On-Demand Tunability of Microphase Separation Structure of 3D Printing Material by Reversible Addition/Fragmentation Chain Transfer Polymerization
Abstract
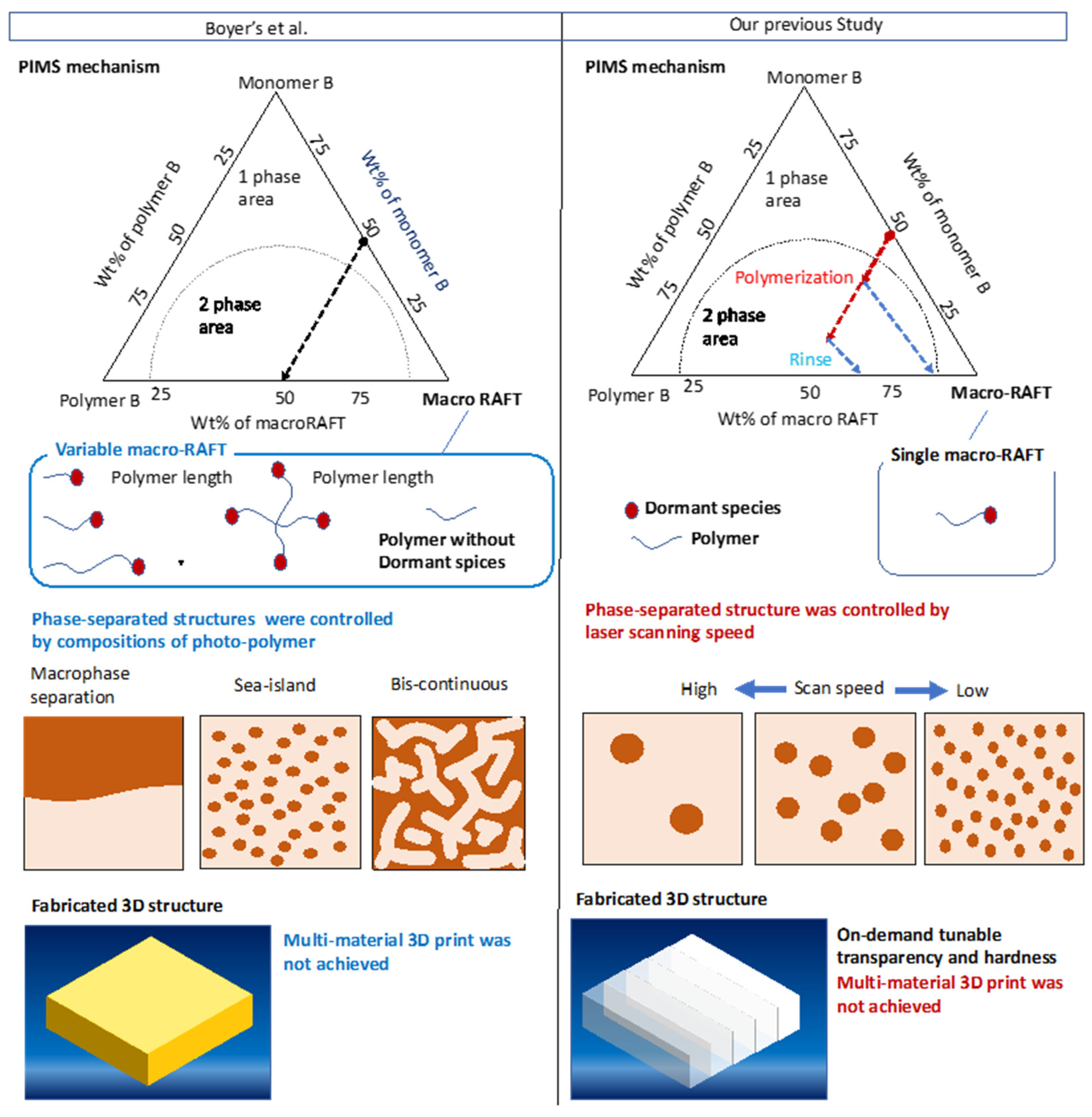
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
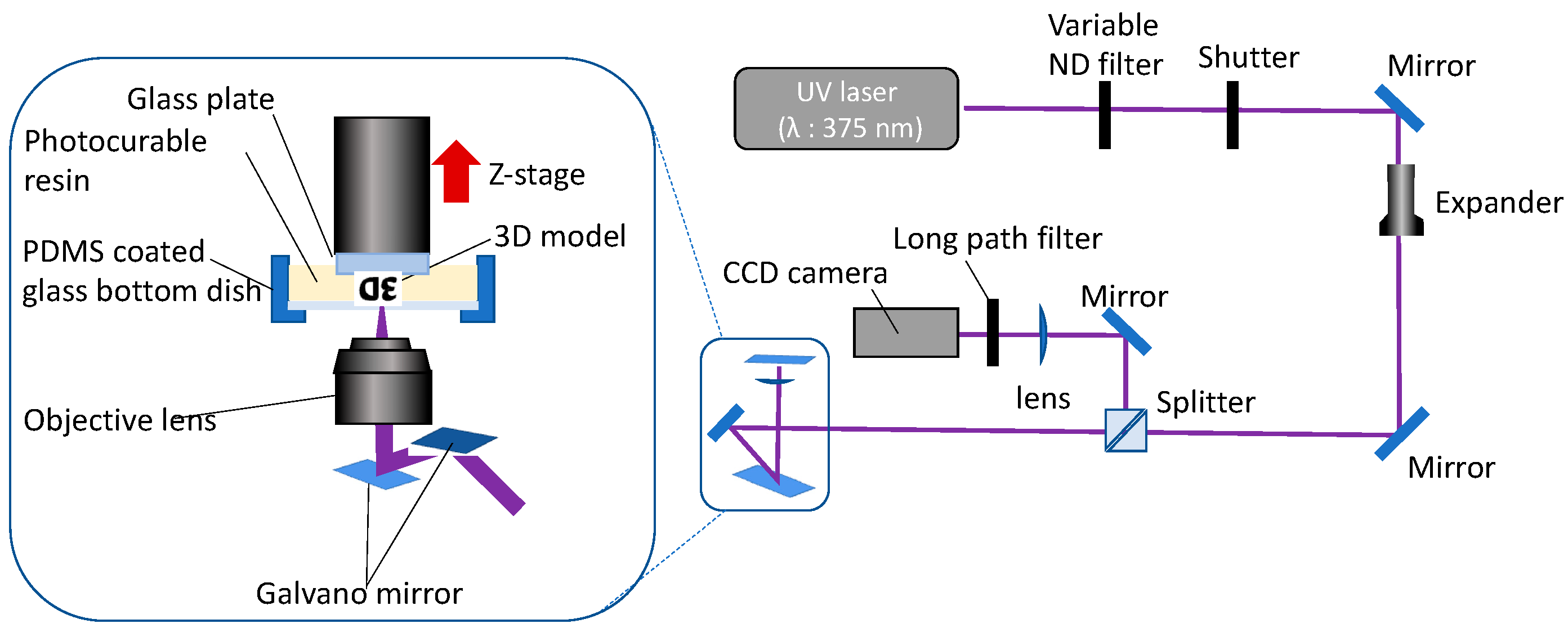
2.3. Stereolithography Systems
2.4. Synthesis of Dithiocarbamate-Terminated Short-Length Poly(n-Butyl Acrylate) (ShortDTC-PBA)
2.5. Synthesis of Dithiocarbamate-Terminated Long-Chain Poly(n-Butyl Acrylate) (LongDTC-PBA)
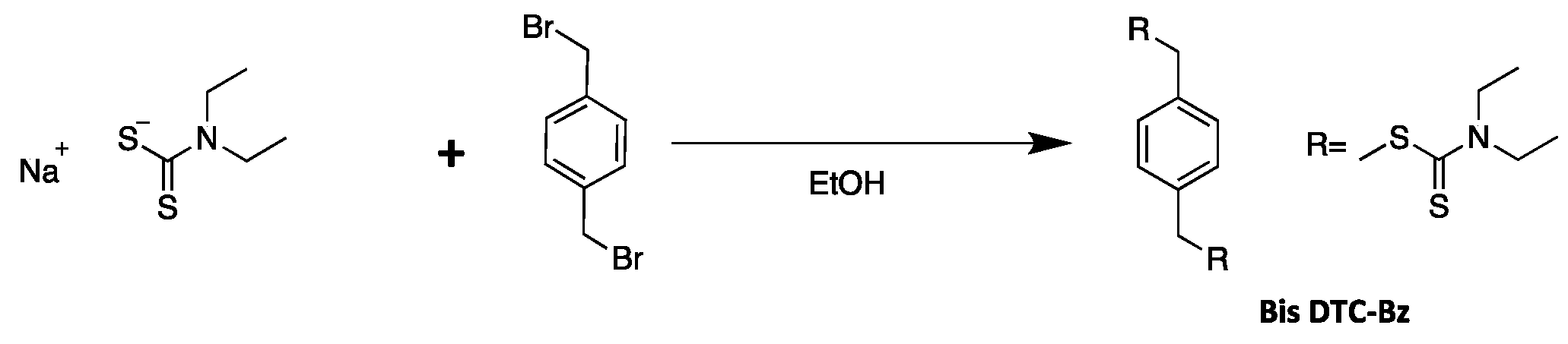
2.6. Synthesis of Bis-Functional RAFT Agent (BisDTC-Bz)
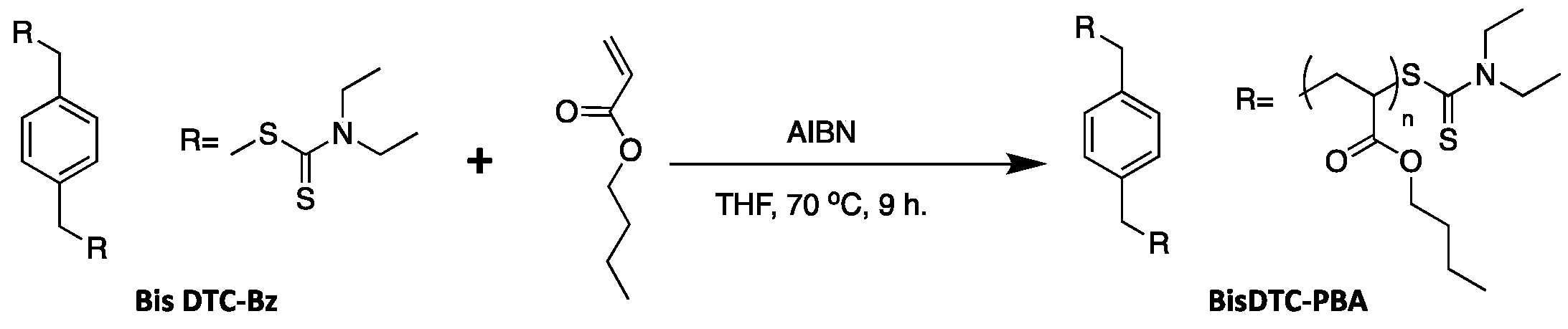
2.7. Synthesis of Dithiocarbamate-Terminated 2-Arm Poly(n-Butyl Acrylate) (BisDTC-PBA)
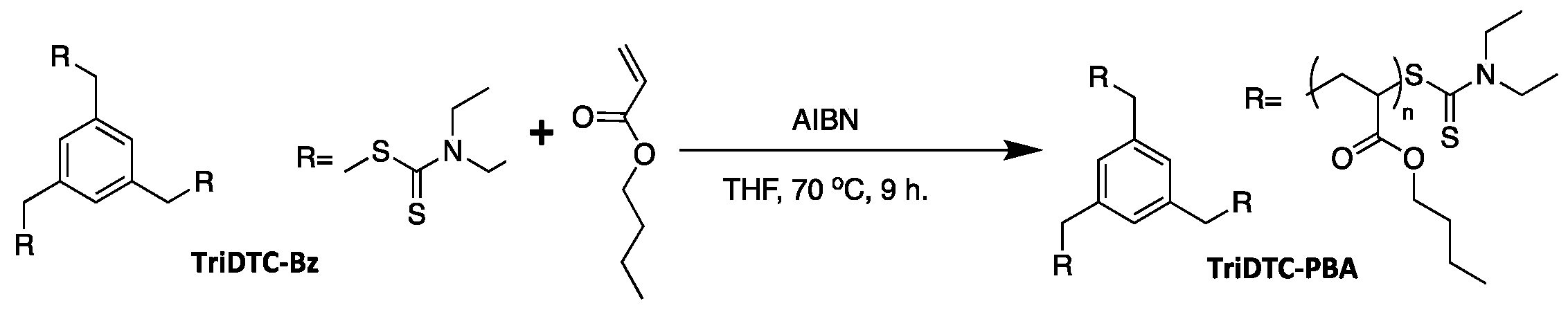
2.8. Synthesis of Tri-Functional RAFT Agent (TriDTC-Bz)
2.9. Synthesis of Dithiocarbamate-Terminated 3-Arm Poly(n-Butyl Acrylate) (TriDTC-PBA)
2.10. Preparation of Photocurable Resin
3. Results and Discussion
3.1. Characterization of Dithiocarbamate-Terminated Macro-RAFT Agent
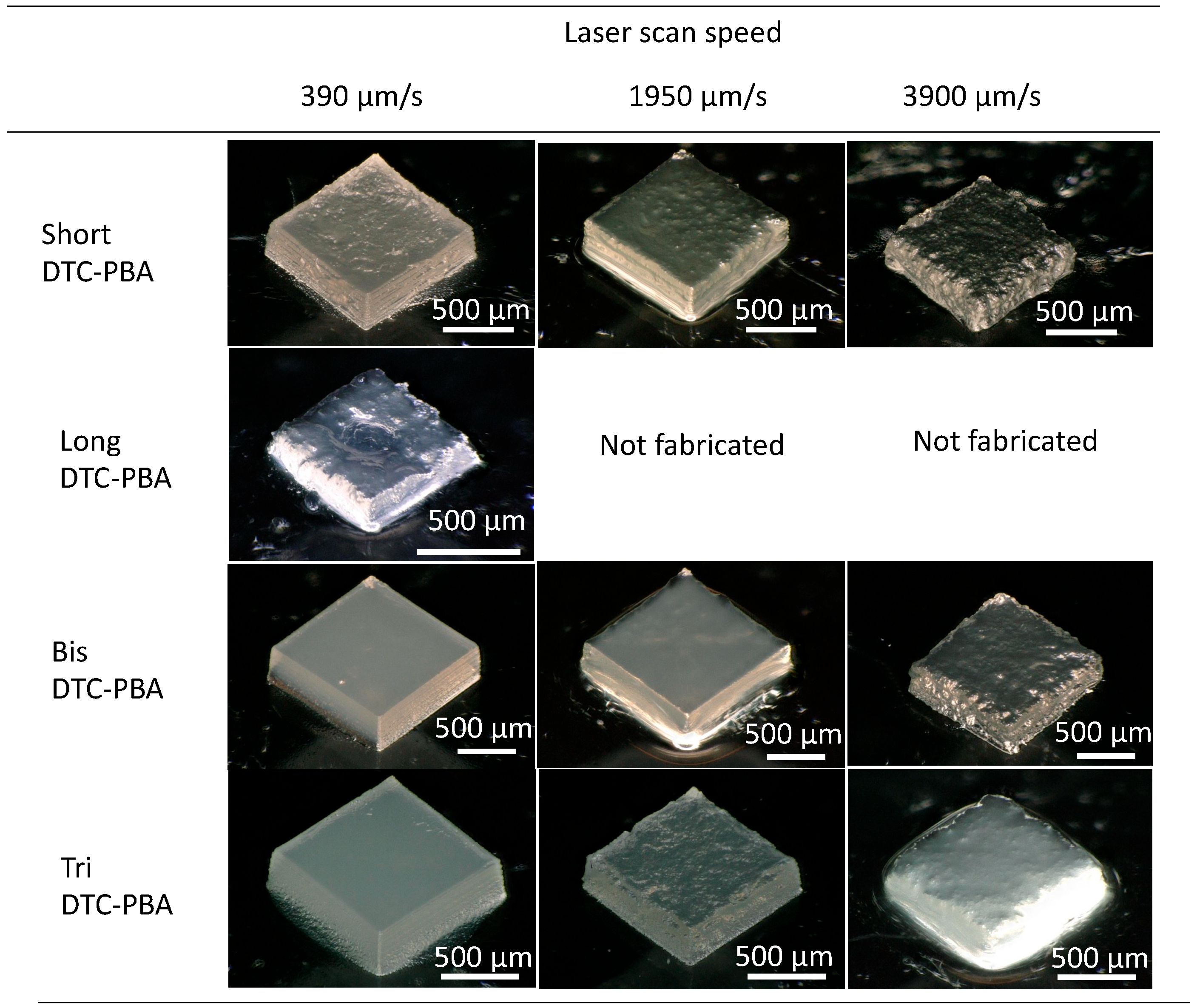
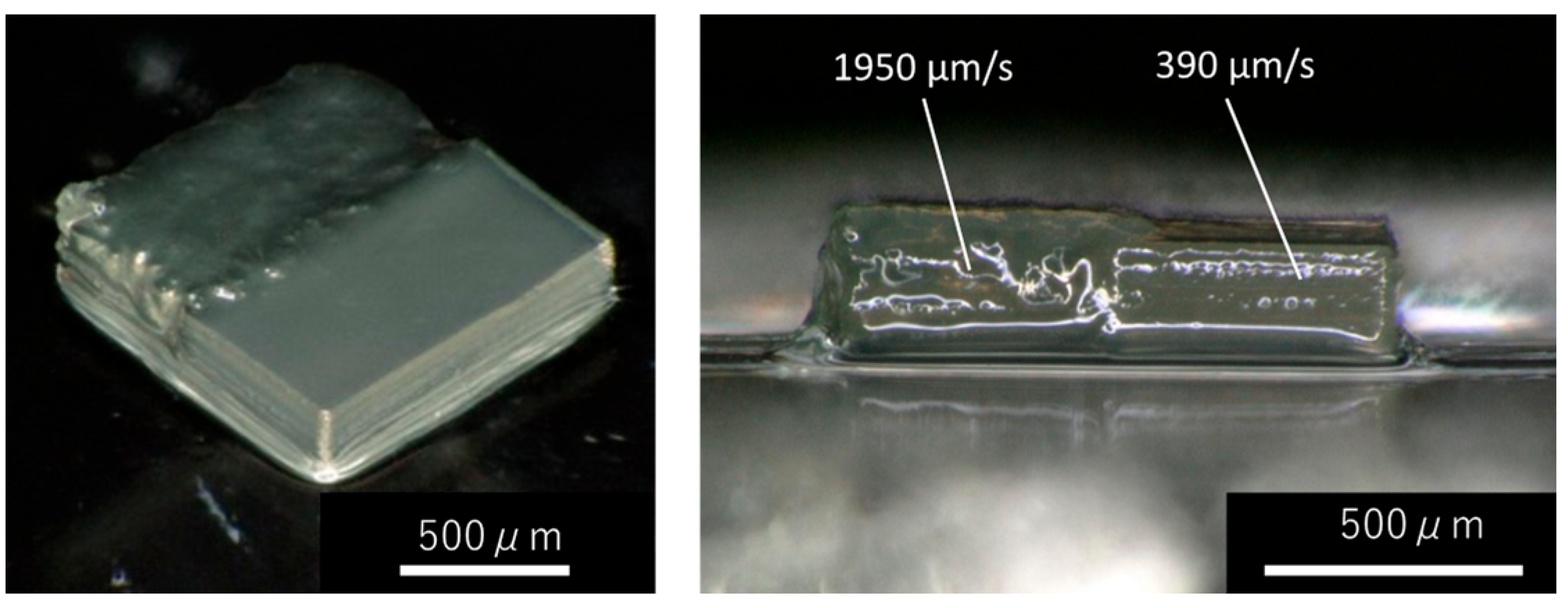
3.2. Variation of Phase-Separated Structure with Macro-RAFT Agent and Scanning Speed
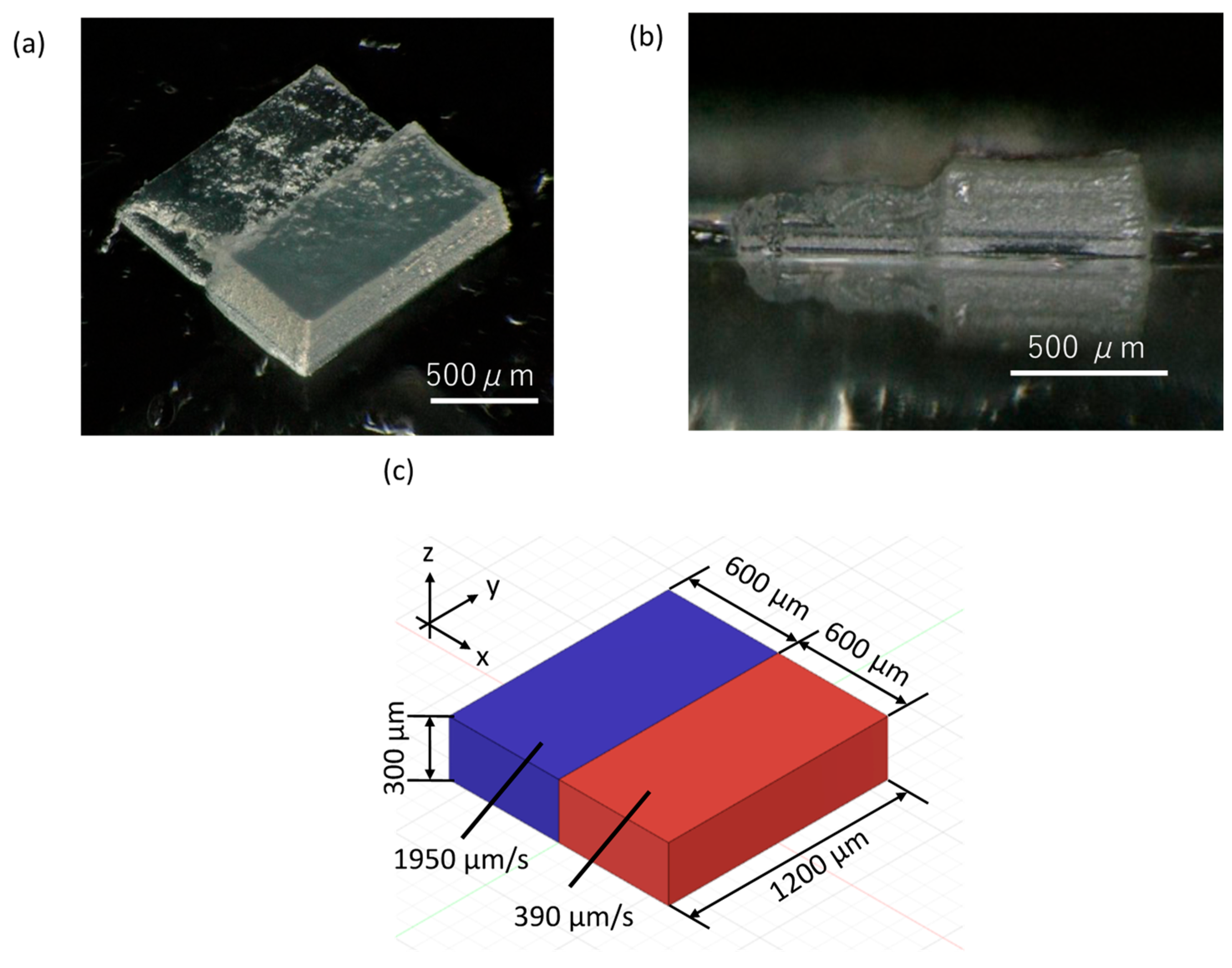
3.3. Shrinkage and Correction of Fabricated Objects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Qin, Q.; Wang, J. A review of stereolithography: Processes and systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Fischer, J.; Wegener, M. Three-dimensional optical laser lithography beyond the diffraction limit. Laser Photonics Rev. 2013, 7, 22–44. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Salonitis, K. 10.03—Stereolithography. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 19–67. [Google Scholar]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Bagheri, A. Application of RAFT in 3D printing: Where are the future opportunities? Macromolecules 2023, 56, 1778–1797. [Google Scholar] [CrossRef]
- Moad, G. A critical survey of dithiocarbamate reversible addition-fragmentation chain transfer (RAFT) agents in radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 216–227. [Google Scholar] [CrossRef]
- Bagheri, A.; Engel, K.E.; Bainbridge, C.W.A.; Xu, J.; Boyer, C.; Jin, J. 3D printing of polymeric materials based on photo-RAFT polymerization. Polym. Chem. 2020, 11, 641–647. [Google Scholar] [CrossRef]
- Zhang, Z.; Corrigan, N.; Bagheri, A.; Jin, J.; Boyer, C. A versatile 3D and 4D printing system through photocontrolled RAFT polymerization. Angew. Chem. Int. Ed. 2019, 58, 17954–17963. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Bainbridge, C.W.A.; Engel, K.E.; Qiao, G.G.; Xu, J.; Boyer, C.; Jin, J. Oxygen tolerant PET-RAFT facilitated 3D printing of polymeric materials under visible LEDs. ACS Appl. Polym. Mater. 2020, 2, 782–790. [Google Scholar] [CrossRef]
- Lee, K.; Corrigan, N.; Boyer, C. Rapid high-resolution 3D printing and surface functionalization via type I photoinitiated RAFT polymerization. Angew. Chem. Int. Ed. 2021, 60, 8839–8850. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J.; Pan, X.; Zhang, Z.; Jin, G.; Zhu, J. Photoinduced free radical promoted cationic RAFT polymerization toward “living” 3D printing. ACS Macro Lett. 2021, 10, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, C.W.A.; Engel, K.E.; Jin, J. 3D printing and growth induced bending based on PET-RAFT polymerization. Polym. Chem. 2020, 11, 4084–4093. [Google Scholar] [CrossRef]
- Bagheri, A.; Asadi-Eydivand, M.; Rosser, A.A.; Fellows, C.M.; Brown, T.C. 3D Printing of customized drug delivery systems with controlled architecture via reversible addition-fragmentation chain transfer polymerization. Adv. Eng. Mater. 2022, 25, 2201785. [Google Scholar] [CrossRef]
- Zhang, Z.; Corrigan, N.; Boyer, C. A Photoinduced dual-wavelength approach for 3D printing and self-healing of thermosetting materials. Angew. Chem. Int. Ed. 2022, 61, e202114111. [Google Scholar] [CrossRef]
- Bagheri, A.; Ling, H.; Bainbridge, C.W.A.; Jin, J. Living Polymer Networks Based on a RAFT Cross-Linker: Toward 3D and 4D Printing Applications. ACS Appl. Polym. Mater. 2021, 3, 2921–2930. [Google Scholar] [CrossRef]
- Bobrin, V.A.; Lee, K.; Zhang, J.; Corrigan, N.; Boyer, C. Nanostructure control in 3D printed materials. Adv. Mater. 2022, 34, 2107643. [Google Scholar] [CrossRef]
- Seo, M.; Hillmyer Marc, A. Reticulated nanoporous polymers by controlled polymerization-induced microphase separation. Science 2012, 336, 1422–1425. [Google Scholar] [CrossRef]
- Oh, J.; Seo, M. photoinitiated polymerization-induced microphase separation for the preparation of nanoporous polymer films. ACS Macro Lett. 2015, 4, 1244–1248. [Google Scholar] [CrossRef]
- Schulze, M.W.; Hillmyer, M.A. Tuning mesoporosity in cross-linked nanostructured thermosets via polymerization-induced microphase separation. Macromolecules 2017, 50, 997–1007. [Google Scholar] [CrossRef]
- Sicher, A.; Ganz, R.; Menzel, A.; Messmer, D.; Panzarasa, G.; Feofilova, M.; Prum, R.O.; Style, R.W.; Saranathan, V.; Rossi, R.M.; et al. Structural color from solid-state polymerization-induced phase separation. Soft Matter 2021, 17, 5772–5779. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Kawazoe, K.; Nakanishi, H.; Norisuye, T.; Tran-Cong-Miyata, Q. Influences of wetting and shrinkage on the phase separation process of polymer mixtures induced by photopolymerization. Soft Matter 2013, 9, 8428–8437. [Google Scholar] [CrossRef]
- Maruyama, T.; Mukai, M.; Sato, R.; Iijima, M.; Sato, M.; Furukawa, T.; Maruo, S. Multifunctional 3D printing of heterogeneous polymer structures by laser-scanning micro-stereolithography using reversible addition–fragmentation chain-transfer polymerization. ACS Appl. Polym. Mater. 2022, 4, 5515–5523. [Google Scholar] [CrossRef]
- Shi, X.; Bobrin, V.A.; Yao, Y.; Zhang, J.; Corrigan, N.; Boyer, C. Designing nanostructured 3D printed materials by controlling macromolecular architecture. Angew. Chem. Int. Ed. 2022, 61, e202206272. [Google Scholar] [CrossRef] [PubMed]
- Bobrin, V.A.; Yao, Y.; Shi, X.; Xiu, Y.; Zhang, J.; Corrigan, N.; Boyer, C. Nano- to macro-scale control of 3D printed materials via polymerization induced microphase separation. Nat. Commun. 2022, 13, 3577. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Kim, H.-C.; Wicker, R. Multi-material stereolithography. J. Mater. Process. Technol. 2011, 211, 318–328. [Google Scholar] [CrossRef]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 1997, 22, 132–134. [Google Scholar] [CrossRef]
- Maruyama, T.; Hirata, H.; Furukawa, T.; Maruo, S. Multi-material microstereolithography using a palette with multicolor photocurable resins. Opt. Mater. Express 2020, 10, 2522–2532. [Google Scholar] [CrossRef]
- Takenouchi, M.; Mukai, M.; Furukawa, T.; Maruo, S. Fabrication of flexible wiring with intrinsically conducting polymers using blue-laser microstereolithography. Polymers 2022, 14, 4949. [Google Scholar] [CrossRef]
- Hahn, V.; Kiefer, P.; Frenzel, T.; Qu, J.; Blasco, E.; Barner-Kowollik, C.; Wegener, M. Rapid assembly of small materials building blocks (voxels) into large functional 3D metamaterials. Adv. Funct. Mater. 2022, 30, 1907795. [Google Scholar] [CrossRef]
- Thiele, S.; Arzenbacher, K.; Gissibl, T.; Giessen, H.; Herkommer, A.M. 3D-printed eagle eye: Compound microlens system for foveated imaging. Sci. Adv. 2017, 3, e1602655. [Google Scholar] [CrossRef]
- Sadeqi, A.; Rezaei Nejad, H.; Owyeung, R.E.; Sonkusale, S. Three-dimensional printing of metamaterial embedded geometrical optics (MEGO). Microsyst. Nanoeng. 2019, 5, 16. [Google Scholar] [CrossRef]
- Moritoki, Y.; Furukawa, T.; Sun, J.; Yokoyama, M.; Shimono, T.; Yamada, T.; Nishiwaki, S.; Kageyama, T.; Fukuda, J.; Mukai, M.; et al. 3D-Printed micro-tweezers with a compliant mechanism designed using topology optimization. Micromachines 2021, 12, 579. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Sarabi, M.R.; Birtek, M.T.; Seyfi, S.; Sitti, M.; Tasoglu, S. 3D-printed microrobots from design to translation. Nat. Commun. 2022, 13, 5875. [Google Scholar] [CrossRef]
- Junkers, T.; Koo, S.P.S.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. Mapping poly(butyl acrylate) product distributions by mass spectrometry in a wide temperature range: Suppression of midchain radical side reactions. Macromolecules 2007, 40, 8906–8912. [Google Scholar] [CrossRef]
- Derboven, P.; Van Steenberge, P.H.M.; Vandenbergh, J.; Reyniers, M.-F.; Junkers, T.; D’Hooge, D.R.; Marin, G.B. Improved livingness and control over branching in RAFT polymerization of acrylates: Could microflow synthesis make the difference? Macromol. Rapid Commun. 2015, 36, 2117. [Google Scholar] [CrossRef]
- Grigoras, M.; Negru, O.-I. Synthesis of star poly(N-vinylcarbazole) by microwave-assisted reversible addition-fragmentation chain transfer polymerization (RAFT). Polymers 2012, 4, 1183–1194. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, P.; Severac, R.; Boutevin, B. Reverse iodine transfer polymerization of methyl acrylate and n-butyl acrylate. Macromolecules 2005, 38, 6299–6309. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-Y.; Wang, C.-C. Thermal degradation kinetics of poly(n-butyl acrylate) initiated by lactams and thiols. Polym. Degrad. Stabil. 2004, 84, 505–514. [Google Scholar] [CrossRef]
- Overney, R.M.; Tsukruk, V.V. Scanning Probe Microscopy in Polymers: Introductory Notes. In Scanning Probe Microscopy of Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; Volume 694, pp. 2–30. [Google Scholar]



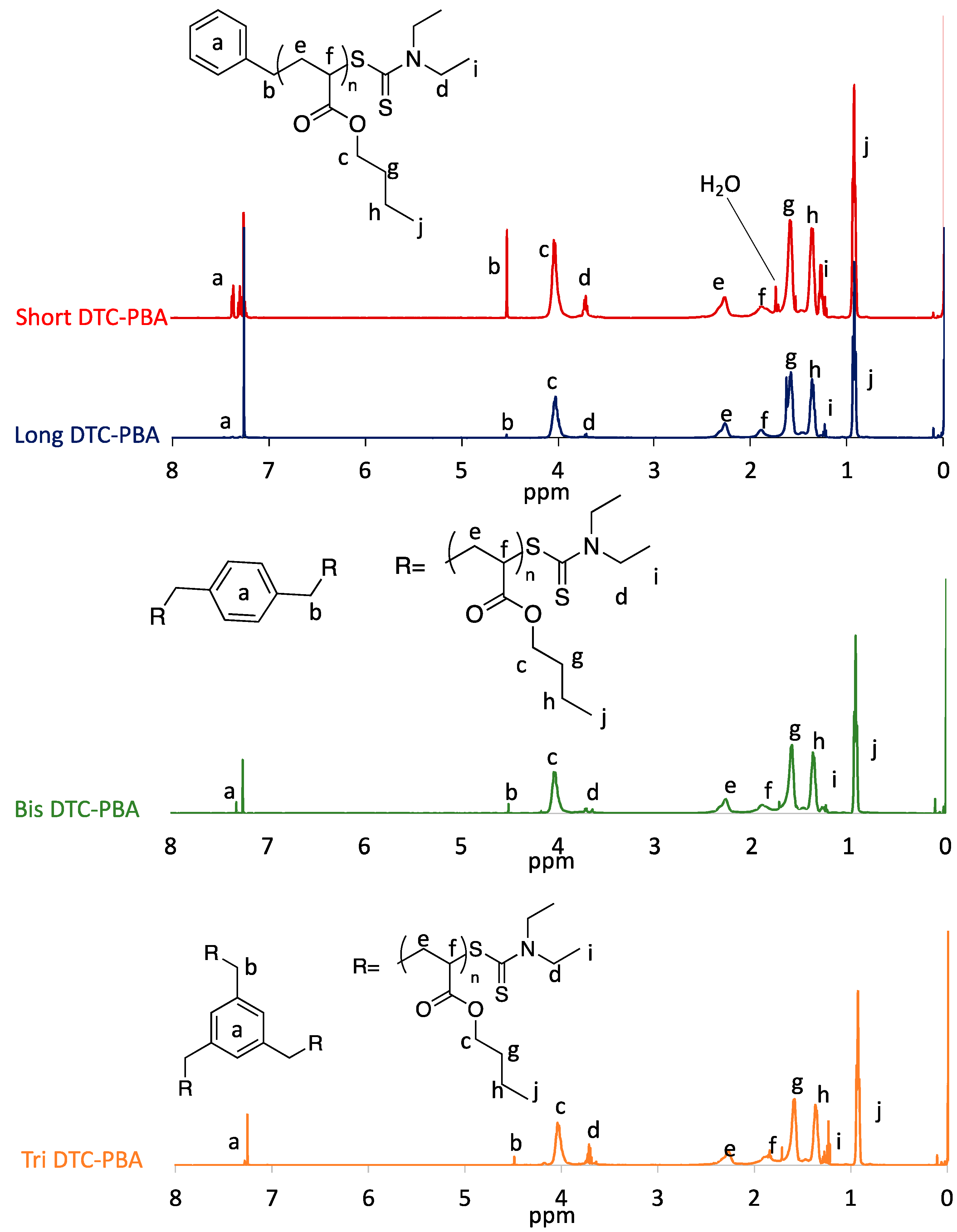


| Sample | Peaks | DP b | [BA]/ [RAFT] | i/a | |
|---|---|---|---|---|---|
| a | i | ||||
| ShortDTC-PBA | - c | 0.67 | 9 | 10 | - |
| StandardDTC-PBA d | - c | 0.14 | 41 | 50 | - |
| LongDTC-PBA | - c | 0.0025 | 240 | 200 | - |
| BisDTC-PBA | 0.029 | 0.091 | 66 | 80 | 3.1 |
| TriDTC-PBA | 0.012 | 0.150 | 40 | 118 | 12.5 |
| Sample | Mn | ĐM |
|---|---|---|
| ShortDTC-PBA | 2.1 k | 2.2 |
| StandardDTC-PBA | 5.0 k | 1.7 |
| LongDTC-PBA | 10.9 k | 1.6 |
| BisDTC-PBA | 3.4 k | 1.8 |
| TriDTC-PBA | 3.6 k | 1.7 |
| Scan Speed (µm/s) | Dimension of 3D CAD Model | ||
|---|---|---|---|
| X: Width (µm) | Y: Length (µm) | Z: Height (µm) | |
| 1950 | 600 | 1200 | 300 |
| 390 | 600 | 1200 | 300 |
| Scan speed (µm/s) | Measured dimensions of fabricated objects | ||
| X: Width | Y: Length | Z: Height | |
| 1950 | 467 µm (79%) | 1117 µm (93%) | 215 µm (71%) |
| 390 | 569 µm (94%) | 1148 µm (96%) | 282 µm (94%) |
| Scan Speed (µm/s) | Dimensions of the 3D CAD Model with Compensation Values | ||
|---|---|---|---|
| X: Width (µm) | Y: Length (µm) | Z: Height (µm) | |
| 1950 | 771 | 1200 | 420 |
| 390 | 663 | 1200 | 330 |
| Scan speed (µm/s) | Measured dimensions of fabricated objects | ||
| X: Width | Y: Length | Z: Height | |
| 1950 | 558 ± 7 µm (93 ± 4%) | 1158 ± 5 µm (96 ± 0.4%) | 309 ± 11 µm (103 ± 4%) |
| 390 | 598 ± 16 µm (100 ± 2%) | 1187 ± 4 µm (99 ± 0.3%) | 284 ± 19 µm (93 ± 6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukai, M.; Sato, M.; Miyadai, W.; Maruo, S. On-Demand Tunability of Microphase Separation Structure of 3D Printing Material by Reversible Addition/Fragmentation Chain Transfer Polymerization. Polymers 2023, 15, 3519. https://doi.org/10.3390/polym15173519
Mukai M, Sato M, Miyadai W, Maruo S. On-Demand Tunability of Microphase Separation Structure of 3D Printing Material by Reversible Addition/Fragmentation Chain Transfer Polymerization. Polymers. 2023; 15(17):3519. https://doi.org/10.3390/polym15173519
Chicago/Turabian StyleMukai, Masaru, Mituki Sato, Wakana Miyadai, and Shoji Maruo. 2023. "On-Demand Tunability of Microphase Separation Structure of 3D Printing Material by Reversible Addition/Fragmentation Chain Transfer Polymerization" Polymers 15, no. 17: 3519. https://doi.org/10.3390/polym15173519
APA StyleMukai, M., Sato, M., Miyadai, W., & Maruo, S. (2023). On-Demand Tunability of Microphase Separation Structure of 3D Printing Material by Reversible Addition/Fragmentation Chain Transfer Polymerization. Polymers, 15(17), 3519. https://doi.org/10.3390/polym15173519






