoriero, M.V.D.; Hermida, L. Aroma finishing of cotton fabrics by means of microencapsulation techniques. J. Ind. Text. 2010, 40, 13–32. [Google Scholar] [CrossRef]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Muangsin, N.; Shah, A.A.; Prapasarakul, N. The efficacy of three double-microencapsulation methods for preservation of probiotic bacteria. Sci. Rep. 2021, 11, 13753. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ju, X.-J.; Xie, R.; Mou, C.-L.; Lin, X.; Chu, L.-Y. Novel cationic pH-responsive poly(N,N-dimethylaminoethyl methacrylate) microcapsules prepared by a microfluidic technique. J. Colloid Interface Sci. 2011, 357, 101–108. [Google Scholar] [CrossRef]
- Tian, K.; Zeng, J.; Zhao, X.; Liu, L.; Jia, X.; Liu, P. Synthesis of multi-functional nanocapsules via interfacial AGET ATRP in miniemulsion for tumor micro-environment responsive drug delivery. Colloids Surface B 2015, 134, 188–195. [Google Scholar] [CrossRef]
- Narita, T.; Takakura, H.; Ogata, N.; Kawakita, H.; Oishi, Y. Self-pulsation observed in pH-sensitive microcapsules. Chem. Commun. 2013, 49, 919–921. [Google Scholar] [CrossRef]
- Mak, W.; Olesen, K.; Sivlér, P.; Lee, C.; Moreno-Jimenez, I.; Edin, J.; Courtman, D.; Skog, M.; Griffith, M. Controlled delivery of human cells by temperature responsive microcapsules. J. Func. Biomater. 2015, 6, 439–453. [Google Scholar] [CrossRef]
- Xiao, D.; Liang, W.; Xie, Z.; Cheng, J.; Du, Y.; Zhao, J. A temperature-responsive release cellulose-based microcapsule loaded with chlorpyrifos for sustainable pest control. J. Hazard. Mater. 2021, 403, 123654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, W.; Guo, K.; Chen, J.; Zhang, P. Synthesis of temperature-responsive poly (N-isopropyl acrylamide)/poly (methyl methacrylate)/silica hybrid capsules from inverse pickering emulsion polymerization and their application in controlled drug release. Langmuir 2010, 26, 7971–7980. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, L.; He, S.; Zhang, J.; Shao, W. Recent progress in PNIPAM-based multi-responsive actuators: A mini-review. Chem. Eng. J. 2022, 433, 133496. [Google Scholar] [CrossRef]
- Poly, R.P. Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J. Colloid Interface Sci. 2010, 348, 673–674. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Cheng, Q.; Jiang, L. A Strong Bio-Inspired Layered PNIPAM-Clay Nanocomposite Hydrogel. Angew. Chem. Int. Ed. 2012, 51, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2018, 121, 1276–1286. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.; Hu, J.; Zhang, J.; Heng, T.; Xu, C.; Wang, X.; Liu, J.; Yu, K. Preparation of pH-responsive dual-compartmental microcapsules via pickering emulsion and their application in multifunctional textiles. ACS Appl. Mater. Interfaces 2021, 13, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, M.; Li, L.; Zhou, L.; He, L.; Deng, W.; Hu, J. Osmanthus fragrance polyurethane/silk fibroin-based double-shell microcapsules for aromatic leather with sustained release fragrance. Flavour Fragr. J. 2023, 38, 73–82. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Microencapsulation and its application in production of functional textiles. Indian J. Fibre Text. Res. 2020, 45, 495–509. [Google Scholar]
- Kert, M.; Tavčer, P.F.; Hladnik, A.; Spasić, K.; Puač, N.; Petrović, Z.L.; Gorjanc, M. Application of fragrance microcapsules onto cotton fabric after treatment with oxygen and nitrogen plasma. Coatings 2021, 11, 1181. [Google Scholar] [CrossRef]
- Blaiszik, B.; Caruso, M.; McIlroy, D.; Moore, J.; White, S.; Sottos, N. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Liu, Y.; Chen, M.; Hu, Y.; Yang, Z. Study on the grafting of chitosan–gelatin microcapsules onto cotton fabrics and its antibacterial effect. Colloid Surface B 2013, 109, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Wickramasinghe, S. Photo-induced graft polymerization of N-isopropyl acrylamide on thin film composite membrane: Produced water treatment and antifouling properties. Sep. Purif. Technol. 2012, 90, 231–238. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Ahmed, E.; Naseem, K.; Ashraf, S.; Sharif, A.; Rehan, R. Catalytic reduction of 4-nitrophenol using silver nanoparticles-engineered poly (N-isopropylacrylamide-co-acrylamide) hybrid microgels. Appl. Organomet. Chem. 2017, 31, e3563. [Google Scholar] [CrossRef]
- Othman, M.B.H.; Khan, A.; Ahmad, Z.; Zakaria, M.R.; Ullah, F.; Akil, H.M. Kinetic investigation and lifetime prediction of Cs–NIPAM–MBA-based thermo-responsive hydrogels. Carbohyd. Polym. 2016, 136, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Mukherjee, M.; Sen, A.K. Studies on thermal and swelling properties of Poly (NIPAM-co-2-HEA) based hydrogels. Adv. Mater. Res. 2012, 1, 269. [Google Scholar] [CrossRef]
- Al-Khafaji, M.A.; Gaál, A.; Wacha, A.; Bóta, A.; Varga, Z. Particle size distribution of bimodal silica nanoparticles: A comparison of different measurement techniques. Materials 2020, 13, 3101. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yang, Q.; Huang, L.; Chen, L.; Ni, Y.; Xiao, H. Temperature and pH responsive cellulose filament/poly (NIPAM-co-AAc) hybrids as novel adsorbent towards Pb (II) removal. Carbohyd. Polym. 2018, 195, 495–504. [Google Scholar] [CrossRef]
- Singhvi, G.; Singh, M. In-vitro drug release characterization models. Int. J. Pharm. Stud. Res. 2012, 2, 77–84. Available online: https://www.researchgate.net/publication/285447890 (accessed on 1 January 2011).
- Gohel, M.C.; Panchal, M.K.; Jogani, V.V. Novel mathematical method for quantitative expression of deviation from the Higuchi model. AAPS PharmSciTech. 2000, 1, 43–48. [Google Scholar] [CrossRef]






| Sample | NIPAM (g) | Gum Arabic (g) | SDS (g) |
|---|---|---|---|
| B0 | 0 | 0.1 | 0.05 |
| B1 | 0.5 | 0.1 | 0.05 |
| B2 | 1.0 | 0.1 | 0.05 |
| B3 | 1.5 | 0.1 | 0.05 |
| B4 | 2.0 | 0.1 | 0.05 |
| Sample | Yield (%) | Encapsulation Efficiency (%) |
|---|---|---|
| B0 | 78 ± 0.57 | 59 ± 1.06 |
| B1 | 68 ± 0.63 | 52 ± 1.14 |
| B2 | 74 ± 0.79 | 61 ± 1.12 |
| B3 | 71 ± 0.45 | 55 ± 1.12 |
| B4 | 65 ± 0.89 | 53 ± 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Hu, G.; Qiu, H.; Mia, R.; Zhang, H.; Pei, L.; Wang, J. Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls: A Study on Preparation and Sustained Release Mechanism. Polymers 2023, 15, 3686. https://doi.org/10.3390/polym15183686
Yang Q, Hu G, Qiu H, Mia R, Zhang H, Pei L, Wang J. Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls: A Study on Preparation and Sustained Release Mechanism. Polymers. 2023; 15(18):3686. https://doi.org/10.3390/polym15183686
Chicago/Turabian StyleYang, Qun, Genghao Hu, Huili Qiu, Rajib Mia, Hongjuan Zhang, Liujun Pei, and Jiping Wang. 2023. "Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls: A Study on Preparation and Sustained Release Mechanism" Polymers 15, no. 18: 3686. https://doi.org/10.3390/polym15183686
APA StyleYang, Q., Hu, G., Qiu, H., Mia, R., Zhang, H., Pei, L., & Wang, J. (2023). Temperature-Sensitive Fragrance Microcapsules with Double Capsule Walls: A Study on Preparation and Sustained Release Mechanism. Polymers, 15(18), 3686. https://doi.org/10.3390/polym15183686








