Influence of Enzymatically Hydrophobized Hemp Protein on Morphology and Mechanical Properties of Bio-Based Polyurethane and Epoxy Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Hydrophobization of Hemp Protein
2.3. Polyurethane Foam Preparation
2.4. Epoxy Foam Preparation
2.5. Characterization of the Hydrophobized Hemp Protein
2.6. Polyurethane and Epoxy Foam Characterization
2.6.1. Characterization of Density and Mechanical Properties
2.6.2. Determination of Moisture Uptake
3. Results and Discussion
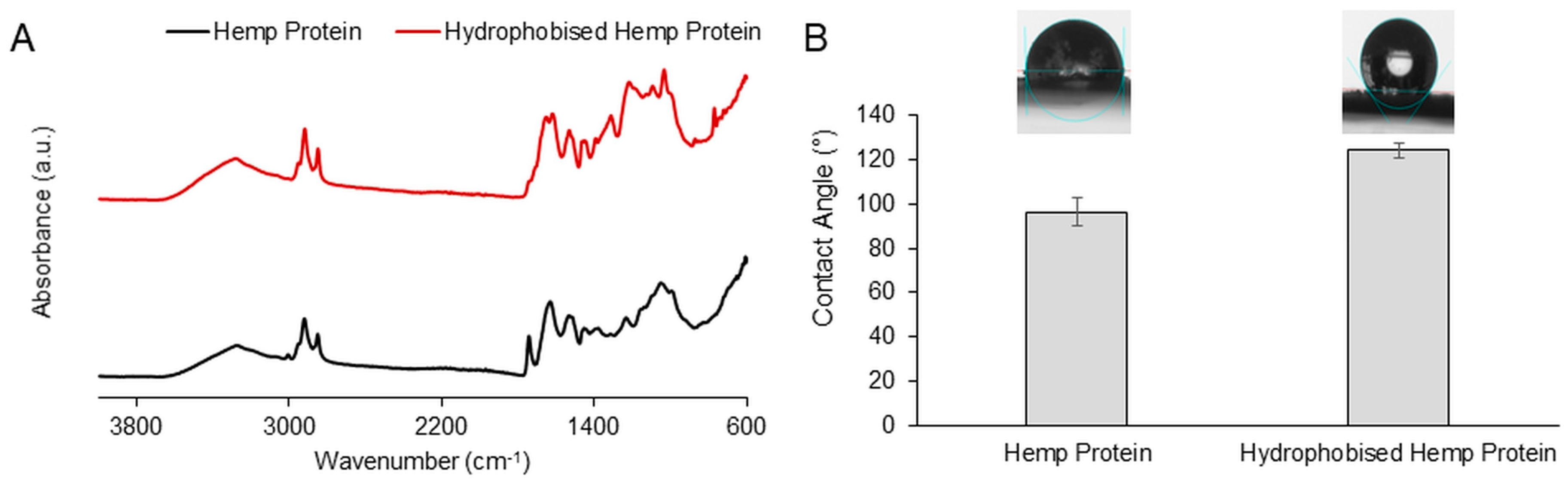
3.1. Characterization of the Hydrophobized Hemp Protein
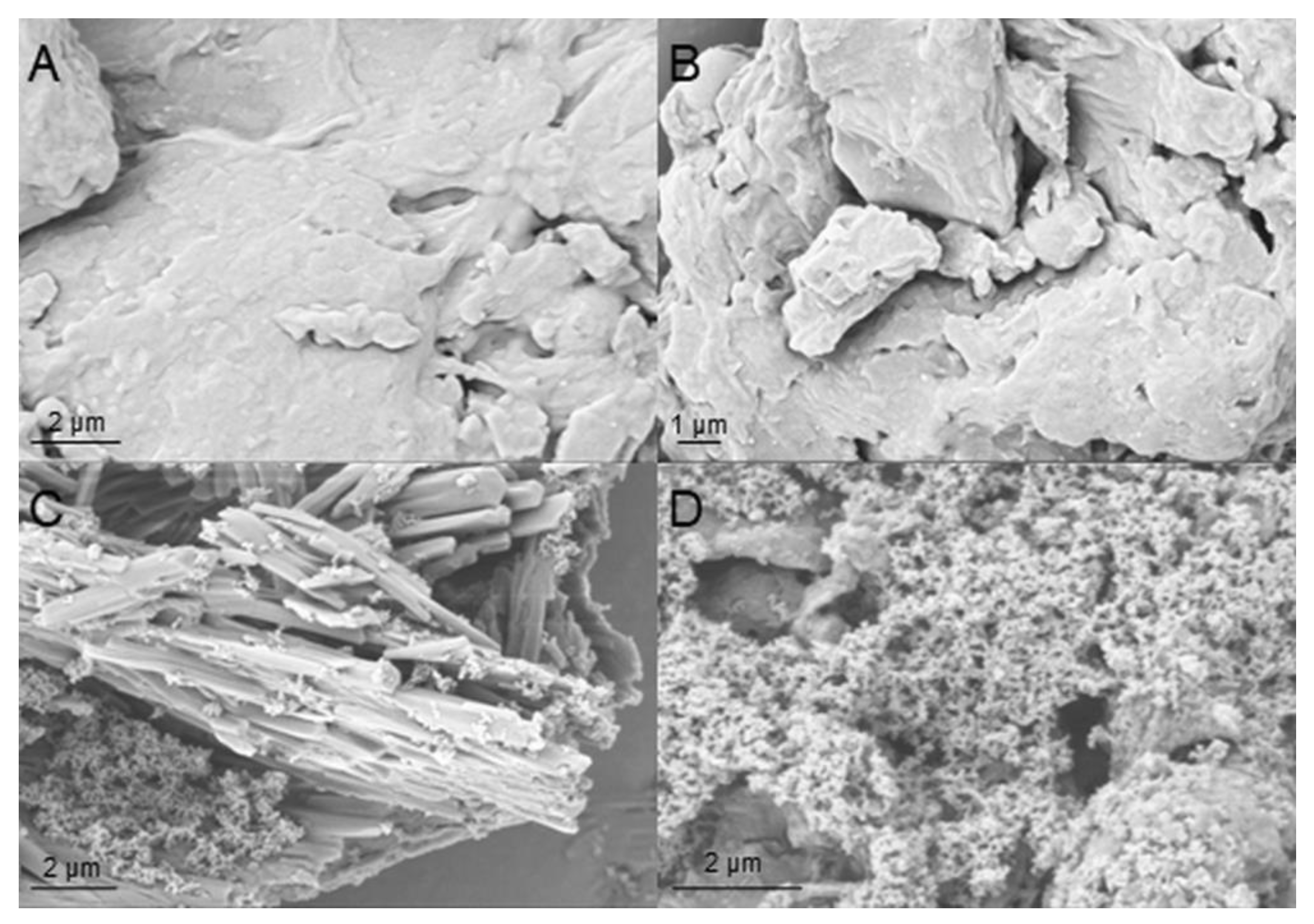
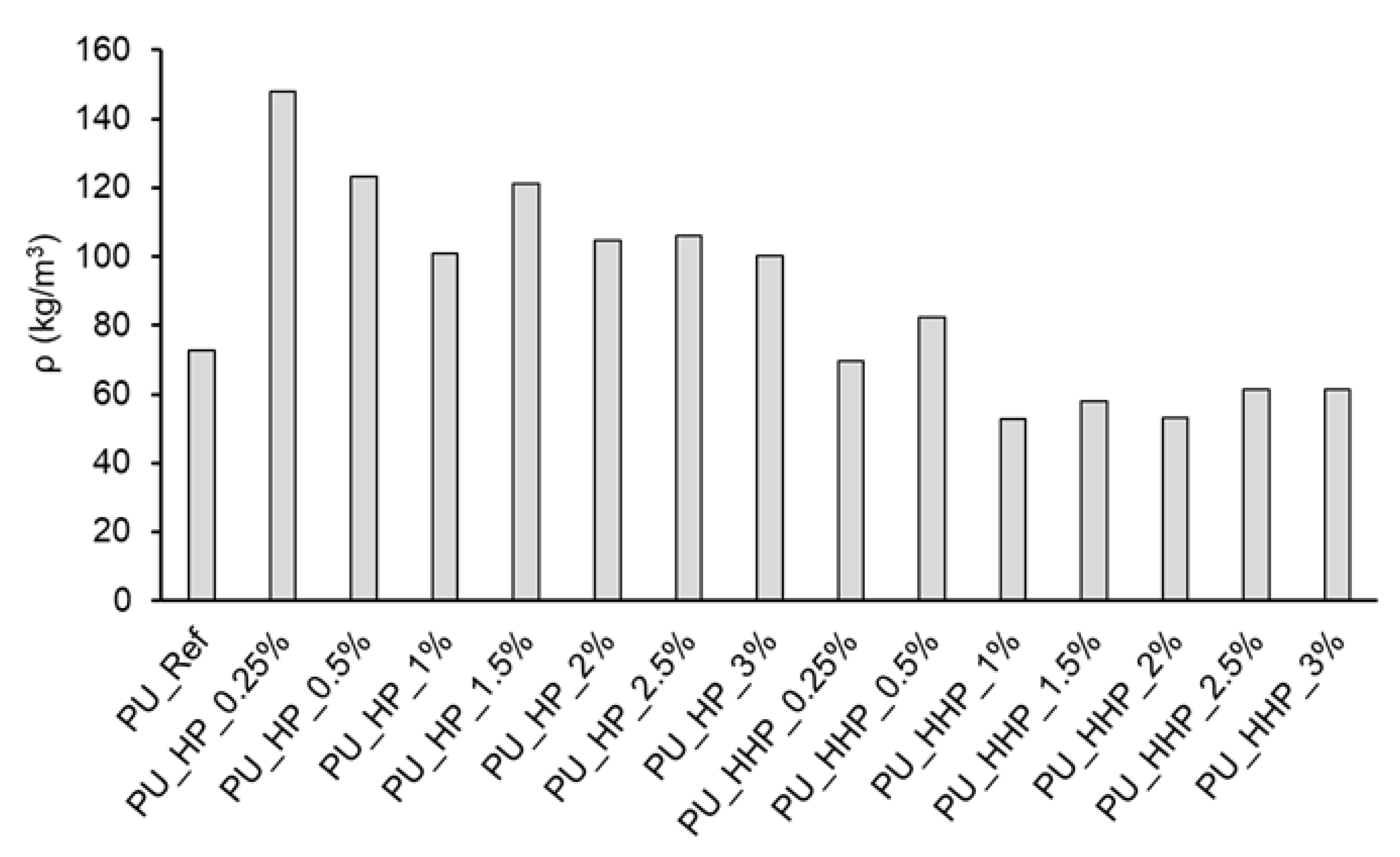
3.2. Polyurethane Foam Characterization
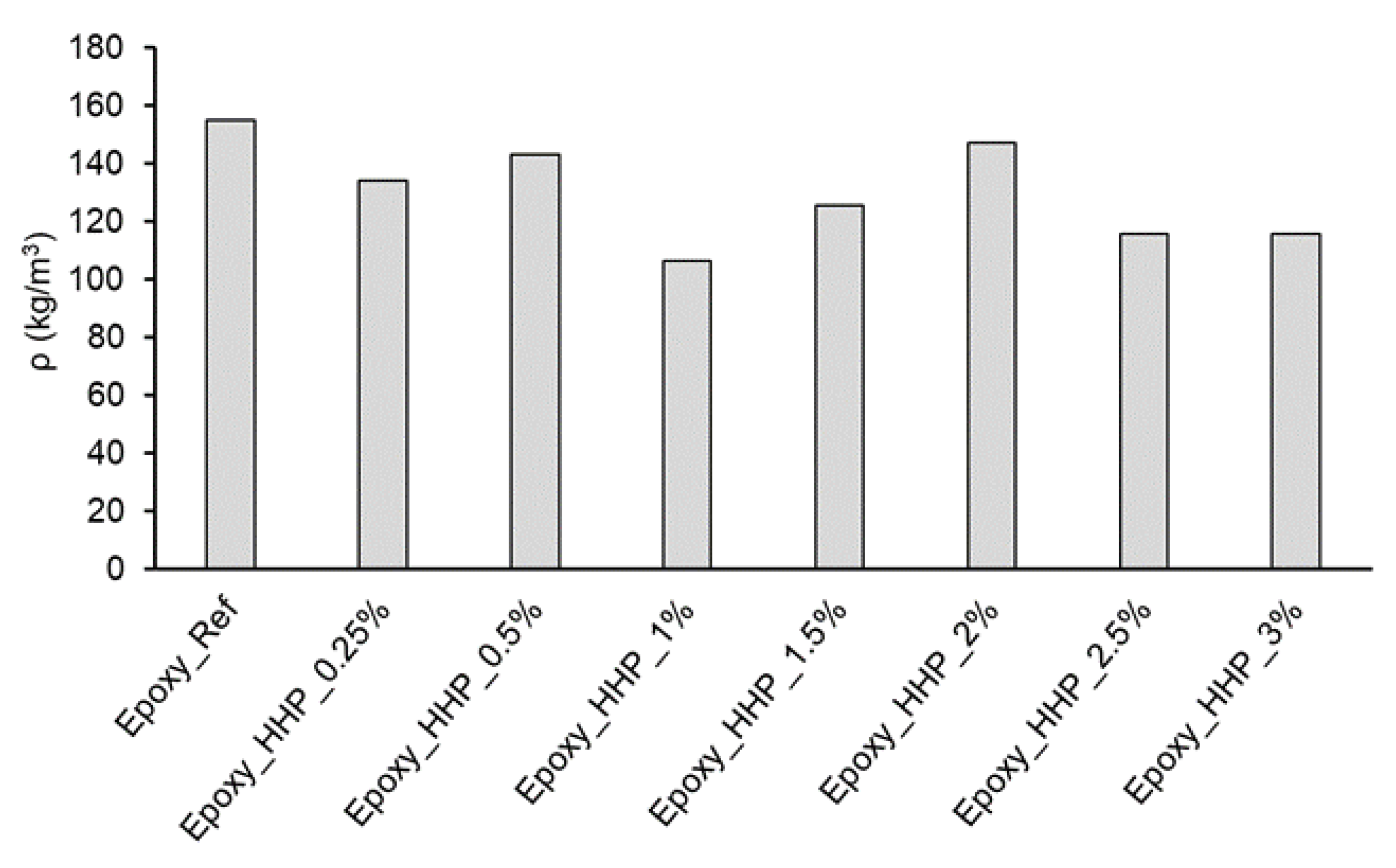
3.3. Epoxy Foam Characterization
3.4. Moisture Uptake of the Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coste, G.; Negrell, C.; Caillol, S. From gas release to foam synthesis, the second breath of blowing agents. Eur. Polym. J. 2020, 140, 110029. [Google Scholar] [CrossRef]
- Kim, B.G.; Gil Lee, D. Development of microwave foaming method for phenolic insulation foams. J. Mater. Process. Technol. 2008, 201, 716–719. [Google Scholar] [CrossRef]
- Basso, M.; Giovando, S.; Pizzi, A.; Celzard, A.; Fierro, V. Tannin/furanic foams without blowing agents and formaldehyde. Ind. Crop. Prod. 2013, 49, 17–22. [Google Scholar] [CrossRef]
- Celzard, A.; Zhao, W.; Pizzi, A.; Fierro, V. Mechanical properties of tannin-based rigid foams undergoing compression. Mater. Sci. Eng. A 2010, 527, 4438–4446. [Google Scholar] [CrossRef]
- Altuna, F.I.; Ruseckaite, R.A.; Stefani, P.M. Biobased Thermosetting Epoxy Foams: Mechanical and Thermal Characterization. ACS Sustain. Chem. Eng. 2015, 3, 1406–1411. [Google Scholar] [CrossRef]
- Ito, A.; Semba, T.; Taki, K.; Ohshima, M. Effect of the molecular weight between crosslinks of thermally cured epoxy resins on the CO2-bubble nucleation in a batch physical foaming process. J. Appl. Polym. Sci. 2014, 131, 40407. [Google Scholar] [CrossRef]
- Omonov, T.S.; Curtis, J.M. Biobased epoxy resin from canola oil. J. Appl. Polym. Sci. 2014, 131, 40142. [Google Scholar] [CrossRef]
- Chen, K.; Tian, C.; Lu, A.; Zhou, Q.; Jia, X.; Wang, J. Effect of SiO2 on rheology, morphology, thermal, and mechanical properties of high thermal stable epoxy foam. J. Appl. Polym. Sci. 2013, 131, 40068. [Google Scholar] [CrossRef]
- Jalalian, M.; Jiang, Q.; Bismarck, A. Air Templated Macroporous Epoxy Foams with Silica Particles as Property-Defining Additive. ACS Appl. Polym. Mater. 2019, 1, 335–343. [Google Scholar] [CrossRef]
- Choe, H.; Lee, J.H.; Kim, J.H. Polyurethane composite foams including CaCO3 fillers for enhanced sound absorption and compression properties. Compos. Sci. Technol. 2020, 194, 108153. [Google Scholar] [CrossRef]
- Wu, G.; Xie, P.; Yang, H.; Dang, K.; Xu, Y.; Sain, M.; Turng, L.-S.; Yang, W. A review of thermoplastic polymer foams for functional applications. J. Mater. Sci. 2021, 56, 11579–11604. [Google Scholar] [CrossRef]
- Zhang, L.; Roy, S.; Chen, Y.; Chua, E.K.; See, K.Y.; Hu, X.; Liu, M. Mussel-Inspired Polydopamine Coated Hollow Carbon Microspheres, a Novel Versatile Filler for Fabrication of High Performance Syntactic Foams. ACS Appl. Mater. Interfaces 2014, 6, 18644–18652. [Google Scholar] [CrossRef]
- Nofar, M. Effects of nano-/micro-sized additives and the corresponding induced crystallinity on the extrusion foaming behavior of PLA using supercritical CO2. Mater. Des. 2016, 101, 24–34. [Google Scholar] [CrossRef]
- Chandni, T.; Anand, K. Utilization of recycled waste as filler in foam concrete. J. Build. Eng. 2018, 19, 154–160. [Google Scholar] [CrossRef]
- Żukowska, W.; Kosmela, P.; Wojtasz, P.; Szczepański, M.; Piasecki, A.; Barczewski, R.; Barczewski, M.; Hejna, A. Comprehensive Enhancement of Prepolymer-Based Flexible Polyurethane Foams’ Performance by Introduction of Cost-Effective Waste-Based Ground Tire Rubber Particles. Materials 2022, 15, 5728. [Google Scholar] [CrossRef]
- Schumacher, A.G.D.; Pequito, S.; Pazour, J. Industrial hemp fiber: A sustainable and economical alternative to cotton. J. Clean. Prod. 2020, 268, 122180. [Google Scholar] [CrossRef]
- Ahmed, A.T.M.F.; Islam, Z.; Mahmud, S.; Sarker, E.; Islam, R. Hemp as a potential raw material toward a sustainable world: A review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; KAIRYTĖ, A. The Impact of Hemp Shives Impregnated with Selected Plant Oils on Mechanical, Thermal, and Insulating Properties of Polyurethane Composite Foams. Materials 2020, 13, 4709. [Google Scholar] [CrossRef]
- Osabohien, E.; Egboh, S.H.O. Utilization of bowstring hemp fiber as a filler in natural rubber compounds. J. Appl. Polym. Sci. 2008, 107, 210–214. [Google Scholar] [CrossRef]
- Yeh, S.-K.; Hsieh, C.-C.; Chang, H.-C.; Yen, C.C.; Chang, Y.-C. Synergistic effect of coupling agents and fiber treatments on mechanical properties and moisture absorption of polypropylene–rice husk composites and their foam. Compos. Part A Appl. Sci. Manuf. 2015, 68, 313–322. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Aranguren, M.I.; Reboredo, M.M. Dependence of the mechanical properties of woodflour-polymer composites on the moisture content. J. Appl. Polym. Sci. 1998, 68, 2069–2076. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and rigid polyurethane foams derived from liquefied lignocellulosic and cellulosic biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Spada, J.C.; Jasper, A.; Tessaro, I.C. Biodegradable Cassava Starch Based Foams Using Rice Husk Waste as Macro Filler. Waste Biomass-Valorization 2020, 11, 4315–4325. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Effect of natural biomass fillers on the stability, degradability, and elasticity of crop straws liquefied polyols-based polyurethane foams. J. Appl. Polym. Sci. 2023, 140, e53324. [Google Scholar] [CrossRef]
- Jonjaroen, V.; Ummartyotin, S.; Chittapun, S. Algal cellulose as a reinforcement in rigid polyurethane foam. Algal Res. 2020, 51, 102057. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Branda, F.; Malucelli, G.; Durante, M.; Piccolo, A.; Mazzei, P.; Costantini, A.; Silvestri, B.; Pennetta, M.; Bifulco, A. Silica Treatments: A Fire Retardant Strategy for Hemp Fabric/Epoxy Composites. Polymers 2016, 8, 313. [Google Scholar] [CrossRef]
- Lei, Z.; Zheng, P.; Niu, L.; Yang, Y.; Shen, J.; Zhang, W.; Wang, C. Ultralight, robustly compressible and super-hydrophobic biomass-decorated carbonaceous melamine sponge for oil/water separation with high oil retention. Appl. Surf. Sci. 2019, 489, 922–929. [Google Scholar] [CrossRef]
- Hwang, U.; Lee, B.; Oh, B.; Shin, H.S.; Lee, S.S.; Kang, S.G.; Kim, D.; Park, J.; Shin, S.; Suhr, J.; et al. Hydrophobic lignin/polyurethane composite foam: An eco-friendly and easily reusable oil sorbent. Eur. Polym. J. 2022, 165, 110971. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.H. Influence of filler surface characteristics on morphological, physical, acoustic properties of polyurethane composite foams filled with inorganic fillers. Compos. Sci. Technol. 2017, 146, 147–154. [Google Scholar] [CrossRef]
- Cusola, O.; Valls, C.; Vidal, T.; Tzanov, T.; Roncero, M.B. Electrochemical Insights on the Hydrophobicity of Cellulose Substrates Imparted by Enzymatically Oxidized Gallates with Increasing Alkyl Chain Length. ACS Appl. Mater. Interfaces 2015, 7, 13834–13841. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.G.; González, M.D.; Monmany, J.M.D.; Tzanov, T. Effects of alkyl chain lengths of gallates upon enzymatic wool functionalisation. J. Mol. Catal. B Enzym. 2010, 67, 231–235. [Google Scholar] [CrossRef]
- Garcia-Ubasart, J.; Colom, J.F.; Vila, C.; Hernández, N.G.; Roncero, M.B.; Vidal, T. A new procedure for the hydrophobization of cellulose fibre using laccase and a hydrophobic phenolic compound. Bioresour. Technol. 2012, 112, 341–344. [Google Scholar] [CrossRef]
- ASTM D1622-03; Standard Test Method for Apparent Density of Rigid Cellular Plastics, Book of Standards Volume: 08.01. ASTM International: West Conshohocken, PA, USA, 2010.
- ISO 844:2021. Rigid Cellular Plastics—Determination of Compression Properties, Technical Committee: ISO/TC 61/SC 10 Cellular plastics. Available online: https://www.iso.org/standard/73560.html (accessed on 1 August 2023).
- Peng, D.; Li, W.; Liang, X.; Zheng, L.; Guo, X. Enzymatic preparation of hydrophobic biomass with one-pot synthesis and the oil removal performance. J. Environ. Sci. 2023, 124, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Moghim, M.H.; Keshavarz, M.; Zebarjad, S.M. Effect of SiO2 nanoparticles on compression behavior of flexible polyure-thane foam. Polym. Bull. 2019, 76, 227–239. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.; Naik, Y. Mechanical, Morphological and Thermal Properties of Rigid Polyurethane Foam: Effect of the Fillers. Cell. Polym. 2007, 26, 245–259. [Google Scholar] [CrossRef]



| Component | Amount, g |
|---|---|
| Cardyon® LC 05 | 16.37 |
| Poly(propylene glycol)4000 | 4.01 |
| Eternacoll UT-200 | 3.04 |
| Polyethylene glycol 600 | 1.96 |
| Water + CMC_2.5% | 2.50 |
| Aspartic acid | 1.02 |
| Exolit® OP 560 | 5.73 |
| Dibutyltin dilaurate | 1.60 |
| Formic acid | 1.39 |
| LED-103 | 0.05 |
| Tween 80 | 1.02 |
| Niax silicone L-6164 | 1.02 |
| Ortegol 500 | 1.23 |
| Poly(methylhydrosiloxane) | 0.82 |
| iso 133/6 | 19.24 |
| Ongronat CO5700 | 39.00 |
| 100.00 |
| Component | Amount, g |
|---|---|
| SR GreenPoxy 56 | 72.46 |
| Cardolite® NX-4001 | 3.62 |
| Cardolite® NC-513 | 3.62 |
| SZ 8525 | 18.12 |
| Poly(methylhydrosiloxane) | 2.18 |
| 100.00 |
| Sample | Moisture Uptake,% | Sample | Moisture Uptake,% | Sample | Moisture Uptake,% |
|---|---|---|---|---|---|
| PU_Ref | 5.06 ± 0.21 | Epoxy_Ref | 1.23 ± 0.27 | ||
| PU_HP_0.25% | 4.99 ± 0.19 | PU_HHP_0.25% | 4.04 ± 0.50 | Epoxy_HHP_0.25% | 1.10 ± 0.58 |
| PU_HP_0.5% | 4.97 ± 0.19 | PU_HHP_0.5% | 3.20 ± 0.44 | Epoxy_HHP_0.5% | 0.95 ± 0.19 |
| PU_HP_1% | 4.91 ± 0.21 | PU_HHP_1% | 2.81 ± 0.34 | Epoxy_HHP_1% | 0.90 ± 0.42 |
| PU_HP_1.5% | 4.90 ± 0.14 | PU_HHP_1.5% | 2.12 ± 0.31 | Epoxy_HHP_1.5% | 0.85 ± 0.30 |
| PU_HP_2% | 4.84 ± 0.23 | PU_HHP_2% | 1.86 ± 0.60 | Epoxy_HHP_2% | 0.82 ± 0.50 |
| PU_HP_2.5% | 4.23 ± 0.16 | PU_HHP_2.5% | 1.28 ± 0.38 | Epoxy_HHP_2.5% | 0.79 ± 0.23 |
| PU_HP_3% | 4.18 ± 0.30 | PU_HHP_3% | 1.04 ± 0.29 | Epoxy_HHP_3% | 0.68 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreres, G.; Pérez-Rafael, S.; Morena, A.G.; Tzanov, T.; Gryshchuk, L. Influence of Enzymatically Hydrophobized Hemp Protein on Morphology and Mechanical Properties of Bio-Based Polyurethane and Epoxy Foams. Polymers 2023, 15, 3608. https://doi.org/10.3390/polym15173608
Ferreres G, Pérez-Rafael S, Morena AG, Tzanov T, Gryshchuk L. Influence of Enzymatically Hydrophobized Hemp Protein on Morphology and Mechanical Properties of Bio-Based Polyurethane and Epoxy Foams. Polymers. 2023; 15(17):3608. https://doi.org/10.3390/polym15173608
Chicago/Turabian StyleFerreres, Guillem, Sílvia Pérez-Rafael, Angela Gala Morena, Tzanko Tzanov, and Liudmyla Gryshchuk. 2023. "Influence of Enzymatically Hydrophobized Hemp Protein on Morphology and Mechanical Properties of Bio-Based Polyurethane and Epoxy Foams" Polymers 15, no. 17: 3608. https://doi.org/10.3390/polym15173608
APA StyleFerreres, G., Pérez-Rafael, S., Morena, A. G., Tzanov, T., & Gryshchuk, L. (2023). Influence of Enzymatically Hydrophobized Hemp Protein on Morphology and Mechanical Properties of Bio-Based Polyurethane and Epoxy Foams. Polymers, 15(17), 3608. https://doi.org/10.3390/polym15173608










