Naturalized Dyes: A New Opportunity for the Wood Coloring
Abstract
1. Introduction
2. Materials and Methods
2.1. Dyes
2.2. Wood Samples
2.3. Wood Dyeing
2.3.1. Dyeing with Aqueous Solution
2.3.2. Staining
2.3.3. Application of the Clear Top Coat
2.4. Water-Based Dyeing Bath Stability over Time
2.5. Dyeing Capacity
2.6. Color Fastness to Water Washout
2.7. Color Fading in Aging Test
3. Results and Discussion
3.1. UV-Vis Analysis on the Water-Based Dyeing Baths
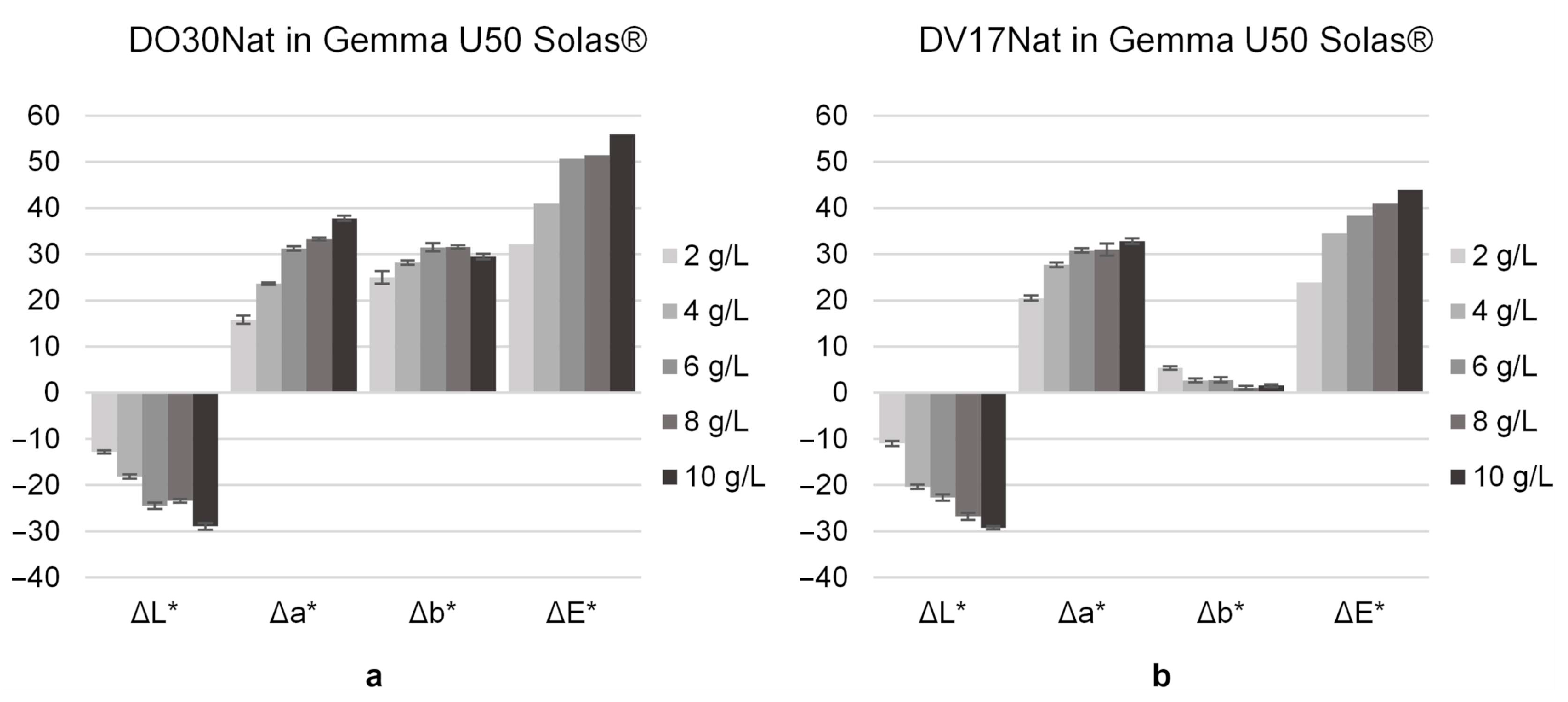
3.2. Effect of ND Concentration on Staining Agent
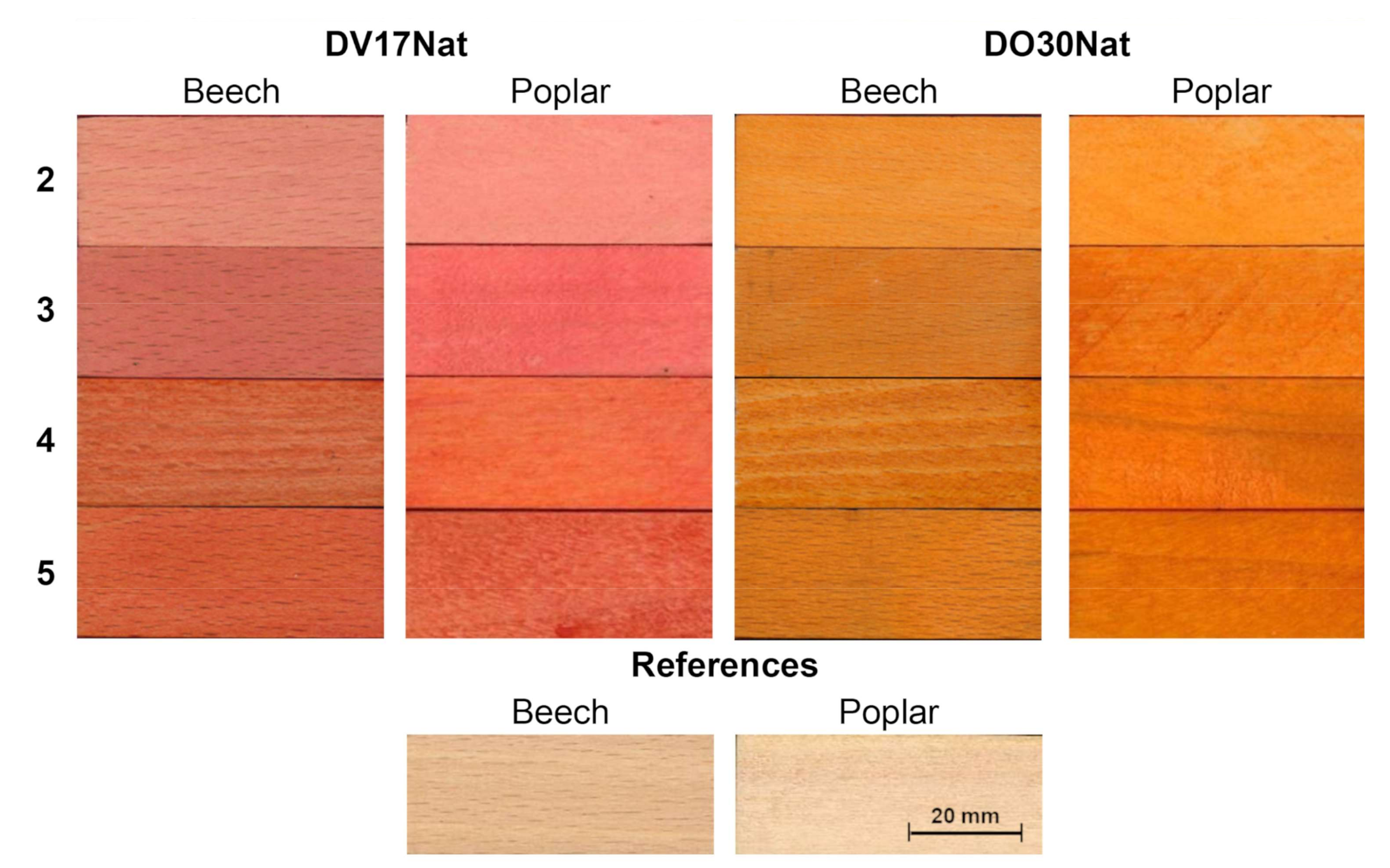
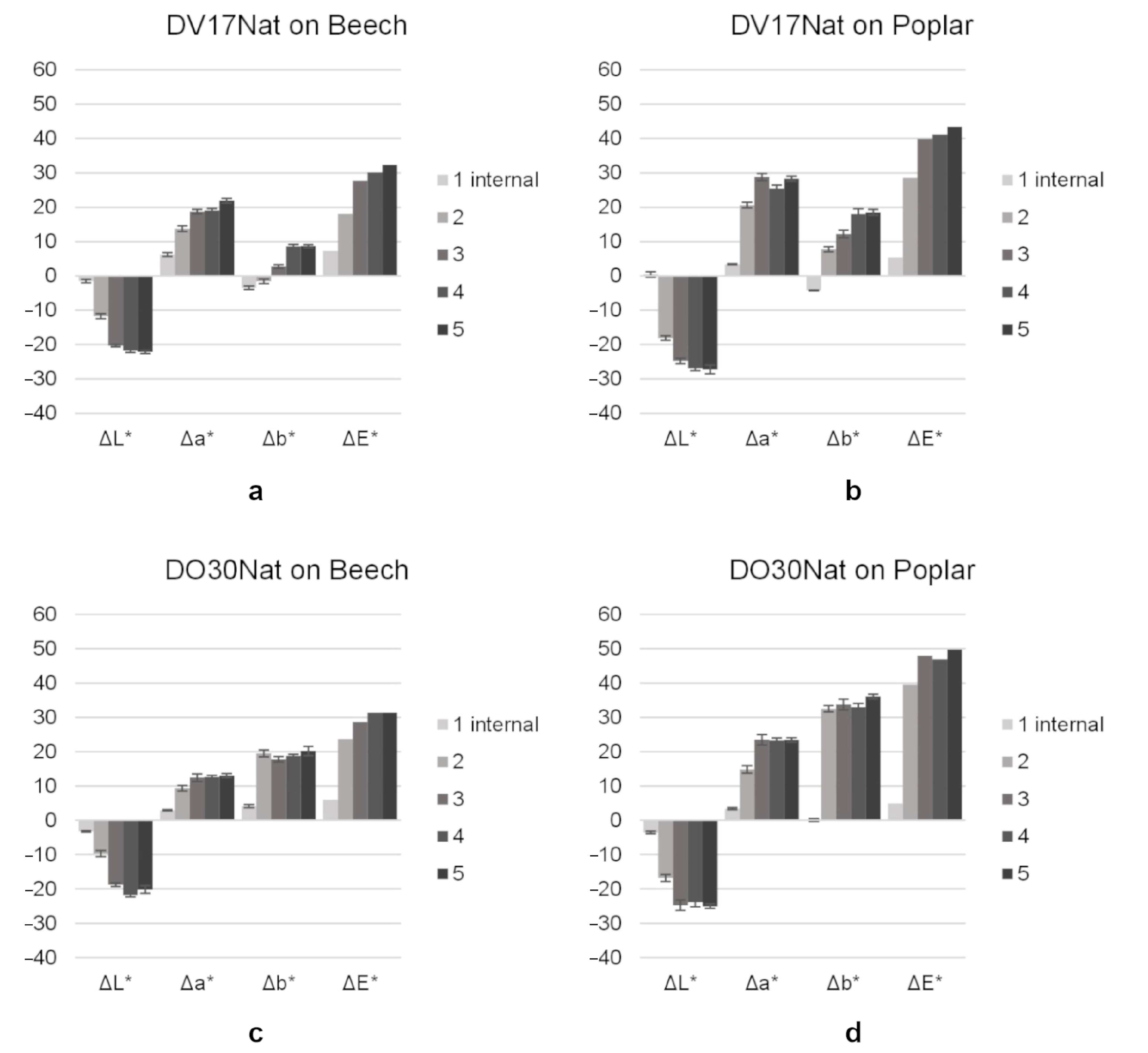
3.3. Wood Coloring
3.4. Fastness Grade (FG)
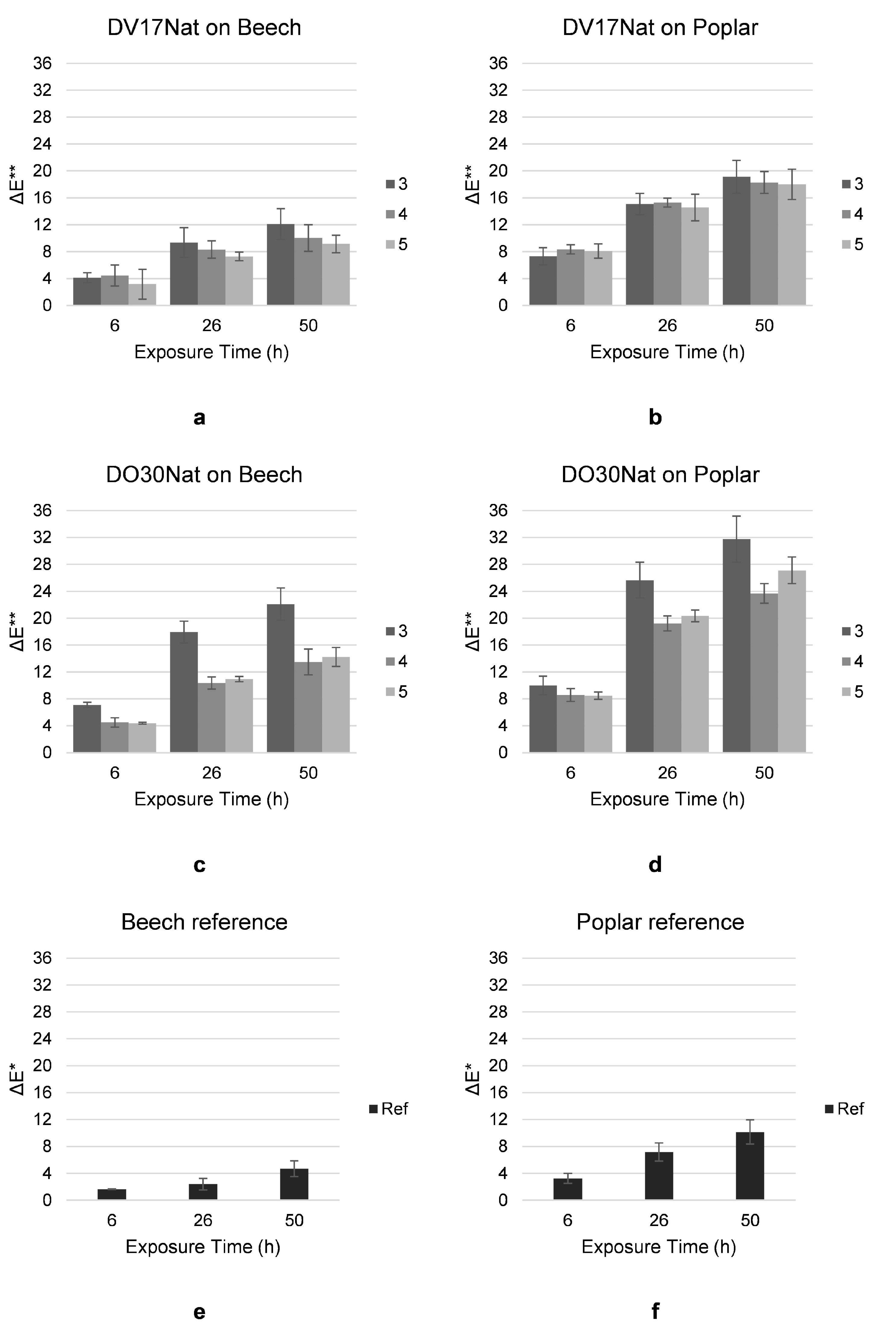
3.5. Photo-Induced Fading
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balter, M. Clothes Make the (Hu) Man. Science 2009, 325, 1329. [Google Scholar] [CrossRef] [PubMed]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Introduction and importance of synthetic organic dyes. In Metal-Free Synthetic Organic Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Aspland, J.R. The structure and properties of disperse dyes and related topics. In Textile Chemist and Colorist; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 1993; Volume 25, pp. 21–25. [Google Scholar]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Jamil, S.; Zahid, M. Biogenic synthesis, characterization and investigation of photocatalytic and antimicrobial activity of manganese nanoparticles synthesized from Cinnamomum verum bark extract. J. Mol. Struct. 2019, 1179, 532–539. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lou, L. Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 281–287. [Google Scholar] [CrossRef]
- Kausar, A.; MacKinnon, G.; Alharthi, A.; Hargreaves, J.; Bhatti, H.N.; Iqbal, M. A green approach for the removal of Sr(II) from aqueous media: Kinetics, isotherms and thermodynamic studies. J. Mol. Liq. 2018, 257, 164–172. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Peng, H.; Li, M.; Zhu, X.; Yao, Y. Nanostructured switchable pH-responsive membranes prepared via spherical polyelectrolyte brushes. J. Membr. Sci. 2019, 580, 117–124. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Hrovatin, K.; Hrovatin, J. Bio-based Methods with Potentials for Application in Wooden Furniture Industry. Drv. Ind. 2020, 71, 301–308. [Google Scholar] [CrossRef]
- Doty, K.; Haar, S.; Kim, J. Black walnut, Osage orange and eastern redcedar sawmill waste as natural dyes: Effect of aluminum mordant on color parameters. Fash. Text. 2016, 3, 22. [Google Scholar] [CrossRef]
- Prabhu, K.H.; Bhute, A.S. Plant based natural dyes and mordants: A Review. J. Nat. Prod. Plant Resour 2012, 2, 649–664. Available online: http://scholarsresearchlibrary.com/archive.html (accessed on 13 November 2022).
- Vega Gutierrez, S.M.; Vega Gutierrez, P.T.; Godinez, A.; Pittis, L.; Huber, M.; Stanton, S.; Robinson, S.C. Feasibility of Coloring Bamboo with the Application of Natural and Extracted Fungal Pigments. Coatings 2016, 6, 37. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Maduarta, I.M.; Howe, J.; Ingram, W.; Jansen, S. Hanging by a thread: Natural metallic mordant processes in traditional Indonesian textiles. Econ. Bot. 2011, 65, 241–259. Available online: http://www.jstor.org/stable/41408235 (accessed on 10 December 2022). [CrossRef]
- İşmal, Ö.E.; Yıldırım, L.; Özdoğan, E. Use of almond shell extracts plus biomordants as effective textile dye. J. Clean. Prod. 2014, 70, 61–67. [Google Scholar] [CrossRef]
- Vankar, P.S. Chemistry of natural dyes. Resonance 2000, 5, 73–80. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, S.; Ren, K.; Chen, J.; Lin, J.; Li, J. Colorability of dyed wood veneer using natural dye extracted from Dalbergia cochinchinensis with different organic solvents. BioResources 2012, 13, 7197–7211. [Google Scholar] [CrossRef]
- D’Ulivo, A.; Bonanni, M.; Mascherpa, M.C.; Bianchini, R.; Bramanti, E.; Corsi, M. Lactose cuts down heavy metal contamination from commercial dyes. La Chim. E L’Industria 2016, Anno XCVIII n 4, 52–55. Available online: http://dx.medra.org/10 (accessed on 20 March 2023).
- Bianchini, R.; Rolla, M.; Isaad, J.; Catelani, G.; Guazzelli, L.; Corsi, M.; Bonanni, M. Efficient double glycoconjugation to naturalize high molecular weight disperse dyes. Carbohydr. Res. 2012, 356, 104–109. [Google Scholar] [CrossRef][Green Version]
- Bartalucci, G.; Bianchini, R.; Catelani, G.; D’Andrea, F.; Guazzelli, L. Naturalised dyes: A simple straightforward synthetic route to a new class of dyes—Glycoazodyes (GADs). Eur. J. Org. Chem. 2007, 4, 588–595. [Google Scholar] [CrossRef]
- Porri, A.; Baroncelli, R.; Guglielminetti, L.; Sarrocco, S.; Guazzelli, L.; Forti, M.; Catelani, G.; Valentini, G.; Bazzichi, A.; Franceschi, M.; et al. Fusarium oxysporum degradation and detoxification of a new textile-glycoconjugate azo dye (GAD). Fungal Biol. 2011, 115, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Szymczak, A.; Wojciechowski, K. Naturalized Dyes—A Way to Increase Susceptibility for Microbiological Degradation. Biotechnol. Bioprocess Eng. 2015, 20, 100–108. [Google Scholar] [CrossRef]
- Bianchini, R.; Corsi, M.; Bonanni, M. Coloring Agents Naturalised with the 6′-Deoxy-6′-(Piperazinyl)Lactose Moiety (WIPO Patent WO2014177528A1). 2014. Available online: https://worldwide.espacenet.com/patent/search/family/048485283/publication/WO2014177528A1?q=WO2014177528A1 (accessed on 11 February 2021).
- Pellegrini, D.; Corsi, M.; Bonanni, M.; Bianchini, R.; D’Ulivo, A.; Bramanti, E. Study of the interaction between collagen and naturalized and commercial dyes by Fourier transform infrared spectroscopy and thermogravimetric analysis. Dyes Pigm. 2015, 116, 65–73. [Google Scholar] [CrossRef]
- Richter, C. Wood Characteristics: Description, Causes, Prevention, Impact on Use and Technological Adaptation; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Zanuttini, R.; Negro, F. Wood-Based Composites: Innovation towards a Sustainable Future. Forests 2021, 12, 1717. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Y.; Shi, S.Q.; Li, J.; Fang, Z. Cuttlebone-inspired magnesium oxychloride cement reinforced by biochar as green adhesive for wood industry. J. Clean. Prod. 2022, 370, 133365. [Google Scholar] [CrossRef]
- Substances Restricted under REACH, Annex XVII, Entry 43: Azocolourants and Azodyes. Available online: https://echa.europa.eu/substances-restricted-under-reach/-/dislist/details/0b0236e1807e2abe (accessed on 4 January 2023).
- Fogli, S. Glycoconjugated Dyes: Dye Removal and Evidence of Self-Assembly in Solution through Spectrophotometric and Scattering Techniques. Ph.D. Thesis, University of Florence, Florence, Italy, 2018. [Google Scholar]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Weigl, M.; Kandelbauer, A.; Hansmann, C.; Pöckl, J.; Müller, U.; Grabner, M. Application of Natural Dyes in the Coloration of Wood. In Handbook of Natural Colorants; Stevens, C.V., Bechtold, T., Mussak, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Mclellan, M.R.; Lind, L.R.; Kime, R.W. Hue angle determinations and statistical analysis for multiquadrant Hunter L,a,b data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- UNI EN 646:2019; Paper and Board Intended to Come into Contact with Foodstuffs—Determination of Colour Fastness of Dyed Paper and Board. European Committee for Standardization (CEN/EN Standards): Brussels, Belgium, 2019.
- UNI EN ISO 105-A03:2019; Textiles. Tests for Colour Fastness—Part A03: Grey Scale for Assessing Staining. International Organization for Standardization (ISO Standards): Geneva, Switzerland, 2019.
- ISO 11341:2004; Paints and Varnishes. Artificial Weathering and Exposure to Artificial Radiation—Exposure to Filtered Xenon-arc Radiation. International Organization for Standardization (ISO Standards): Geneva, Switzerland, 2004.
- Bulian, F.; Graystone, J. Wood Coatings Theory and Practice; Elsevier Science: Amsterdam, The Netherlands, 2009; ISBN 978-0-444-52840-7. [Google Scholar] [CrossRef]
- Badea, G.; Lăcătuşu, I.; Badea, N.; Ott, C.; Meghea, A. Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Rosu, D.; Mustata, F.R.; Rosu, L.; Varganici, C.D. Photochemical Aging of Eco-Friendly Wood Coatings Derived from Vegetable Oils. ACS Appl. Polym. Mater. 2021, 3, 6303–6314. [Google Scholar] [CrossRef]
- Barili, P.L.; Catelani, G.; D’Andrea, F.; De Rensis, F.; Falcini, P. Improved preparation of 2,3:5,6:30,40-tri-O-isopropylidenelactose dimethyl acetal and its 60-O-(1-methoxy-1-methylethyl) derivative. Carbohydr. Res. 1997, 298, 75–84. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Varma, M.; Varma, R.S.; Srivastava, P.C.; Knapp, F.F., Jr. The tosylation of alcohols. J. Org. Chem. 1986, 51, 2386–2388. [Google Scholar] [CrossRef]
- Attolino, E.; Catelani, G.; D’Andrea, F. Regiospecific synthesis of 4-deoxy-D-threo-hex-3-enopyranosides by simultaneous activation–elimination of the talopyranoside axial 4-OH with the NaH/ImS2O2 system: Manifestation of the stereoelectronic effect. Eur. J. Org. Chem. 2006, 23, 5279–5292. [Google Scholar] [CrossRef]
- Catelani, G.; Corsaro, A.; D’Andrea, F.; Mariani, M.; Pistarà, V.; Vittorino, E. Convenient preparation of l-arabinohexos-5-ulose derivatives from lactose. Carbohydr. Res. 2003, 338, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, M.; Kawachi, C.; Iwasaki, F.; Terao, K.; Tani, S. Synthesis and characterization of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM). Tetrahedron Lett. 1999, 40, 5327–5330. [Google Scholar] [CrossRef]
- Baughman, T.W.; Sworen, J.C.; Wagener, K.B. The facile preparation of alkenyl metathesis synthons. Tetrahedron 2004, 60, 10943–10948. [Google Scholar] [CrossRef]
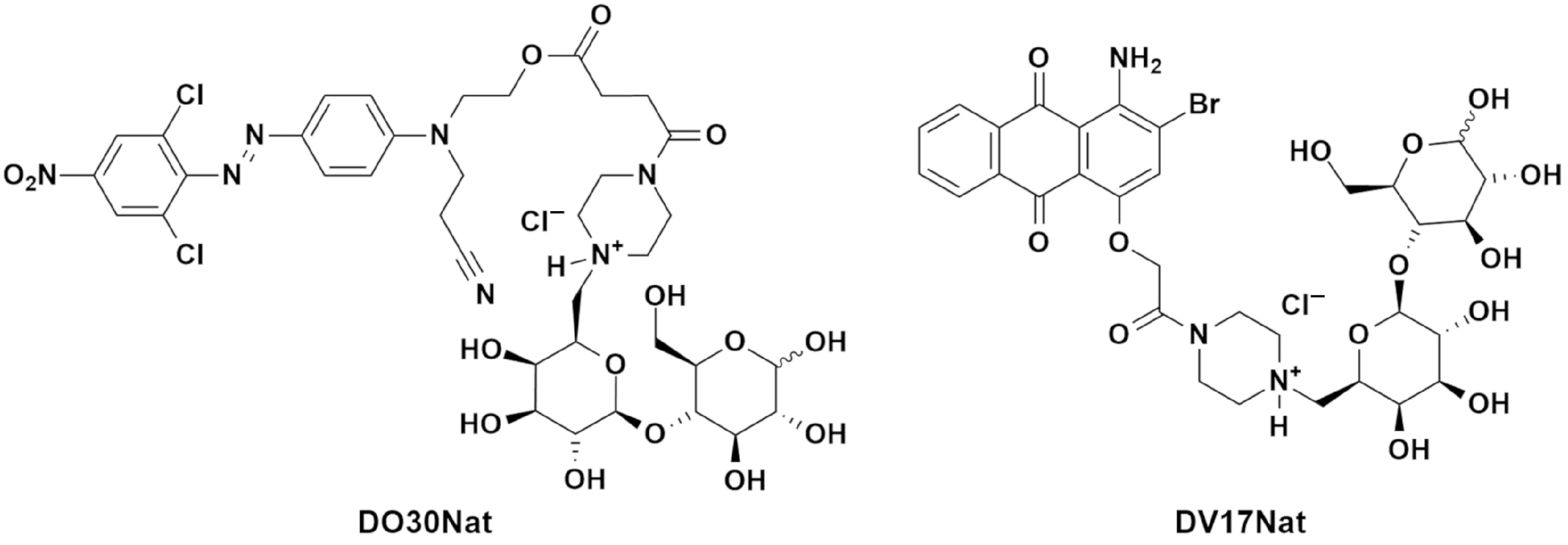



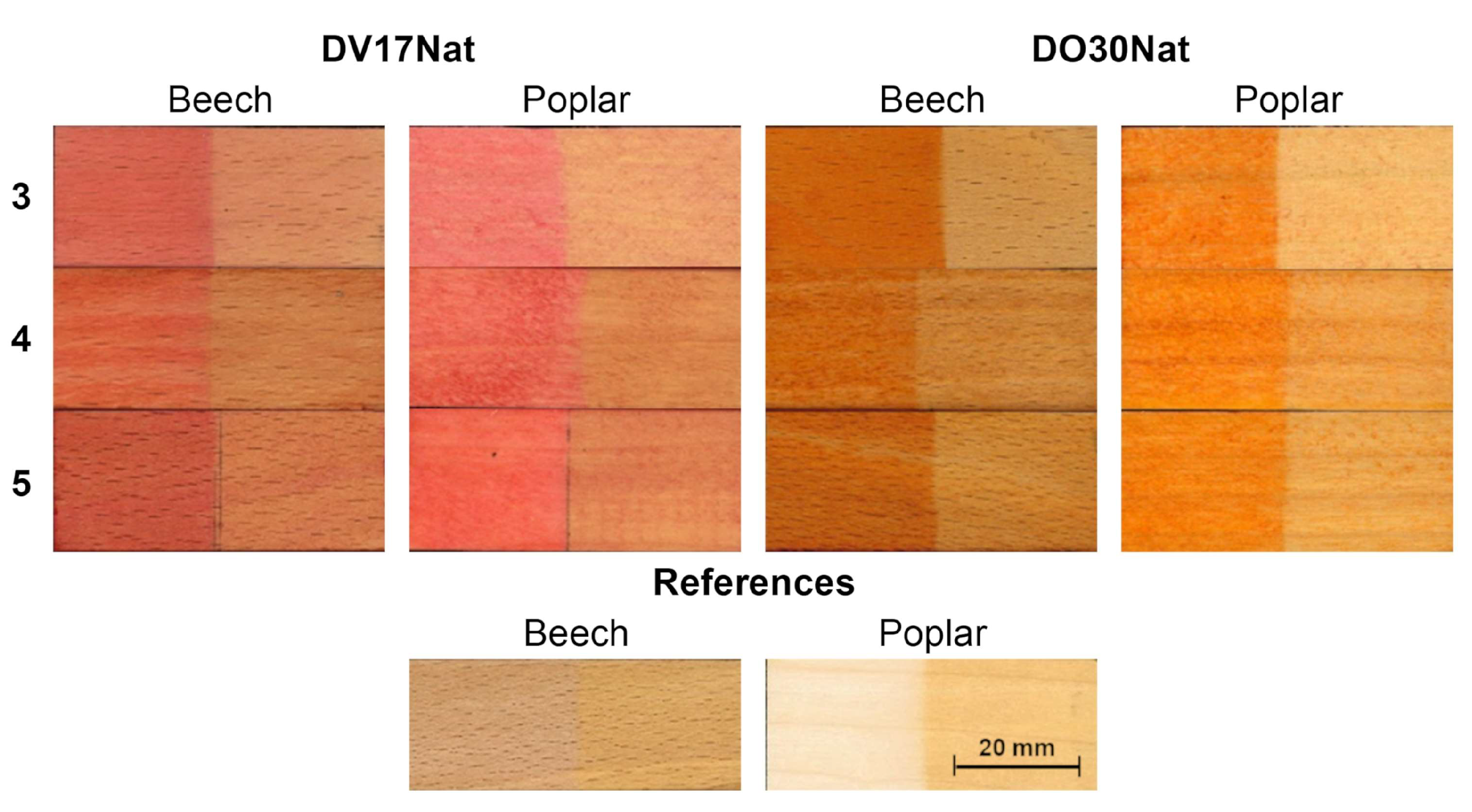
| Type of Wood | Type of ND | Coloring Method | L* | C* | h (°) |
|---|---|---|---|---|---|
| Beech | None | 74.3 ± 0.2 | 20.1 ± 0.2 | 57.2 ± 1.1 | |
| DO30Nat | 2 | 64.6 ± 0.7 | 41.6 ± 0.3 | 61.9 ± 1.1 | |
| 3 | 55.6 ± 0.3 | 41.8 ± 0.7 | 57.1 ± 1.0 | ||
| 4 | 52.6 ± 0.4 | 42.7 ± 0.2 | 57.6 ± 0.2 | ||
| 5 | 54.2 ± 0.9 | 44.1 ± 1.0 | 58.1 ± 0.5 | ||
| DV17Nat | 2 | 62.6 ± 0.5 | 28.7 ± 0.3 | 33.2 ± 1.1 | |
| 3 | 54.0 ± 0.1 | 35.3 ± 0.4 | 34.6 ± 0.3 | ||
| 4 | 52.7 ± 0.4 | 39.1 ± 0.3 | 41.4 ± 0.6 | ||
| 5 | 52.2 ± 0.2 | 41.3 ± 0.3 | 38.8 ± 0.5 | ||
| Poplar | None | 82.3 ± 0.4 | 8.7 ± 0.5 | 57.2 ± 1.1 | |
| DO30Nat | 2 | 65.5 ± 0.6 | 44.4 ± 0.6 | 63.9 ± 0.8 | |
| 3 | 57.6 ± 1.1 | 49.8 ± 0.4 | 55.5 ± 1.8 | ||
| 4 | 58.4 ± 0.9 | 49.0 ± 0.5 | 55.3 ± 0.8 | ||
| 5 | 57.3 ± 0.2 | 51.7 ± 0.4 | 58.1 ± 0.5 | ||
| DV17Nat | 2 | 64.2 ± 0.2 | 29.5 ± 0.2 | 30.7 ± 1.1 | |
| 3 | 57.6 ± 0.4 | 38.8 ± 0.3 | 30.2 ± 1.4 | ||
| 4 | 55.5 ± 0.3 | 39.4 ± 0.4 | 40.0 ± 1.7 | ||
| 5 | 55.1 ± 0.9 | 41.9 ± 0.5 | 38.0 ± 0.5 | ||
| Coloring Method | DO30Nat | DV17Nat | ||||||
|---|---|---|---|---|---|---|---|---|
| Beech | Poplar | Beech | Poplar | |||||
| ΔE* | FG | ΔE* | FG | ΔE* | FG | ΔE* | FG | |
| 3 | 3.2 ± 0.8 | 4 | 2.7 ± 0.7 | 4 | 3.4 ± 0.8 | 4 | 3.0 ± 0.9 | 4 |
| 4 | 0.5 ± 0.2 | 5 | 0.5 ± 0.1 | 5 | 1.7 ± 0.4 | 4–5 | 1.7 ± 0.4 | 4–5 |
| 5 | 0.2 ± 0.1 | 5 | 0.5 ± 0.3 | 5 | 0.6 ± 0.1 | 5 | 0.6 ± 0.2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vespignani, L.; Bonanni, M.; Marradi, M.; Pizzo, B.; Bianchini, R.; Goli, G. Naturalized Dyes: A New Opportunity for the Wood Coloring. Polymers 2023, 15, 3632. https://doi.org/10.3390/polym15173632
Vespignani L, Bonanni M, Marradi M, Pizzo B, Bianchini R, Goli G. Naturalized Dyes: A New Opportunity for the Wood Coloring. Polymers. 2023; 15(17):3632. https://doi.org/10.3390/polym15173632
Chicago/Turabian StyleVespignani, Laura, Marco Bonanni, Marco Marradi, Benedetto Pizzo, Roberto Bianchini, and Giacomo Goli. 2023. "Naturalized Dyes: A New Opportunity for the Wood Coloring" Polymers 15, no. 17: 3632. https://doi.org/10.3390/polym15173632
APA StyleVespignani, L., Bonanni, M., Marradi, M., Pizzo, B., Bianchini, R., & Goli, G. (2023). Naturalized Dyes: A New Opportunity for the Wood Coloring. Polymers, 15(17), 3632. https://doi.org/10.3390/polym15173632







