Ni(II) and Pb(II) Removal Using Bacterial Cellulose Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BC Synthesis
2.2.1. Bacterial Growth
2.2.2. Production of Bacterial Cellulose Membranes
2.3. BC Characterization
2.4. Batch Adsorption Tests
2.4.1. Experimental Methodology: Equilibrium Experiments
- pH optimization. Fixed conditions: 25 mL of sample volume, 5 mg·L−1 of initial metal concentration and 400 mg·L−1 of dried BC dosage; variable conditions: operating pH 2–8;
- Dosage of BC optimization. Fixed conditions: 25 mL of sample volume, 5 mg·L−1 of initial metal concentration and operating pH of 4; variable conditions: dried BC dosage 10–400 mg·L−1;
- The initial concentration of metal optimization (isotherm experiments). Fixed conditions: 25 mL of sample volume, operating pH of 4 and 400 mg·L−1 of dried BC dosage; variable conditions: initial metal concentration 1.5–150 mg·L−1 for Pb(II)) and 1.5–175 mg·L−1 for Ni(II).
2.4.2. Experimental Methodology: Equilibrium Experiments
- Temperature effect. Fixed conditions: 25 mL of sample volume, 1.5 mg·L−1 of initial metal concentration, operation pH of 4; 400 mg·L−1 of dried BC dosage; variable conditions: operating temperature 25–50 °C, contact time 1–180 min.
2.4.3. Experimental Methodology: Determination of Metal Concentration
2.4.4. Data Analysis
3. Results and Discussion
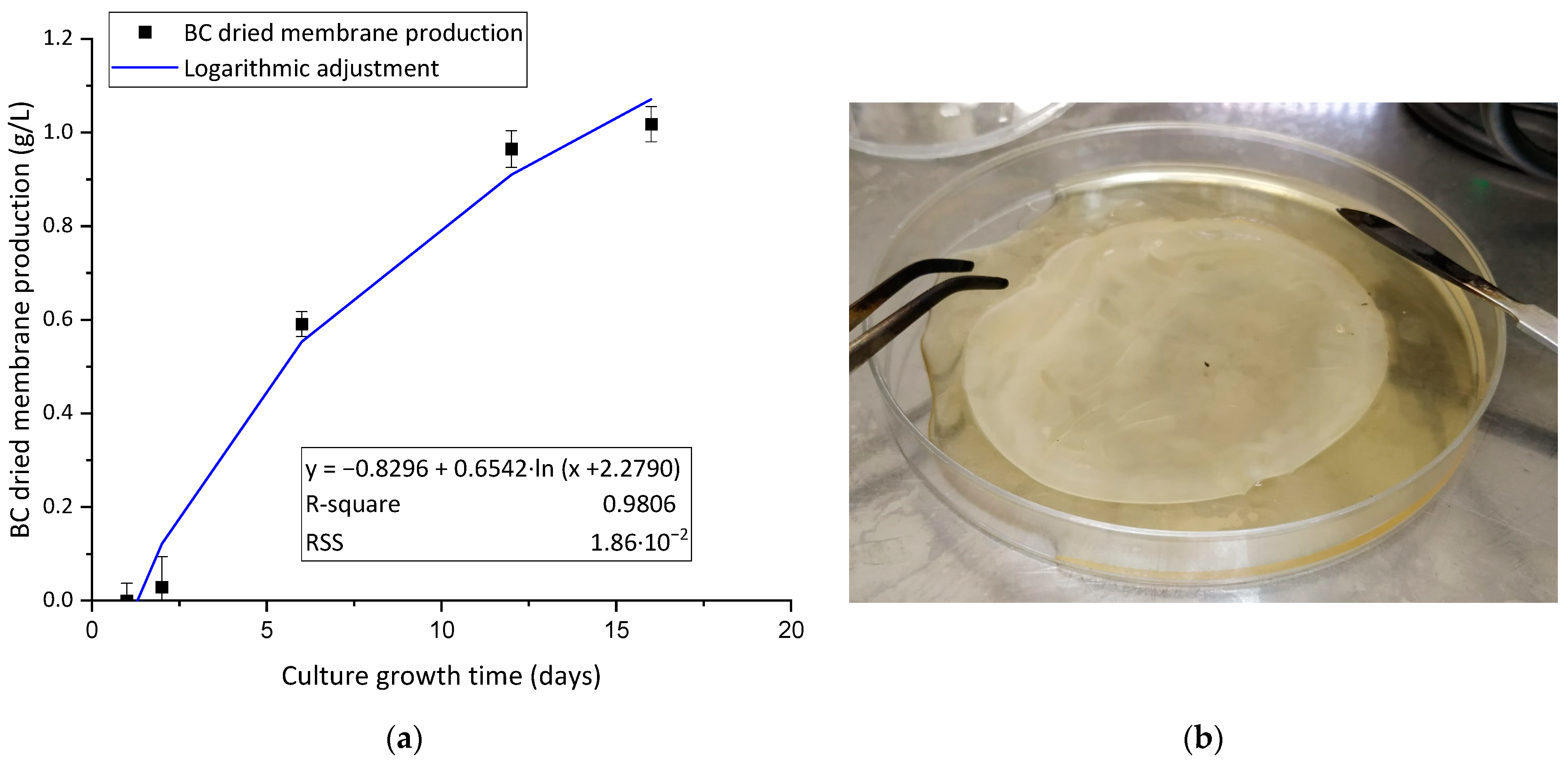
3.1. BC Characterization
3.2. Adsorption Tests Experimental Results
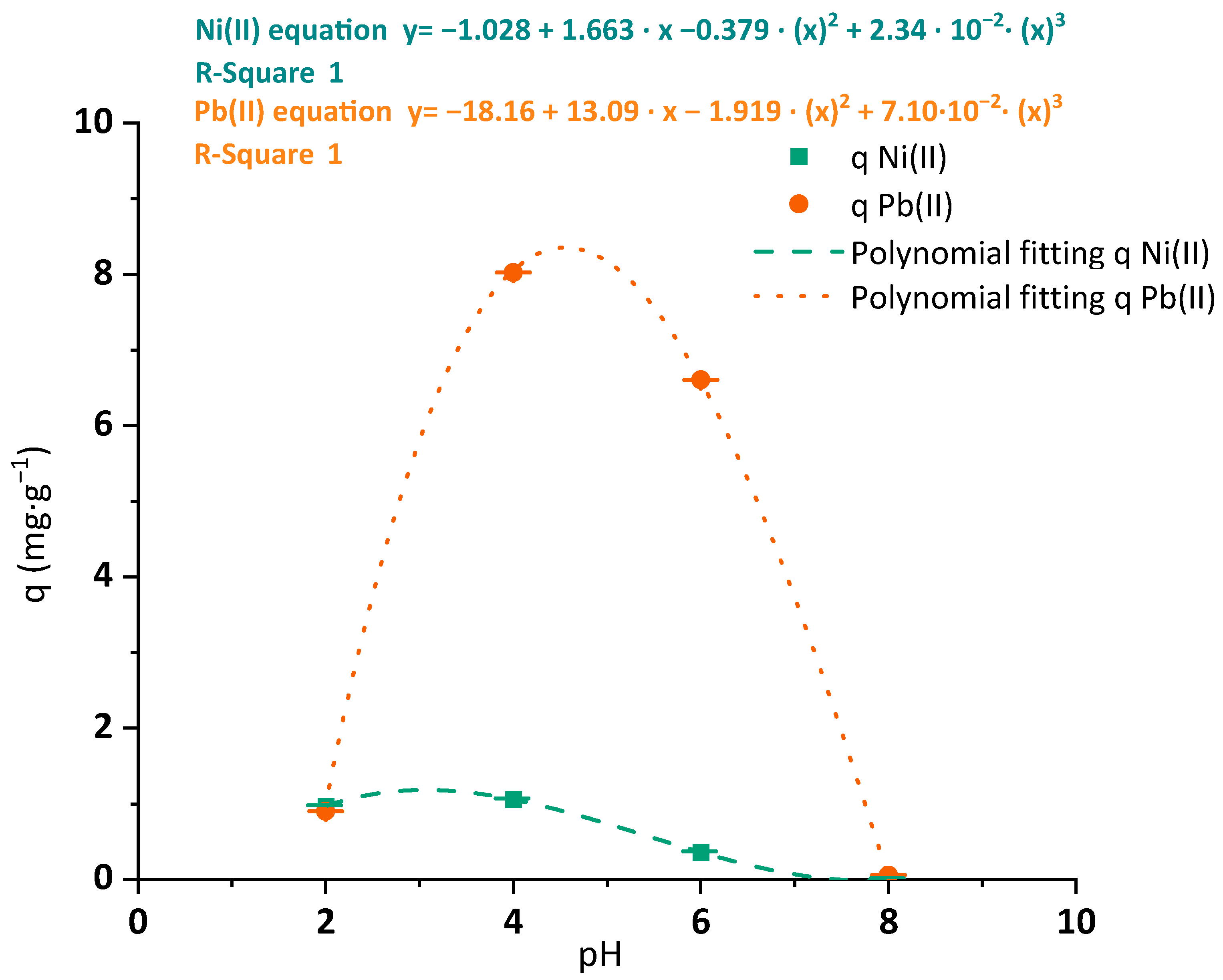
3.2.1. pH Optimization
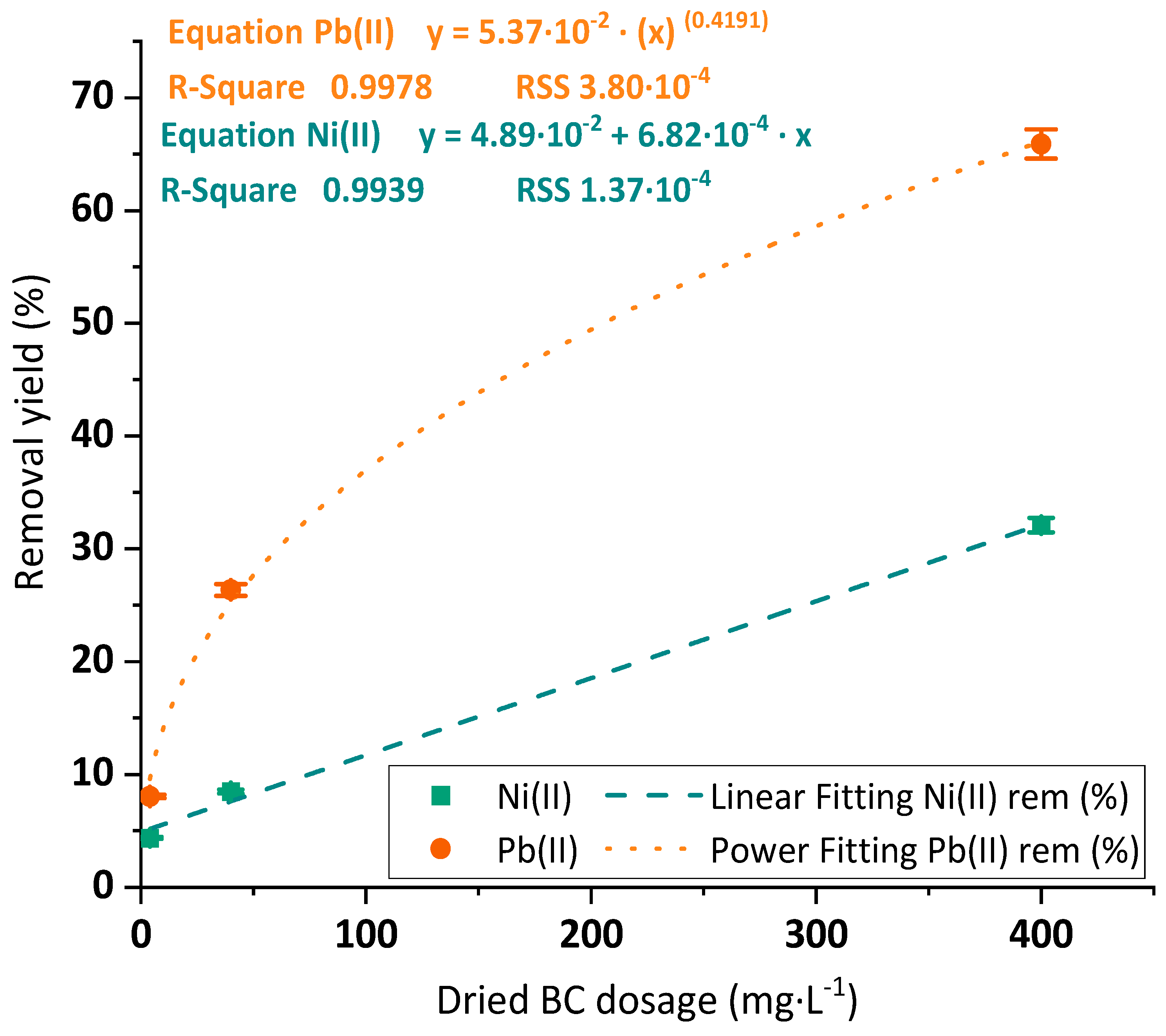
3.2.2. Dosage Optimization
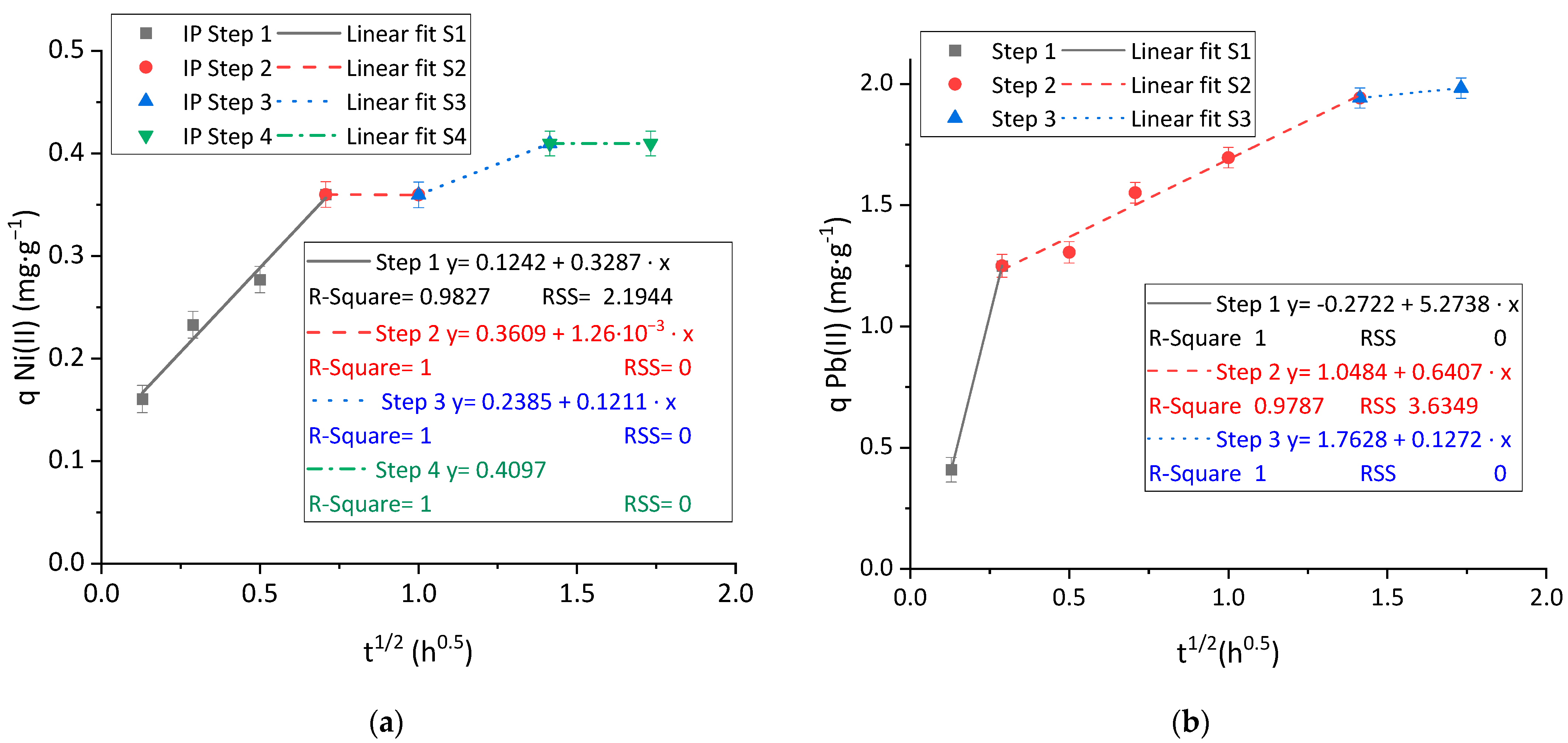
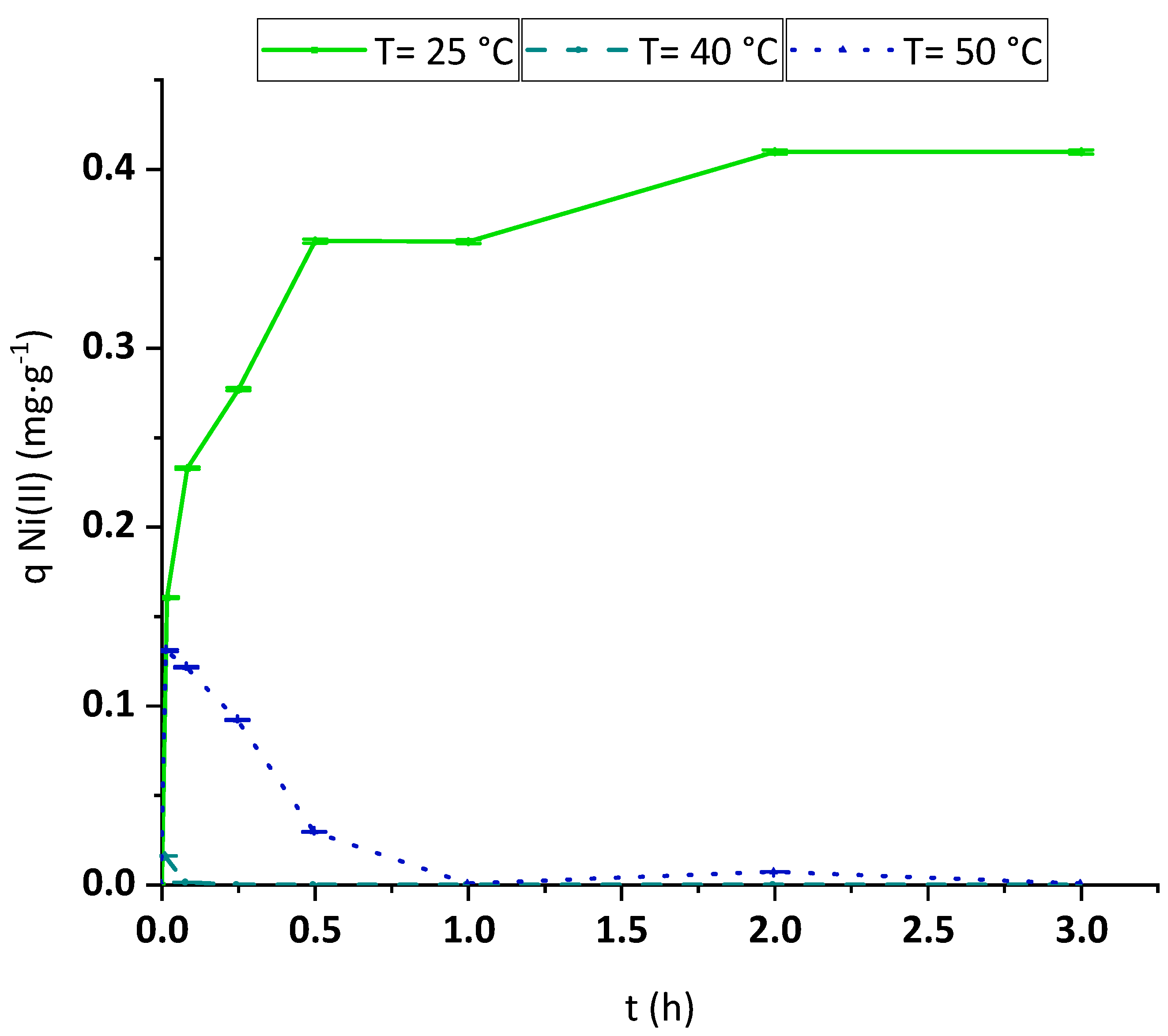
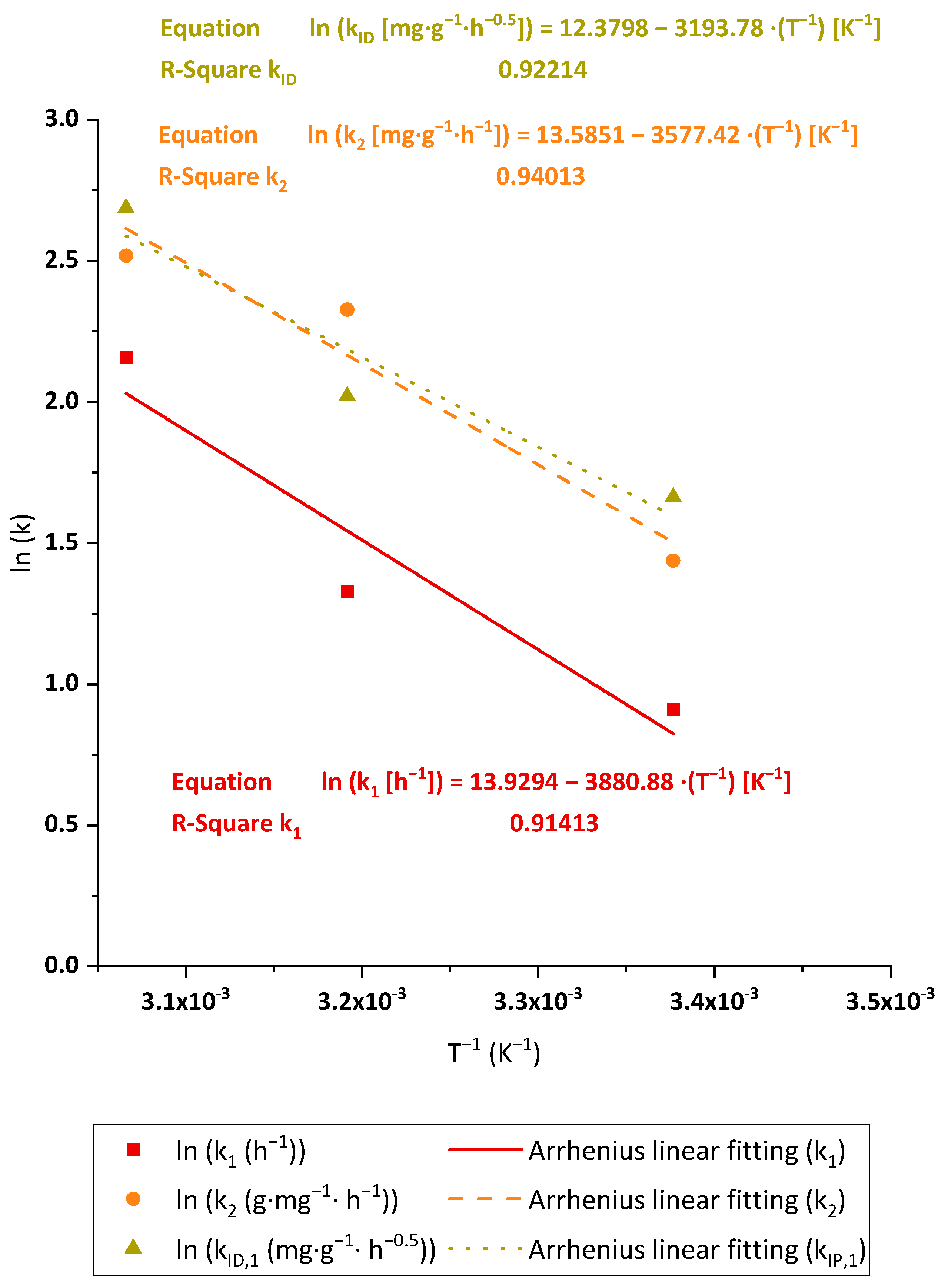
3.2.3. Kinetic Analysis
3.2.4. Temperature Effect
3.2.5. Isotherm Analysis
3.2.6. Adsorption Thermodynamics
3.3. Mechanism Proposal for Ni(II) and Pb(II) Removal through BC Membranes
3.4. Advantages and Disadvantages of BC Implementation for Ni(II) and Pb(II) Treatment in Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Li, X.; Xiong, Z.; Wang, M.; Liu, Q. Environmental Pollution Effect Analysis of Lead Compounds in China Based on Life Cycle. Int. J. Environ. Res. Public. Health 2020, 17, 2184. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Gul, T. Global EV Outlook 2023; International Energy Agency: Paris, France, 2023; 140p. [Google Scholar]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Muñoz, P.Q. Hazardous Substances and Limits of Discharge for Indirect Dumpings in Autonomic Legislation; FCCA: Puerto de la Cruz, Spain, 2015; 45p. (In Spanish) [Google Scholar]
- Kumar, V.; Dwivedi, S.K. A review on accessible techniques for removal of hexavalent Chromium and divalent Nickel from industrial wastewater: Recent research and future outlook. J. Clean. Prod. 2021, 295, 126229. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Qiao, A.; Cui, M.; Huang, R.; Ding, G.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef]
- Minceva, M.; Fajgar, R.; Markovska, L.; Meshko, V. Comparative Study of Zn2+, Cd2+, and Pb2+ Removal from Water Solution Using Natural Clinoptilolitic Zeolite and Commercial Granulated Activated Carbon. Equilibrium of Adsorption. Sep. Sci. Technol. 2008, 43, 2117–2143. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Sridevi, M.; Nirmala, C.; Jawahar, N.; Arthi, G.; Vallinayagam, S.; Sharma, V.K. Role of nanomaterial’s as adsorbent for heterogeneous reaction in waste water treatment. J. Mol. Struct. 2021, 1241, 130596. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sindhu, R.; Sirohi, R.; Awasthi, M.K.; Pandey, A.; Binod, P. Nanocellulose as green material for remediation of hazardous heavy metal contaminants. J. Hazard. Mater. 2022, 424, 127516. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xu, D.; Luo, L.; Zhou, Y.; Yan, W.; Leng, X.; Dai, D.; Zhou, Y.; Ahmad, H.; Rao, J.; et al. Overview of nanocellulose as additives in paper processing and paper products. Nanotechnol. Rev. 2021, 10, 264–281. [Google Scholar] [CrossRef]
- Faiz Norrrahim, M.N.; Mohd Kasim, N.A.; Knight, V.F.; Mohamad Misenan, M.S.; Janudin, N.; Ahmad Shah, N.A.; Kasim, N.; Wan Yusoff, W.Y.; Mohd Noor, S.A.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Chawla, P.; Bajaj, I.; Survase, S.; Singhal, R. Microbial Cellulose: Fermentative Production and Applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Ashjaran, A.; Yazdanshenas, M.E.; Rashidi, A.; Khajavi, R.; Rezaee, A. Overview of bio nanofabric from bacterial cellulose. J. Text. Inst. 2013, 104, 121–131. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, S.Y.; Shi, S.K.; Li, X.; Zhang, X.; Hu, W.L.; Wang, H.P. Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr. Polym. 2009, 75, 110–114. [Google Scholar] [CrossRef]
- Chen, S.Y.; Shen, W.; Yu, F.; Hu, W.L.; Wang, H.P. Preparation of Amidoximated Bacterial Cellulose and Its Adsorption Mechanism for Cu2+ and Pb2+. J. Appl. Polym. Sci. 2010, 117, 8–15. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. Bacterial cellulose of Gluconoacetobacter hansenii as a potential bioadsorption agent for its green environment applications. J. Biomater. Sci. Polym. Ed. 2014, 25, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Carbajo, J.; Villar, J. The Effect of Carbon and Nitrogen Sources on Bacterial Cellulose Production and Properties from Gluconacetobacter sucrofermentans CECT 7291 Focused on its use in Degraded Paper Restoration. Bioresources 2013, 8, 3630. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Gómez, N.; Ladero, M.; Villar, J.C. Modification of Bacterial Cellulose Biofilms with Xylan Polyelectrolytes. Bioengineering 2017, 4, 93. [Google Scholar] [CrossRef]
- Seniya, D.; Verma, S.; Trivedia, S.; Verma, R.; Vijayarti, H.S.; Vyas, S. Metal Stress and Antibiotic Susceptibility Profileof Some Bacterial and Fungal Strains. J. Pure Appl. Microbiol. 2012, 6, 1727–1734. [Google Scholar]
- Balea, A.; Sanchez-Salvador, J.L.; Monte, M.C.; Merayo, N.; Negro, C.; Blanco, A. In Situ Production and Application of Cellulose Nanofibers to Improve Recycled Paper Production. Molecules 2019, 24, 1800. [Google Scholar] [CrossRef]
- Hosseini Talari, M.; Tabrizi, N.S.; Babaeipour, V.; Halek, F. Adsorptive removal of organic pollutants from water by carbon fiber aerogel derived from bacterial cellulose. J. Sol-Gel Sci. Technol. 2022, 101, 345–355. [Google Scholar] [CrossRef]
- Ojembarrena, F.d.B.; Sammaraie, H.; Campano, C.; Blanco, A.; Merayo, N.; Negro, C. Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals. Nanomaterials 2022, 12, 4172. [Google Scholar] [CrossRef] [PubMed]
- DIN 38402-51:2017-05; Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung–Allgemeine Angaben (Gruppe A)–Teil 51: Kalibrierung von Analysenverfahren–Lineare Kalibrierfunktion (A 51). DIN: Berlin, Germany, 2017.
- DIN 32645:2008-11; Chemische Analytik—Nachweis-, Erfassungs- und Bestimmungsgrenze unter Wiederholbedingungen—Begriffe, Verfahren, Auswertung. DIN: Berlin, Germany, 2008.
- DIN ISO 5725-1:1994-12; Accuracy (trueness and precision) of measurement methods and results—Part 1: General principles and definitions. DIN: Berlin, Germany, 1994.
- Ojembarrena, F.d.B.; Sánchez-Salvador, J.L.; Mateo, S.; Balea, A.; Blanco, A.; Merayo, N.; Negro, C. Modeling of Hexavalent Chromium Removal with Hydrophobically Modified Cellulose Nanofibers. Polymers 2022, 14, 3425. [Google Scholar] [CrossRef] [PubMed]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohydr. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of Nanocomposite Film Comprising of Polyvinyl Alcohol (PVA) Incorporated with Bacterial Cellulose Nanocrystals and Magnetite Nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, A. In situ production of bacterial cellulose to economically improve recycled paper properties. Int. J. Biol. Macromol. 2018, 118, 1532–1541. [Google Scholar] [CrossRef]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef]
- Putro, J.; Kurniawan, A.; Ismadji, S.; Ju, Y.-H. Nanocellulose based biosorbents for wastewater treatment: Study of isotherm, kinetic, thermodynamic and reusability. Environ. Nanotechnol. Monit. Manag. 2017, 8, 134–149. [Google Scholar] [CrossRef]
- Dotto, G.L.; Pinto, L.A.A. Adsorption of food dyes acid blue 9 and food yellow 3 onto chitosan: Stirring rate effect in kinetics and mechanism. J. Hazard. Mater. 2011, 187, 164–170. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Miller, A.; Wildeman, T.; Figueroa, L. Zinc and nickel removal in limestone based treatment of acid mine drainage: The relative role of adsorption and co-precipitation. Appl. Geochem. 2013, 37, 57–63. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Balsamo, M.; Montagnaro, F. Liquid–Solid Mass Transfer in Adsorption Systems—An Overlooked Resistance? Ind. Eng. Chem. Res. 2020, 59, 22007–22016. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Gauden, P.; Terzyk, A.P.; Kowalczyk, P.; Aranovich, G.L.; Ćwiertnia, M.; Furmaniak, S.; Rychlicki, G. Giles’ classification of solute adsorption isotherms for binary non-electrolyte solutions via lattice DFT supported by experimental sorption data from aqueous solutions on carbonaceous materials. In Carbon Materials: Theory and Practice; Research Signpost: Kerala, India, 2008; pp. 517–571. [Google Scholar]
- Lawrence, M.A.M.; Davies, N.A.; Edwards, P.A.; Taylor, M.G.; Simkiss, K. Can adsorption isotherms predict sediment bioavailability? Chemosphere 2000, 41, 1091–1100. [Google Scholar] [CrossRef]
- Brandes, R.; Belosinschi, D.; Brouillette, F.; Chabot, B. A new electrospun chitosan/phosphorylated nanocellulose biosorbent for the removal of cadmium ions from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103477. [Google Scholar] [CrossRef]
- Iqbal, D.; Zhao, Y.; Zhao, R.; Russell, S.J.; Ning, X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers 2022, 14, 2343. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Anderson, B.D. Maintenance of supersaturation II: Indomethacin crystal growth kinetics versus degree of supersaturation. J. Pharm. Sci. 2013, 102, 1544–1553. [Google Scholar] [CrossRef]
- Vinogradov, M.I.; Makarov, I.S.; Golova, L.K.; Gromovykh, P.S.; Kulichikhin, V.G. Rheological Properties of Aqueous Dispersions of Bacterial Cellulose. Processes 2020, 8, 423. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Campano, C.; Negro, C.; Monte, M.C.; Blanco, A. Increasing the Possibilities of TEMPO-Mediated Oxidation in the Production of Cellulose Nanofibers by Reducing the Reaction Time and Reusing the Reaction Medium. Adv. Sustain. Syst. 2021, 5, 2000277. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, T.; Huang, X.; Wei, W. Dispersion and beating of bacterial cellulose and their influence on paper properties. BioResources 2016, 11, 9290–9301. [Google Scholar] [CrossRef]






| Model | Parameters [Units] | Values | |
|---|---|---|---|
| Ni(II) | Pb(II) | ||
| Langmuir | kL [L·mg−1] | 2.13 × 10−3 | 3.09 × 10−2 |
| qmax,L [mg·g−1] | 107.29 | 10.14 | |
| R2 | 0.9971 | 0.9886 | |
| RSS | 3.69 | 1.41 | |
| Freundlich | kF [mg(1−1/n)·L(1/n)·g−1] | 1.08 | 0.58 |
| nF | 0.26 | 1.78 | |
| R2 | 0.9947 | 0.9933 | |
| RSS | 7.92 | 1.22 | |
| Sips | kS [L(1/nS)·mol−(1/nS)] | 1.49 × 10−3 | 2.73 × 10−2 |
| nS | 0.69 | 1.50 | |
| qmax,S [mg·g−1] | 39.23 | 20.02 | |
| R2 | 0.9989 | 0.9975 | |
| RSS | 1.45 | 0.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojembarrena, F.d.B.; García, S.; Merayo, N.; Blanco, A.; Negro, C. Ni(II) and Pb(II) Removal Using Bacterial Cellulose Membranes. Polymers 2023, 15, 3684. https://doi.org/10.3390/polym15183684
Ojembarrena FdB, García S, Merayo N, Blanco A, Negro C. Ni(II) and Pb(II) Removal Using Bacterial Cellulose Membranes. Polymers. 2023; 15(18):3684. https://doi.org/10.3390/polym15183684
Chicago/Turabian StyleOjembarrena, Francisco de Borja, Sergio García, Noemi Merayo, Angeles Blanco, and Carlos Negro. 2023. "Ni(II) and Pb(II) Removal Using Bacterial Cellulose Membranes" Polymers 15, no. 18: 3684. https://doi.org/10.3390/polym15183684
APA StyleOjembarrena, F. d. B., García, S., Merayo, N., Blanco, A., & Negro, C. (2023). Ni(II) and Pb(II) Removal Using Bacterial Cellulose Membranes. Polymers, 15(18), 3684. https://doi.org/10.3390/polym15183684








