Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ɛ-caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. BCP/PCL-Based Scaffold Preparation and Characterization
2.2. PCL-Based and BCP/PCL-Based 3D Scaffold Biodegradability Test
- m0 = initial mass of the sample
- mx= mass of the dried sample after immersion at time x
2.3. Cell Viability Assays by Direct-Contact Assay
2.4. In Vitro Antibacterial Assays
2.5. Statistical Analysis
3. Results and Discussion
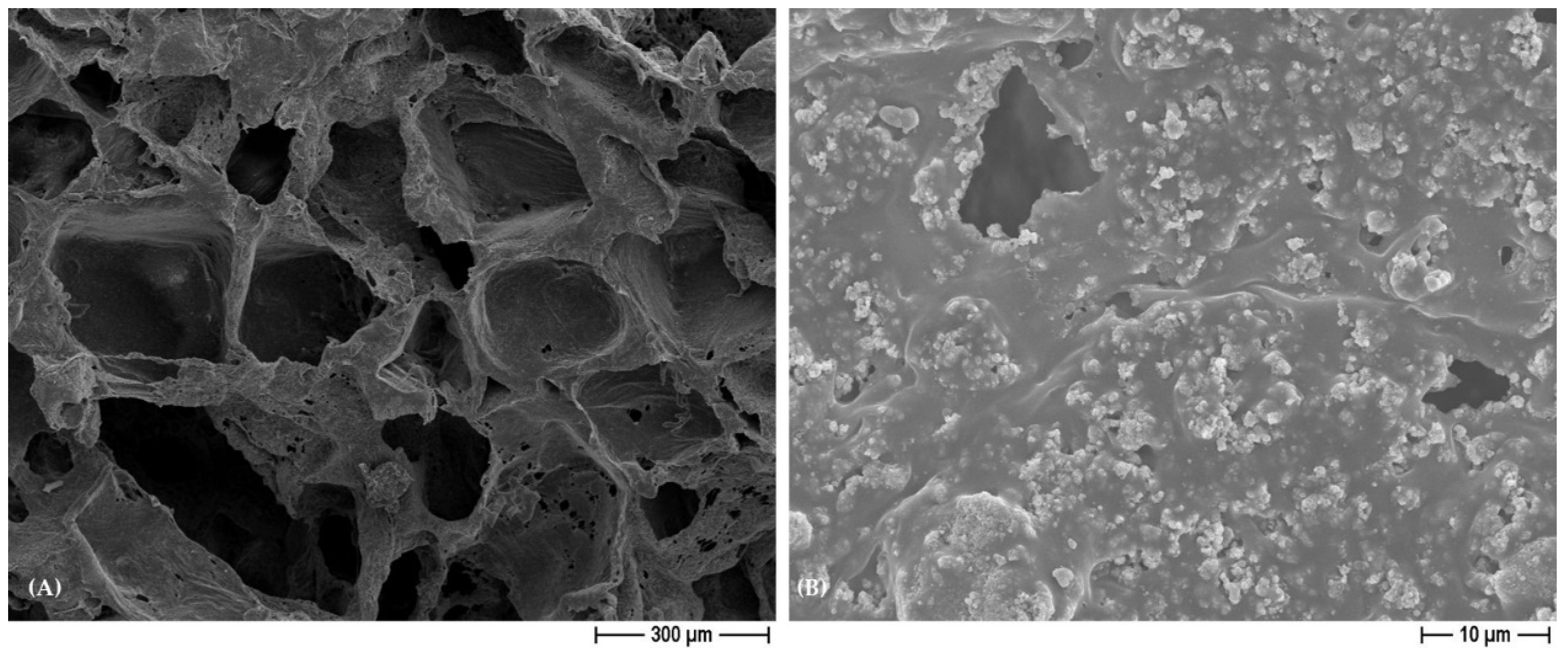
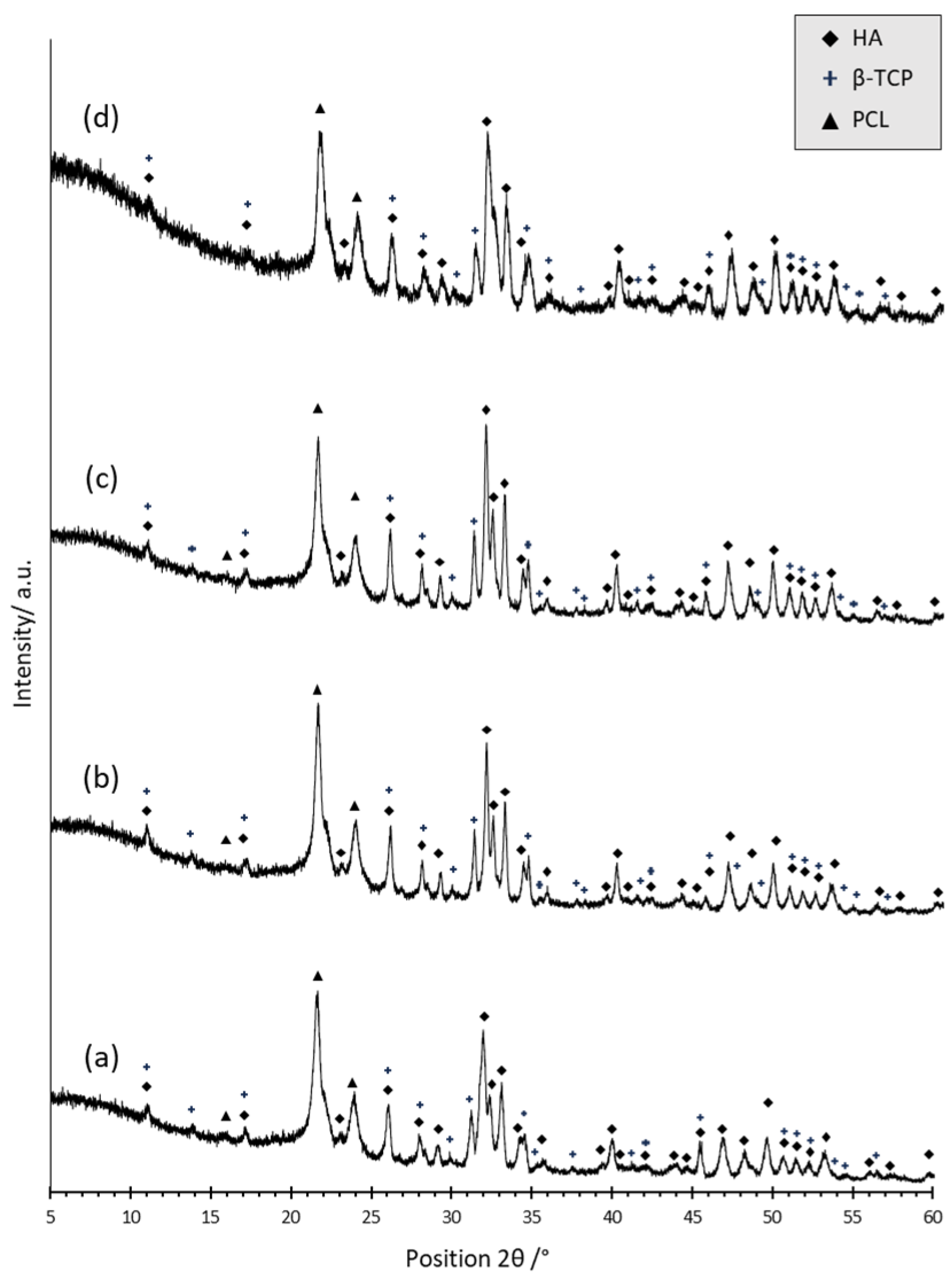
3.1. Characterization of PCL- and BCP/PCL-Based Biomaterials
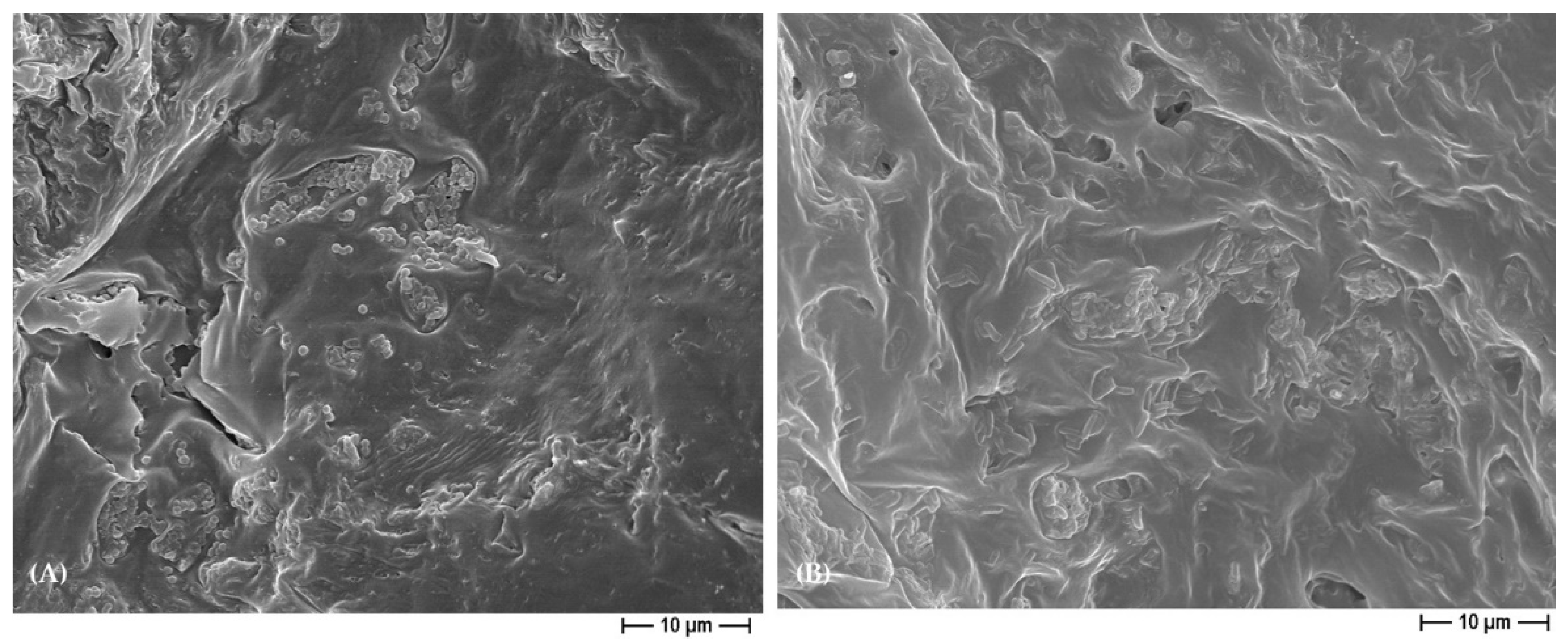
3.2. PCL- and BCP/PCL-Based 3D Scaffold Biodegradability Degree
3.3. In Vitro Saos-2 Cell Viability/Proliferation Assay
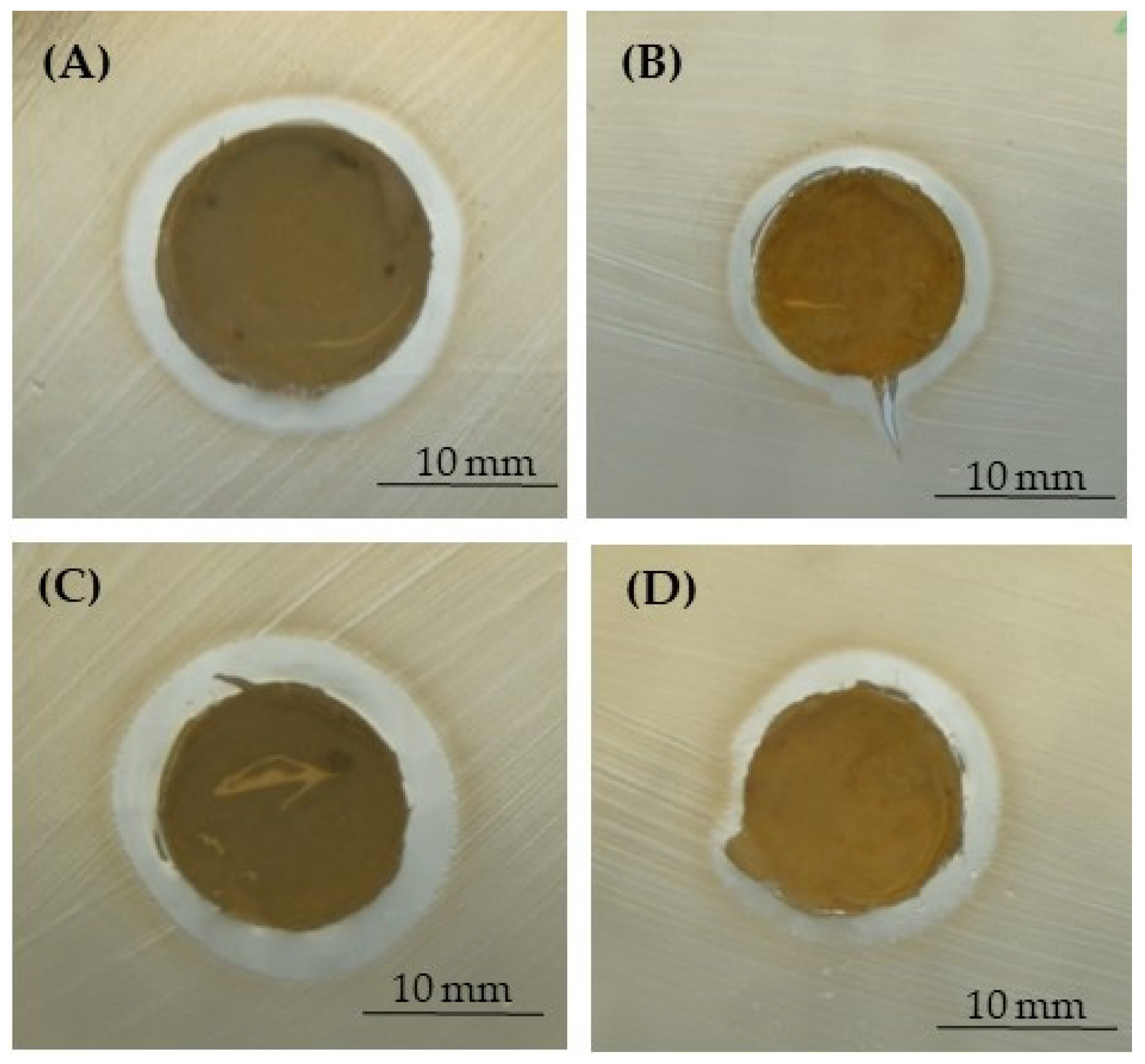
3.4. Antibacterial Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.-J.; Sarkar, S.K.; Kim, W.-J.; Kim, B.-R.; Park, J.-S.; Lee, B.-T. Bone Regeneration by Multichannel Cylindrical Granular Bone Substitute for Regeneration of Bone in Cases of Tumor, Fracture, and Arthroplasty. Int. J. Environ. Res. Public Health 2022, 19, 8228. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic Potential of the Growth Factors and Bioactive Molecules in Bone Regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Goh, C.; Shrestha, A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21, e2000365. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Sugishita, Y.; Suzuki-Takahashi, Y. Bone Development and Regeneration 2.0. Int. J. Mol. Sci. 2023, 24, 8761. [Google Scholar] [CrossRef]
- Holešová, S.; Čech Barabaszová, K.; Hundáková, M.; Ščuková, M.; Hrabovská, K.; Joszko, K.; Antonowicz, M.; Gzik-Zroska, B. Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties. Polymers 2021, 13, 3193. [Google Scholar] [CrossRef]
- Hajduga, M.B.; Bobinski, R.; Dutka, M.; Bujok, J.; Cwiertnia, M.; Pajak, C.; Kurowska, A.; Rajzer, I. The Influence of Graphene Content on the Antibacterial Properties of Polycaprolactone. Int. J. Mol. Sci. 2022, 23, 10899. [Google Scholar] [CrossRef] [PubMed]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-Specific 3D Scanned and 3D Printed Antimicrobial Polycaprolactone Wound Dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Allizond, V.; Banche, G.; Salvoni, M.; Malandrino, M.; Cecone, C.; Cuffini, A.M.; Bracco, P. Facile One-Step Electrospinning Process to Prepare AgNPs-Loaded PLA and PLA/PEO Mats with Antibacterial Activity. Polymers 2023, 15, 1470. [Google Scholar] [CrossRef] [PubMed]
- Bou-Francis, A.; Piercey, M.; Al-Qatami, O.; Mazzanti, G.; Khattab, R.; Ghanem, A. Polycaprolactone Blends for Fracture Fixation in Low Load-Bearing Applications. J. Appl. Polym. Sci. 2020, 137, 48940. [Google Scholar] [CrossRef]
- Hou, Z.; Zhou, W.; Guo, X.; Zhong, R.; Wang, A.; Li, J.; Cen, Y.; You, C.; Tan, H.; Tian, M. Poly(ϵ-Caprolactone)-Methoxypolyethylene Glycol (PCL-MPEG)-Based Micelles for Drug-Delivery: The Effect of PCL Chain Length on Blood Components, Phagocytosis, and Biodistribution. Int. J. Nanomed. 2022, 17, 1613–1632. [Google Scholar] [CrossRef]
- Comini, S.; Sparti, R.; Coppola, B.; Mohammadi, M.; Scutera, S.; Menotti, F.; Banche, G.; Cuffini, A.M.; Palmero, P.; Allizond, V. Novel Silver-Functionalized Poly(ε-Caprolactone)/Biphasic Calcium Phosphate Scaffolds Designed to Counteract Post-Surgical Infections in Orthopedic Applications. Int. J. Mol. Sci. 2021, 22, 10176. [Google Scholar] [CrossRef]
- Comini, S.; Scutera, S.; Sparti, R.; Banche, G.; Coppola, B.; Bertea, C.M.; Bianco, G.; Gatti, N.; Cuffini, A.M.; Palmero, P.; et al. Combination of Poly(ε-Caprolactone) Biomaterials and Essential Oils to Achieve Anti-Bacterial and Osteo-Proliferative Properties for 3D-Scaffolds in Regenerative Medicine. Pharmaceutics 2022, 14, 1873. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional Scaffolds with Improved Antimicrobial Properties and Osteogenicity Based on Piezoelectric Electrospun Fibers Decorated with Bioactive Composite Microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef]
- Florea, D.A.; Grumezescu, V.; Bîrcă, A.C.; Vasile, B.Ș.; Mușat, M.; Chircov, C.; Stan, M.S.; Grumezescu, A.M.; Andronescu, E.; Chifiriuc, M.C. Design, Characterization, and Antibacterial Performance of MAPLE-Deposited Coatings of Magnesium Phosphate-Containing Silver Nanoparticles in Biocompatible Concentrations. Int. J. Mol. Sci. 2022, 23, 7910. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Bassous, N.; Harb, S.; Palo-Nieto, C.; Ruiz-Esparza, G.U.; Marciano, F.R.; Webster, T.J.; Furtado, A.S.A.; Lobo, A.O. Advances in Dual Functional Antimicrobial and Osteoinductive Biomaterials for Orthopaedic Applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102143. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Vale, A.C.; Cunha, E.P.F.; C Paiva, M.; Reis, R.L.; Vaquette, C.; Alves, N.M. 3D-Printed Cryomilled Poly(ε-Caprolactone)/Graphene Composite Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 961–972. [Google Scholar] [CrossRef]
- Tullio, V.; Mandras, N.; Scalas, D.; Allizond, V.; Banche, G.; Roana, J.; Greco, D.; Castagno, F.; Cuffini, A.M.; Carlone, N.A. Synergy of Caspofungin with Human Polymorphonuclear Granulocytes for Killing Candida Albicans. Antimicrob. Agents Chemother. 2010, 54, 3964–3966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toth, J.M.; Hirthe, W.M.; Hubbard, W.G.; Brantley, W.A.; Lynch, K.L. Determination of the Ratio of HA/TCP Mixtures by x-Ray Diffraction. J. Appl. Biomater. 1991, 2, 37–40. [Google Scholar] [CrossRef]
- Robu, A.; Antoniac, A.; Grosu, E.; Vasile, E.; Raiciu, A.D.; Iordache, F.; Antoniac, V.I.; Rau, J.V.; Yankova, V.G.; Ditu, L.M.; et al. Additives Imparting Antimicrobial Properties to Acrylic Bone Cements. Materials 2021, 14, 7031. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.Y.; Leng, Y.; Chow, K.L.; Ren, F.; Ge, X.; Wang, K.; Lu, X. Cell Culture Medium as an Alternative to Conventional Simulated Body Fluid. Acta Biomater. 2011, 7, 2615–2622. [Google Scholar] [CrossRef]
- Petit, C.; Tulliani, J.-M.; Tadier, S.; Meille, S.; Chevalier, J.; Palmero, P. Novel Calcium Phosphate/PCL Graded Samples: Design and Development in View of Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 336–346. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Allizond, V.; Bertea, C.M.; Novara, C.; Cochis, A.; Geobaldo, F.; Bistolfi, A.; Cuffini, A.M.; Rimondini, L.; et al. Grafting of the Peppermint Essential Oil to a Chemically Treated Ti6Al4V Alloy to Counteract the Bacterial Adhesion. Surf. Coat. Technol. 2019, 378, 125011. [Google Scholar] [CrossRef]
- Allizond, V.; Comini, S.; Cuffini, A.M.; Banche, G. Current Knowledge on Biomaterials for Orthopedic Applications Modified to Reduce Bacterial Adhesive Ability. Antibiotics 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Contreras, A.; Torres, D.; Piñera-Avellaneda, D.; Pérez-Palou, L.; Ortiz-Hernández, M.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Dual-Action Effect of Gallium and Silver Providing Osseointegration and Antibacterial Properties to Calcium Titanate Coatings on Porous Titanium Implants. Int. J. Mol. Sci. 2023, 24, 8762. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Paterson, T.E.; Shi, R.; Tian, J.; Harrison, C.J.; De Sousa Mendes, M.; Hatton, P.V.; Li, Z.; Ortega, I. Electrospun Scaffolds Containing Silver-Doped Hydroxyapatite with Antimicrobial Properties for Applications in Orthopedic and Dental Bone Surgery. J. Funct. Biomater. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Catauro, M.; Sorrentino, A.; Guadagno, L. Thermal and Mechanical Characterization of Complex Electrospun Systems Based on Polycaprolactone and Gelatin. J. Therm. Anal. Calorim. 2022, 147, 5391–5399. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E. Nanofibrillated Chitosan/Polycaprolactone Bionanocomposite Scaffold with Improved Tensile Strength and Cellular Behavior. Nanomed. J. 2018, 5, 77–89. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable Polymer Matrix Nanocomposites for Tissue Engineering: A Review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Biocompatibility and Biodegradation Studies of PCL/β-TCP Bone Tissue Scaffold Fabricated by Structural Porogen Method. J. Mater. Sci. Mater. Med. 2012, 23, 2217–2226. [Google Scholar] [CrossRef]
- Hedayati, S.K.; Behravesh, A.H.; Hasannia, S.; Kordi, O.; Pourghaumi, M.; Saed, A.B.; Gashtasbi, F. Additive Manufacture of PCL/NHA Scaffolds Reinforced with Biodegradable Continuous Fibers: Mechanical Properties, in-Vitro Degradation Profile, and Cell Study. Eur. Polym. J. 2022, 162, 110876. [Google Scholar] [CrossRef]
- Muhammad, K.B.; Abas, W.A.B.W.; Kim, K.H.; Pingguan-Murphy, B.; Zain, N.M.; Akram, H. In Vitro Comparative Study of White and Dark Polycaprolactone Trifumarate in Situ Cross-Linkable Scaffolds Seeded with Rat Bone Marrow Stromal Cells. Clinics 2012, 67, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, G.; Kim, I.G.; Chung, E.-J.; Noh, I. Comparative Studies on Thin Polycaprolactone-Tricalcium Phosphate Composite Scaffolds and Its Interaction with Mesenchymal Stem Cells. Biomater. Res. 2019, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Musciacchio, L.; Mardirossian, M.; Guagnini, B.; Raffini, A.; Rizzo, M.; Trombetta, C.; Liguori, G.; Turco, G.; Porrelli, D. Rifampicin-Loaded Electrospun Polycaprolactone Membranes: Characterization of Stability, Antibacterial Effects and Urotheliocytes Proliferation. Mater. Des. 2022, 224, 111286. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A Phenotypic Comparison of Osteoblast Cell Lines versus Human Primary Osteoblasts for Biomaterials Testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef]
- Dvorakova, J.; Wiesnerova, L.; Chocholata, P.; Kulda, V.; Landsmann, L.; Cedikova, M.; Kripnerova, M.; Eberlova, L.; Babuska, V. Human Cells with Osteogenic Potential in Bone Tissue Research. BioMed. Eng. OnLine 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fuh, J.; Ozbolat, I.T. Chapter 4—Bioprinting of Bone. In 3D Bioprinting in Tissue and Organ Regeneration; Wu, Y., Fuh, J., Ozbolat, I.T., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 95–118. ISBN 978-0-12-824291-9. [Google Scholar]
- Ashe, S.; Nayak, D.; Kumari, M.; Nayak, B. Ameliorating Effects of Green Synthesized Silver Nanoparticles on Glycated End Product Induced Reactive Oxygen Species Production and Cellular Toxicity in Osteogenic Saos-2 Cells. ACS Appl. Mater. Interfaces 2016, 8, 30005–30016. [Google Scholar] [CrossRef]
- Ashe, S.; Behera, S.; Dash, P.; Nayak, D.; Nayak, B. Gelatin Carrageenan Sericin Hydrogel Composites Improves Cell Viability of Cryopreserved SaOS-2 Cells. Int. J. Biol. Macromol. 2020, 154, 606–620. [Google Scholar] [CrossRef]
- Sokołowska, P.; Siatkowska, M.; Białkowska, K.; Rosowski, M.; Komorowski, P.; Walkowiak, B. Osteosarcoma Cells in Early and Late Stages as Cancer in Vitro Progression Model for Assessing the Responsiveness of Cells to Silver Nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1319–1334. [Google Scholar] [CrossRef]
- Michalakis, K.; Bakopoulou, A.; Papachristou, E.; Vasilaki, D.; Tsouknidas, A.; Michailidis, N.; Johnstone, E. Evaluation of the Response of HOS and Saos-2 Osteosarcoma Cell Lines When Exposed to Different Sizes and Concentrations of Silver Nanoparticles. BioMed Res. Int. 2021, 2021, e5013065. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible Particle-Specific Toxicity Mechanism of Silver Nanoparticles: The Role of Ag+ Ion Release in the Cytosol. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Pinto, S.A.; de Nadai Dias, F.J.; Brasil Camargo Cardoso, G.; Dos Santos Junior, A.R.; de Aro, A.A.; Pino, D.S.; Meneghetti, D.H.; Vitti, R.P.; Dos Santos, G.M.T.; de Carvalho Zavaglia, C.A. Polycaprolactone/Beta-Tricalcium Phosphate Scaffolds Obtained via Rotary Jet-Spinning: In Vitro and in Vivo Evaluation. Cells Tissues Organs 2021, 211, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Lama, S.; Luce, A.; Bitti, G.; Chacon-Millan, P.; Itro, A.; Ferranti, P.; D’Auria, G.; Cammarota, M.; Nicoletti, G.F.; Ferraro, G.A.; et al. Polydatin Incorporated in Polycaprolactone Nanofibers Improves Osteogenic Differentiation. Pharmaceuticals 2022, 15, 727. [Google Scholar] [CrossRef]
- Siljander, M.P.; Sobh, A.H.; Baker, K.C.; Baker, E.A.; Kaplan, L.M. Multidrug-Resistant Organisms in the Setting of Periprosthetic Joint Infection—Diagnosis, Prevention, and Treatment. J. Arthroplast. 2018, 33, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and Management of Fracture-Related Infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Koepf, U.S.; Scheidt, S.; Hischebeth, G.T.R.; Strassburg, C.P.; Wirtz, D.C.; Randau, T.M.; Lutz, P. Increased Rate of Enteric Bacteria as Cause of Periprosthetic Joint Infections in Patients with Liver Cirrhosis. BMC Infect. Dis. 2022, 22, 389. [Google Scholar] [CrossRef]
- Rudelli, B.A.; Giglio, P.N.; de Carvalho, V.C.; Pécora, J.R.; Gurgel, H.M.C.; Gobbi, R.G.; Vicente, J.R.N.; Lima, A.L.L.M.; Helito, C.P. Bacteria Drug Resistance Profile Affects Knee and Hip Periprosthetic Joint Infection Outcome with Debridement, Antibiotics and Implant Retention. BMC Musculoskelet. Disord. 2020, 21, 574. [Google Scholar] [CrossRef]
- Imagama, T.; Seki, K.; Seki, T.; Matsuki, Y.; Yamazaki, K.; Sakai, T. Low Frequency of Local Findings in Periprosthetic Hip Infection Caused by Low-Virulent Bacteria Compared to Periprosthetic Knee Infection. Sci. Rep. 2021, 11, 11714. [Google Scholar] [CrossRef]
- Alipour, M.; Pouya, B.; Aghazadeh, Z.; SamadiKafil, H.; Ghorbani, M.; Alizadeh, S.; Aghazadeh, M.; Dalir Abdolahinia, E. The Antimicrobial, Antioxidative, and Anti-Inflammatory Effects of Polycaprolactone/Gelatin Scaffolds Containing Chrysin for Regenerative Endodontic Purposes. Stem Cells Int. 2021, 2021, e3828777. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, Z.; Zheng, L.; Song, R.; Zhao, Y. Fabrication and Characterization of Electrospun Polycaprolactone Blended with Chitosan-Gelatin Complex Nanofibrous Mats. J. Nanomater. 2014, 2014, e964621. [Google Scholar] [CrossRef]
- Doherty, C.; Byrne, C.V.; Baqader, S.; El-Chami, C.; McBain, A.J.; Thomason, H.A. Anti-Biofilm Effects and Healing Promotion by Silver Oxynitrate-Based Dressings. Sci. Rep. 2023, 13, 2014. [Google Scholar] [CrossRef] [PubMed]







| Types of 3D Scaffolds | Salt Used as Pore Former | Composition of the 3D Scaffolds |
|---|---|---|
| pure PCL | NaCl | poly(ε-caprolactone) |
| pure BCP/PCL | NaCl | biphasic calcium phosphates/poly(ε-caprolactone) |
| PCL + Ag 1% | NaCl | poly(ε-caprolactone) + 1% of silver |
| PCL + Ag 1.2% | NaCl | poly(ε-caprolactone) + 1.2% of silver |
| BCP/PCL + Ag 1% | NaCl | biphasic calcium phosphates/poly(ε-caprolactone) + 1% of silver |
| BCP/PCL + Ag 1.2% | NaCl | biphasic calcium phosphates/poly(ε-caprolactone) + 1.2% of silver |
| pure PCL | NaNO3 | poly(ε-caprolactone) |
| pure BCP/PCL | NaNO3 | biphasic calcium phosphates/poly(ε-caprolactone) |
| PCL + Ag 0.79% | NaNO3 | poly(ε-caprolactone) + 0.79% of silver |
| PCL + Ag 1% | NaNO3 | poly(ε-caprolactone) + 1% of silver |
| BCP/PCL + Ag 0.79% | NaNO3 | biphasic calcium phosphates/poly(ε-caprolactone) + 0.79% of silver |
| BCP/PCL + Ag 1% | NaNO3 | biphasic calcium phosphates/poly(ε-caprolactone) + 1% of silver |
| Morphological Parameters | Statistical Analysis | |||
|---|---|---|---|---|
| A | Diameter (mm) | Height (mm) | Density (mg/mm3) | Student’s t-Test |
| Scaffold Type | ||||
| PCL | 18.31 ± 0.11 | 11.27 ± 0.16 | 0.126 ± 0.003 | weight and density PCL vs. BCP/PCL p < 0.001 |
| BCP/PCL | 18.98 ± 0.10 | 10.80 ± 0.29 | 0.204 ± 0.005 | |
| PCL + Ag 1% | 18.31 ± 0.21 | 10.58 ± 0.49 | 0.133 ± 0.009 | |
| PCL + Ag 1.2% | 18.38 ± 0.22 | 11.18 ± 0.49 | 0.132 ± 0.005 | |
| BCP/PCL + Ag 1% | 18.73 ± 0.12 | 11.68 ± 0.13 | 0.213 ± 0.004 | |
| BCP/PCL + Ag 1.2% | 18.92 ± 0.13 | 12.15 ± 0.16 | 0.221 ± 0.003 | |
| B | ||||
| PCL | 18.10 ± 0.13 | 10.10 ± 0.39 | 0.127 ± 0.003 | weight and density PCL vs. BCP/PCL p < 0.001 |
| BCP/PCL | 18.74 ± 0.10 | 9.87 ± 0.41 | 0.205 ± 0.005 | |
| PCL + Ag 0.79% | 18.61 ± 0.13 | 11.11 ± 0.65 | 0.133 ± 0.009 | |
| PCL + Ag 1% | 18.42 ± 0.22 | 11.81 ± 0.46 | 0.132 ± 0.005 | |
| BCP/PCL + Ag 0.79% | 18.63 ± 0.11 | 9.64 ± 0.25 | 0.213 ± 0.004 | |
| BCP/PCL + Ag 1% | 18.99 ± 0.07 | 11.25 ± 0.16 | 0.220 ± 0.003 | |
| Average Diameter ± SEM (mm) | |||
|---|---|---|---|
| A | S. aureus | S. epidermidis | E. coli |
| Scaffold Type pored with NaCl | |||
| PCL + Ag 1% | 22.65 ± 0.16 | 27.41 ± 0.21 | 22.02 ± 0.36 |
| PCL + Ag 1.2% | 22.85 ± 0.32 | 30.82 ± 0.30 | 22.19 ± 0.13 |
| BCP/PCL + Ag 1% | 22.79 ± 0.24 | 28.13 ± 0.12 | 21.32 ± 0.16 |
| BCP/PCL + Ag 1.2% | 23.06 ± 0.11 | 31.65 ± 0.22 | 22.87 ± 0.41 |
| B | |||
| Scaffold Type pored with NaNO3 | |||
| PCL + Ag 0.79% | 23.12 ± 0.20 | 27.06 ± 0.38 | 21.11 ± 0.13 |
| PCL + Ag 1% | 23.13 ± 0.31 | 29.76 ± 0.18 | 22.29 ± 0.23 |
| BCP/PCL + Ag 0.79% | 24.09 ± 0.50 | 27.89 ± 0.22 | 21.51 ± 0.30 |
| BCP/PCL + Ag 1% | 24.23 ± 0.12 | 30.03 ± 0.47 | 22.77 ± 0.21 |
| Number of Adhered Bacteria as log10 CFU/mL (Means ± Standard Error of the Means) | Statistical Analysis | |||
|---|---|---|---|---|
| A | S. aureus | S. epidermidis | E. coli | Student’s t-Test |
| Scaffold Type pored with NaCl | ||||
| PCL | 2.16 × 109 ± 3.56 × 108 | 1.55 × 107 ± 5.50 × 106 | 1.45 × 108 ± 1.14 × 106 | PCL and BCP/PCL vs. PCL + Ag and BCP/PCL + Ag p < 0.001 |
| BCP/PCL | 3.05 × 109 ± 6.65 × 108 | 2.15 × 107 ± 4.00 × 106 | 1.24 × 108 ± 1.10 × 106 | |
| PCL + Ag 1% | 2.36 × 103 ± 1.88 × 102 | 4.67 × 102 ± 1.40 × 101 | 2.43 × 102 ± 1.15 × 101 | |
| PCL + Ag 1.2% | 2.01 × 103 ± 6.61 × 101 | 2.27 × 102 ± 1.83 × 101 | 2.86 × 102 ± 1.69 × 101 | |
| BCP/PCL + Ag 1% | 2.66 × 103 ± 8.54 × 101 | 3.32 × 102 ± 1.35 × 101 | 2.52 × 102 ± 1.35 × 101 | |
| BCP/PCL + Ag 1.2% | 1.55 × 103 ± 6.53 × 101 | 2.42 × 102 ± 1.76 × 101 | 1.42 × 102 ± 1.55 × 101 | |
| B | ||||
| Scaffold Type pored with NaNO3 | ||||
| PCL | 1.48 × 109 ± 3.80 × 108 | 2.66 × 107 ± 1.34 × 106 | 2.19 × 108 ± 1.41 × 106 | PCL and BCP/PCL vs. PCL + Ag and BCP/PCL + Ag p < 0.001 |
| BCP/PCL | 2.82 × 109 ± 9.60 × 107 | 2.65 × 107 ± 1.89 × 106 | 1.78 × 108 ± 1.76 × 106 | |
| PCL + Ag 0.79% | 3.43 × 103 ± 2.18 × 102 | 2.30 × 102 ± 1.66 × 101 | 3.12 × 102 ± 1.83 × 101 | |
| PCL + Ag 1% | 2.31 × 103 ± 2.30 × 102 | 2.01 × 102 ± 3.10 × 101 | 1.06 × 102 ± 1.28 × 101 | |
| BCP/PCL + Ag 0.79% | 6.18 × 103 ± 2.86 × 102 | 2.52 × 102 ± 1.89 × 101 | 2.89 × 102 ± 1.50 × 101 | |
| BCP/PCL + Ag 1% | 4.96 × 103 ± 1.22 × 102 | 2.44 × 102 ± 2.17 × 101 | 1.60 × 102 ± 1.12 × 101 | |
| Number of Planktonic Bacteria as log10 CFU/mL (Means ± Standard Error of the Means) | Statistical Analysis | |||
|---|---|---|---|---|
| A | S. aureus | S. epidermidis | E. coli | Student’s t-Test |
| Scaffold Type Pored with NaCl | ||||
| PCL | 2.80 × 109 ± 2.82 × 108 | 2.64 × 108 ± 6.62 × 106 | 1.74 × 109 ± 3.13 × 108 | PCL and BCP/PCL vs. PCL + Ag and BCP/PCL + Ag p < 0.001 |
| BCP/PCL | 2.35 × 109 ± 7.25 × 107 | 3.09 × 108 ± 1.21 × 107 | 1.07 × 109 ± 9.35 × 107 | |
| PCL + Ag 1% | 1.28 × 105 ± 1.18 × 104 | 3.32 × 104 ± 1.28 × 103 | 1.67 × 103 ± 1.14 × 102 | |
| PCL + Ag 1.2% | 1.06 × 105 ± 5.53 × 103 | 2.82 × 104 ± 1.80 × 103 | 1.30 × 103 ± 1.60 × 102 | |
| BCP/PCL + Ag 1% | 1.65 × 105 ± 1.64 × 104 | 2.80 × 104± 1.55 × 103 | 3.50 × 103 ± 2.51 × 102 | |
| BCP/PCL + Ag 1.2% | 1.58 × 105 ± 2.40 × 104 | 1.72 × 104 ± 1.39 × 103 | 2.62 × 103± 1.64 × 102 | |
| B | ||||
| Scaffold Type pored with NaNO3 | ||||
| PCL | 2.16 × 109 ± 5.81 × 108 | 2.02 × 108 ± 2.00 × 107 | 1.42 × 109 ± 1.56 × 108 | PCL and BCP/PCL vs. PCL + Ag and BCP/PCL + Ag p < 0.001 |
| BCP/PCL | 3.67 × 109 ± 3.22 × 107 | 3.51 × 108 ± 2.75 × 107 | 1.34 × 109 ± 4.33 × 107 | |
| PCL + Ag 0.79% | 2.78 × 105 ± 2.76 × 104 | 2.79 × 104 ± 2.13 × 103 | 1.48 × 103 ± 1.07 × 102 | |
| PCL + Ag 1% | 1.57 × 105 ± 1.46 × 104 | 2.61 × 104 ± 2.03 × 103 | 1.24 × 103 ± 1.33 × 102 | |
| BCP/PCL + Ag 0.79% | 3.35 × 105 ± 8.26 × 103 | 2.76 × 104± 1.02 × 103 | 2.24 × 103 ± 8.05 × 101 | |
| BCP/PCL + Ag 1% | 2.32 × 105 ± 4.21 × 104 | 2.23 × 104 ± 5.80 × 102 | 2.06 × 103± 1.00 × 102 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menotti, F.; Scutera, S.; Coppola, B.; Longo, F.; Mandras, N.; Cavallo, L.; Comini, S.; Sparti, R.; Fiume, E.; Cuffini, A.M.; et al. Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ɛ-caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering. Polymers 2023, 15, 3618. https://doi.org/10.3390/polym15173618
Menotti F, Scutera S, Coppola B, Longo F, Mandras N, Cavallo L, Comini S, Sparti R, Fiume E, Cuffini AM, et al. Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ɛ-caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering. Polymers. 2023; 15(17):3618. https://doi.org/10.3390/polym15173618
Chicago/Turabian StyleMenotti, Francesca, Sara Scutera, Bartolomeo Coppola, Fabio Longo, Narcisa Mandras, Lorenza Cavallo, Sara Comini, Rosaria Sparti, Elisa Fiume, Anna Maria Cuffini, and et al. 2023. "Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ɛ-caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering" Polymers 15, no. 17: 3618. https://doi.org/10.3390/polym15173618
APA StyleMenotti, F., Scutera, S., Coppola, B., Longo, F., Mandras, N., Cavallo, L., Comini, S., Sparti, R., Fiume, E., Cuffini, A. M., Banche, G., Palmero, P., & Allizond, V. (2023). Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ɛ-caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering. Polymers, 15(17), 3618. https://doi.org/10.3390/polym15173618















