Preparation and Characterization of Silica-Based Ionogel Electrolytes and Their Application in Solid-State Lithium Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Preparation of Ionic Liquids (IL)
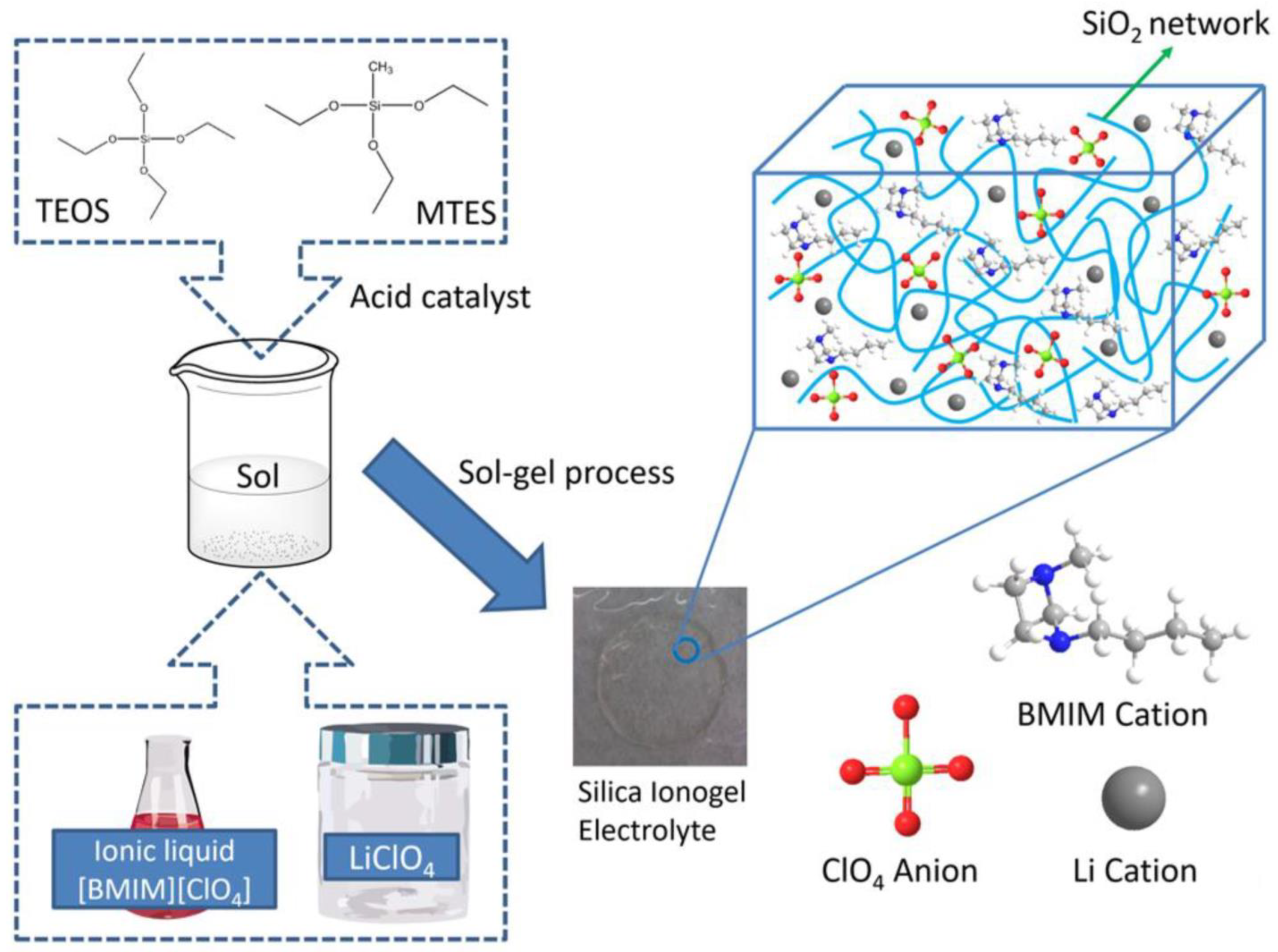
2.4. Preparation of Silica Ionogel Electrolytes
2.5. Material Identification and Analysis Methods
2.6. Descriptions of Electrochemical Measurements
3. Results and Discussion
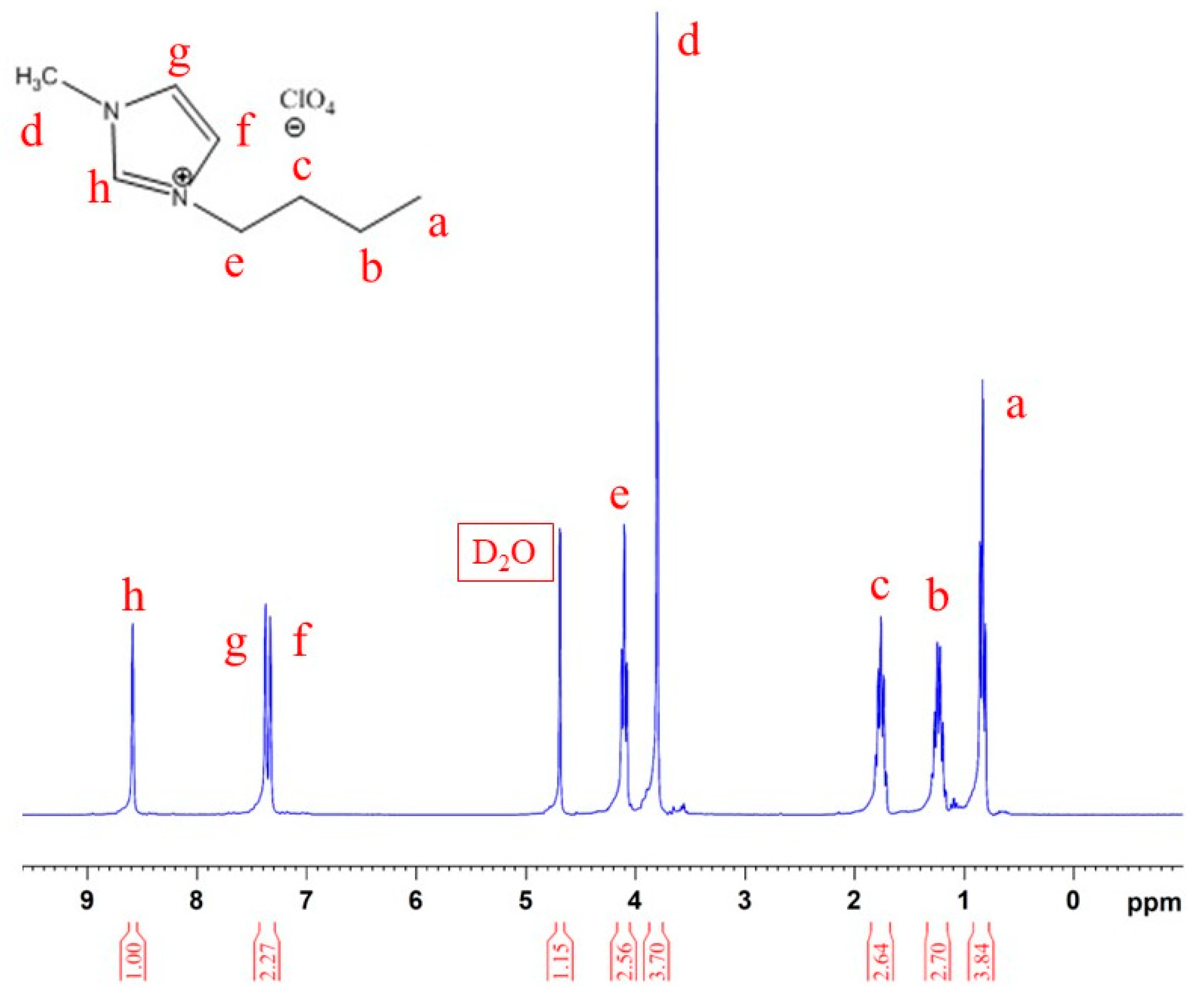
3.1. Identification of BMIC and BMIM-ClO4 Ionic Liquid
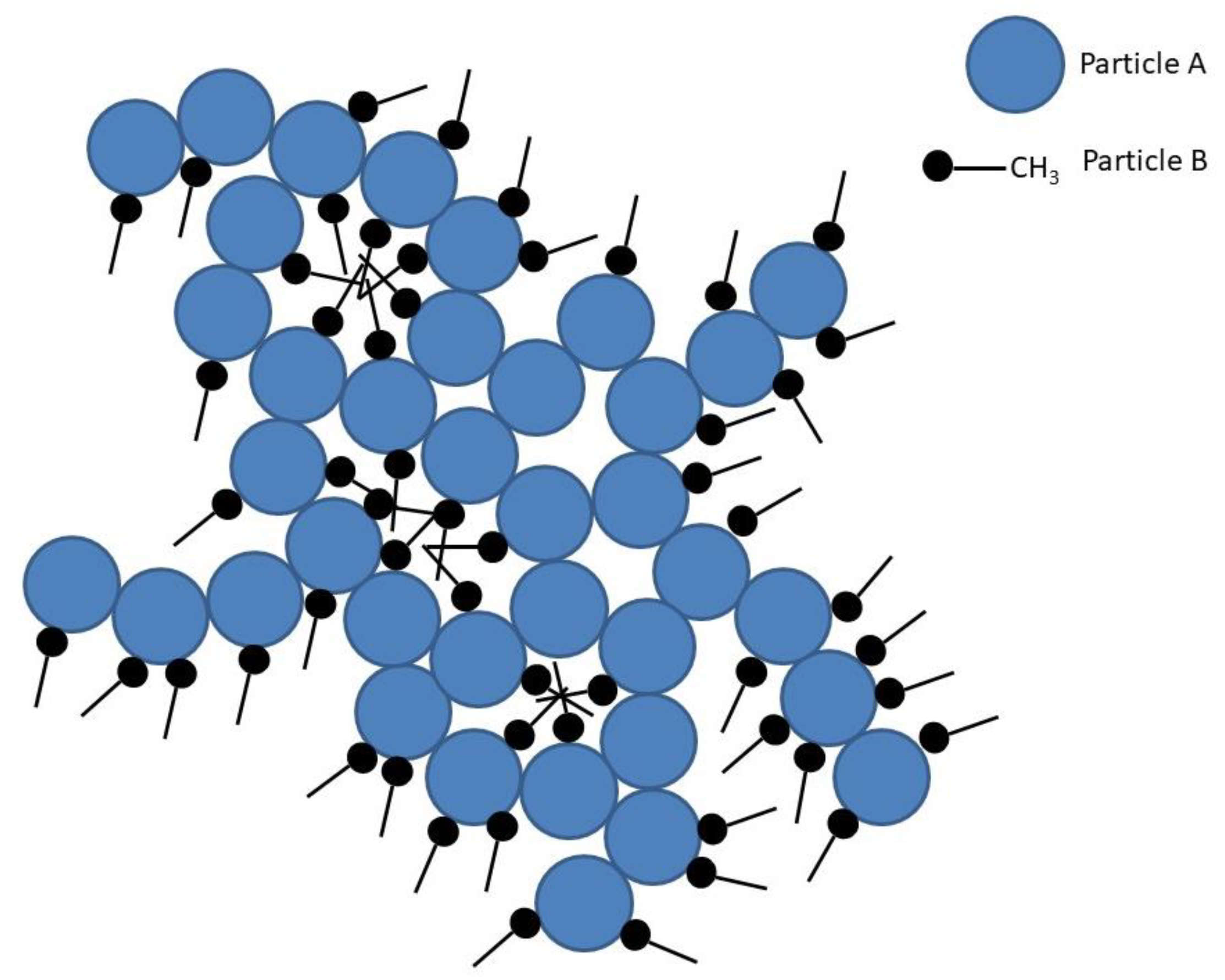
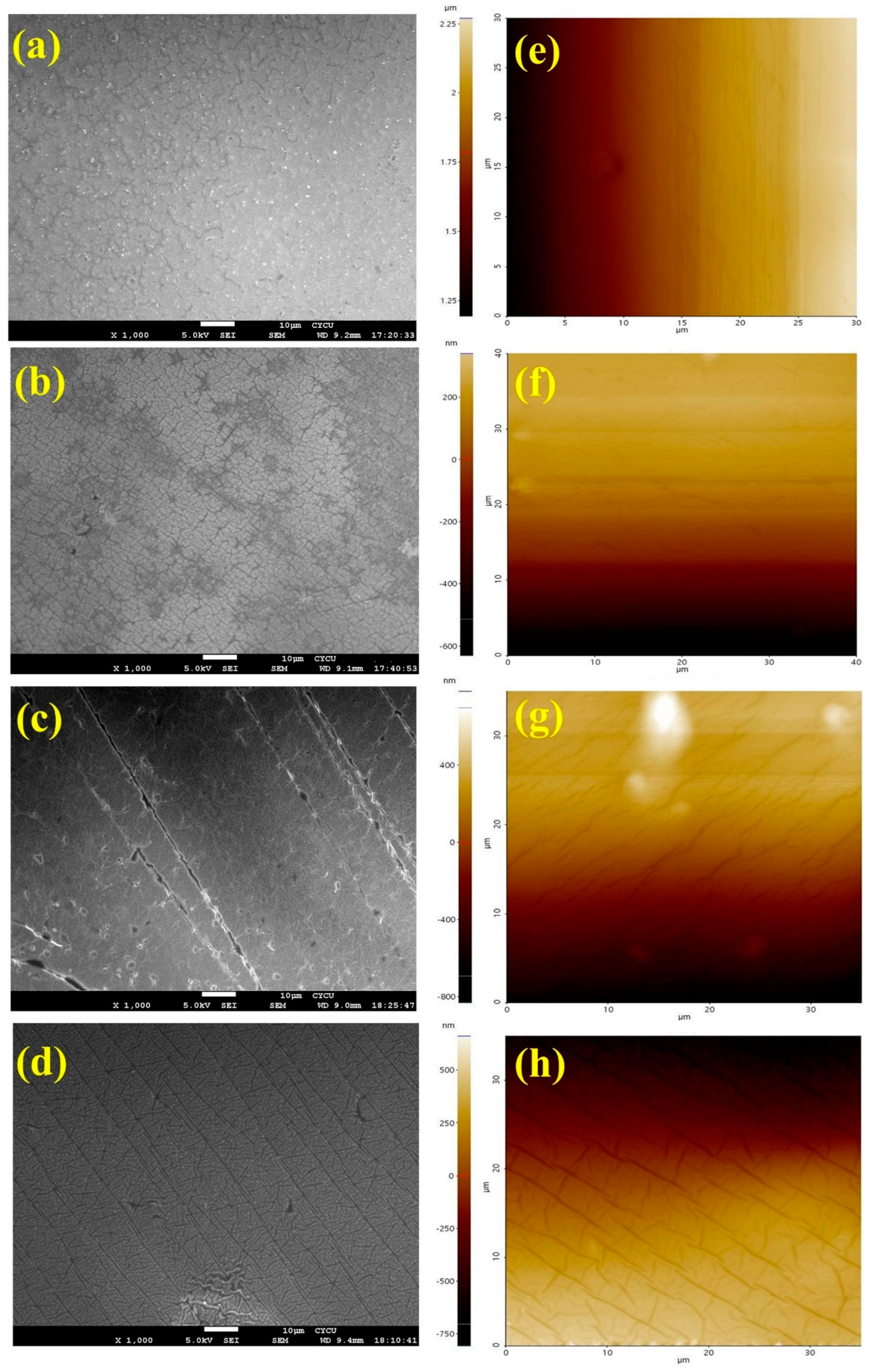
3.2. Identification and Analysis of Silica Ionogel Electrolyte
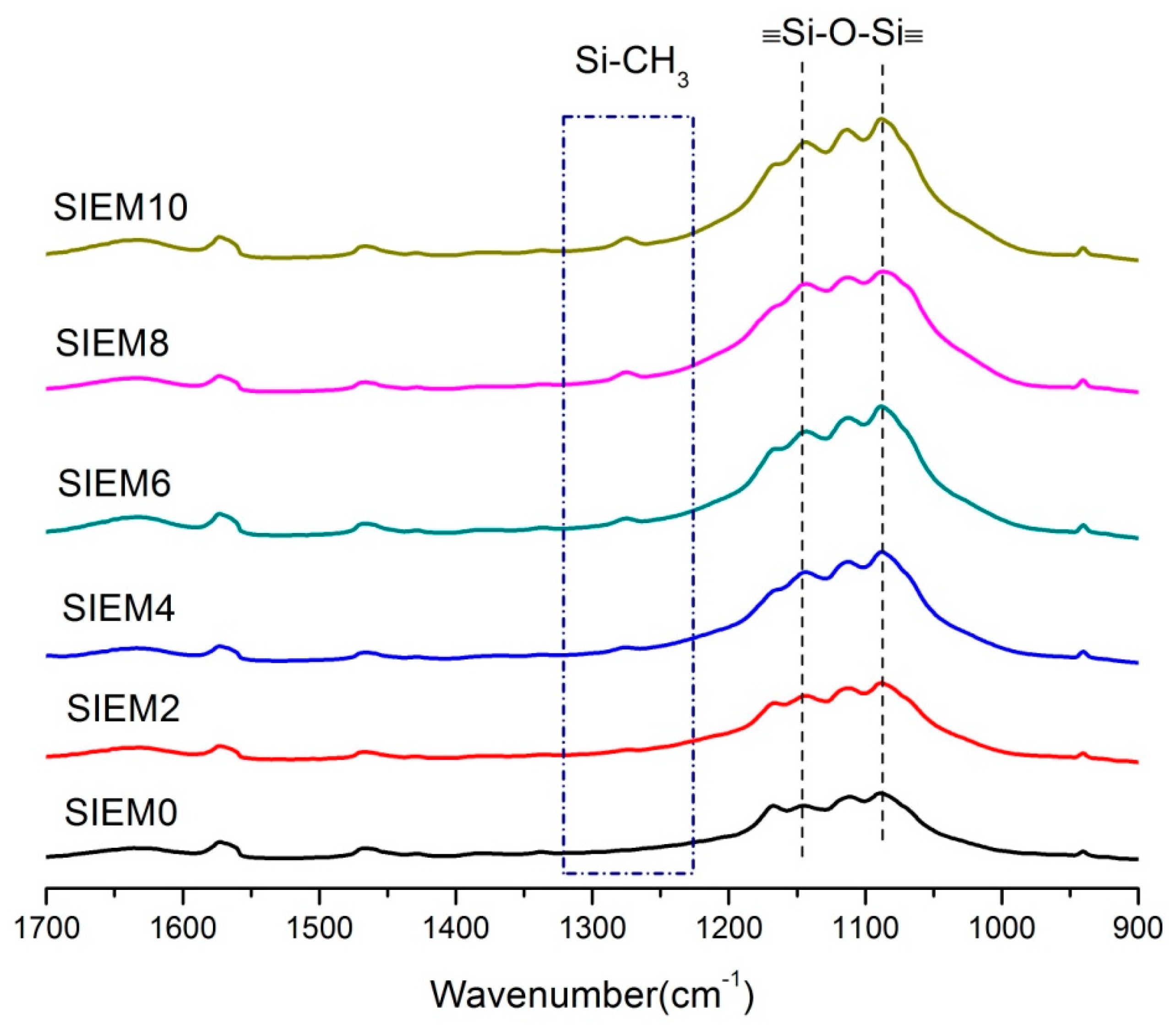
3.2.1. Fourier Transform Infrared Spectroscopy (FTIR) for Detection and Analysis
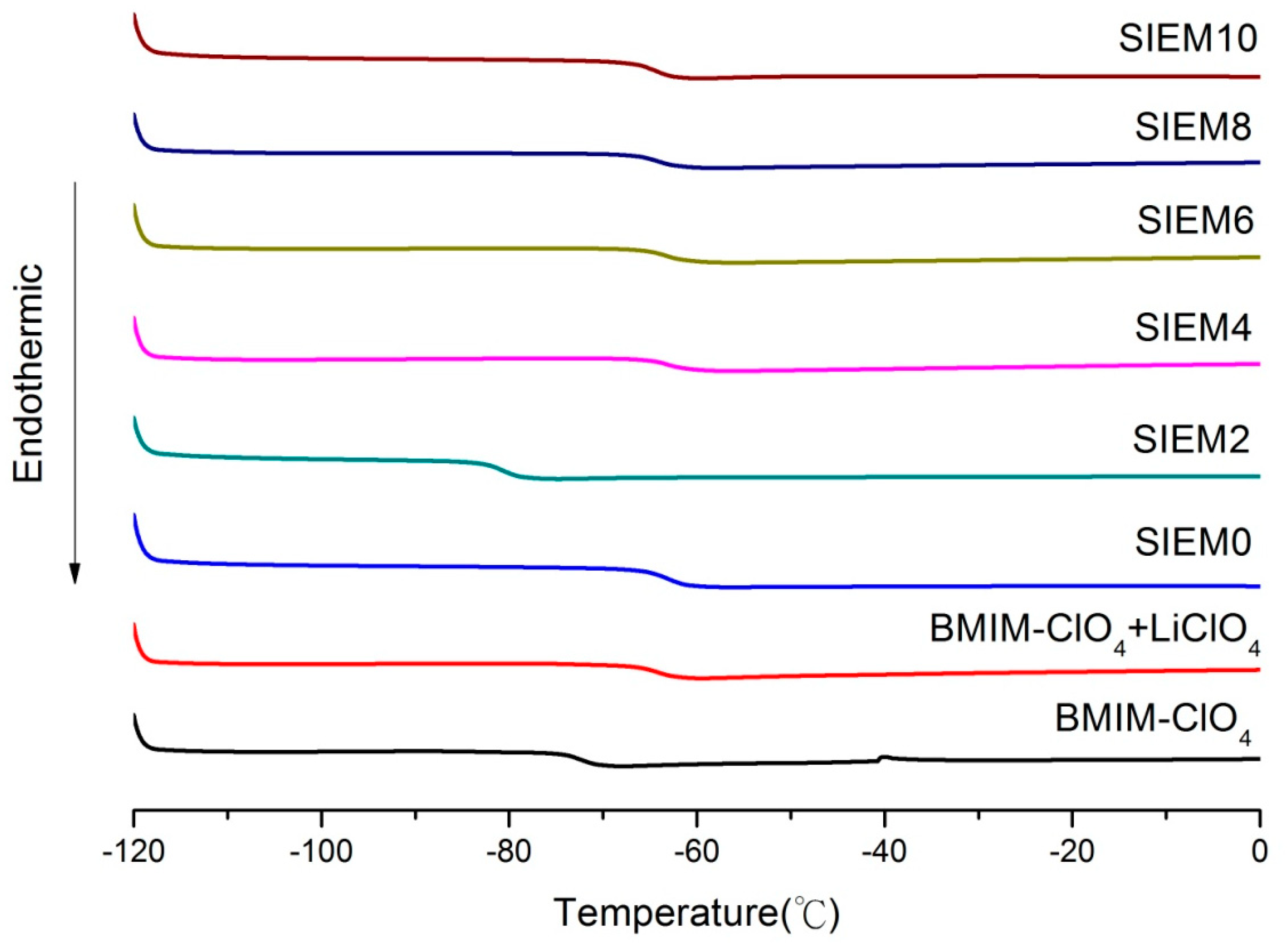
3.2.2. Detection and Analysis of Glass Transition Temperature (Tg) Using Differential Scanning Calorimeter (DSC)
3.2.3. Identification by Solid-State 29Si MAS NMR Spectroscopy
3.2.4. Ionic Conductivity Analysis of Ionogel Electrolytes
3.2.5. The Relationship between Ionic Conductivity and Temperature
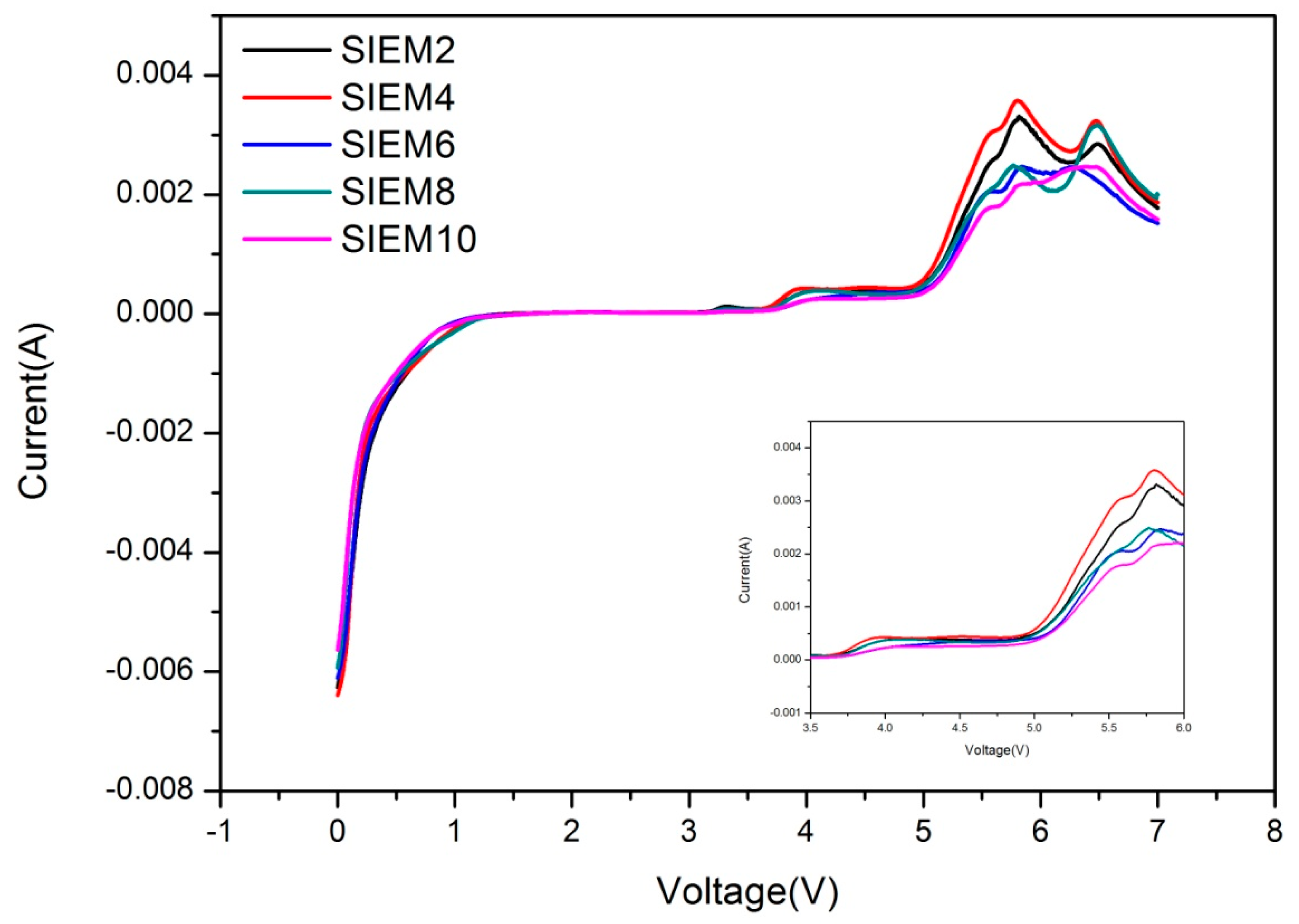
3.2.6. Linear Scanning Voltammetry (LSV) Measurement of Ionogel Electrolytes
3.2.7. Electrochemical Impedance Spectroscopy (EIS) of Ionogel Electrolytes
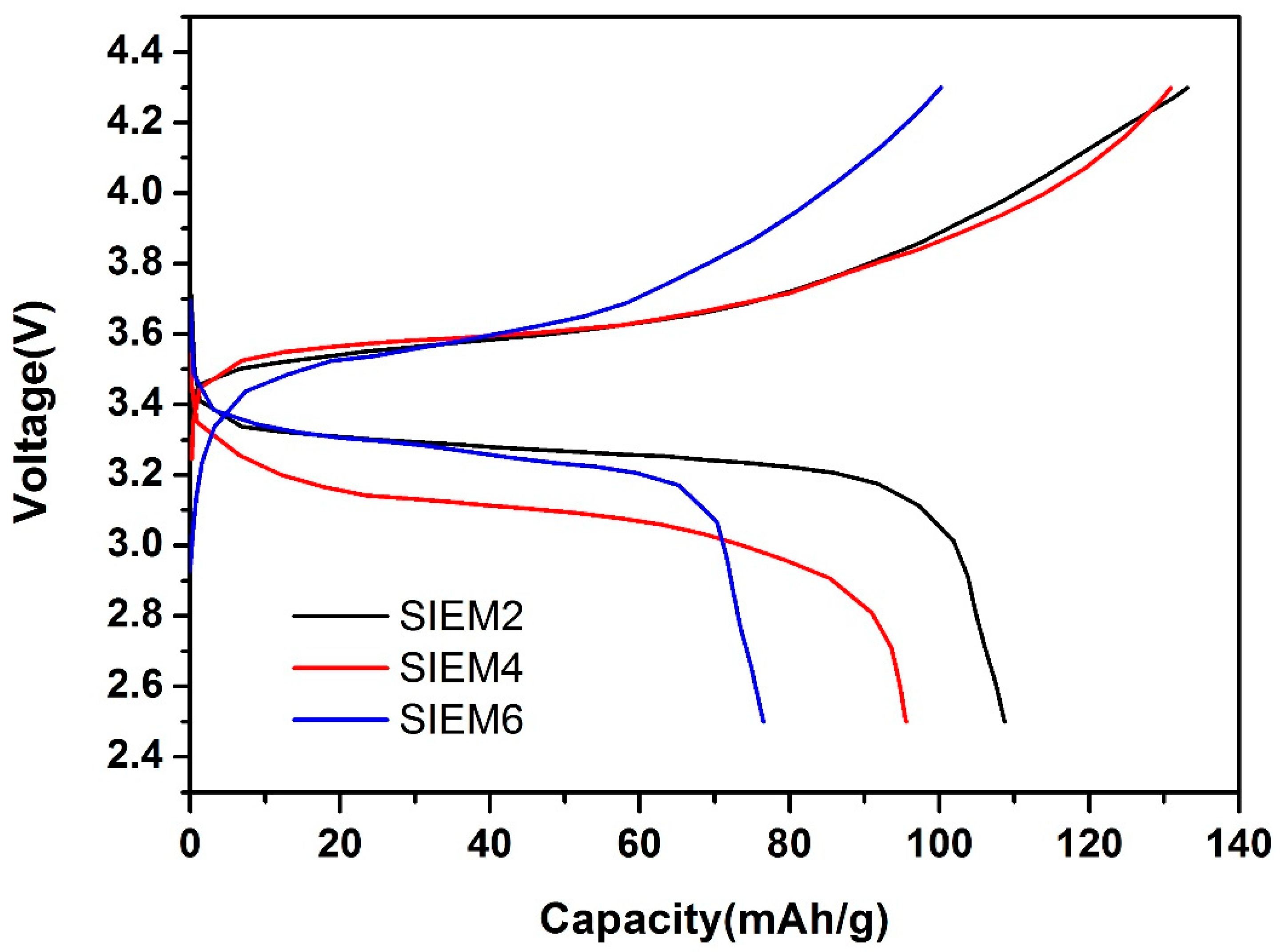
3.3. Charge and Discharge Test of Solid-State Lithium Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Mo, F.; Li, H.; Zhu, M.; Wang, Z.; Liang, G.; Zhi, C. Advances in flexible and wearable energy-storage textiles. Small Methods 2018, 2, 1800124. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible energy-storage devices: Design consideration and recent progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Honda, W.; Harada, S.; Arie, T.; Akita, S. Toward flexible and wearable human-interactive health-monitoring devices. Adv. Healthc. Mater. 2014, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Rajendran, S.; Tang, Z.; George, A.; Cannon, A.; Neumann, C.; Sawas, A.; Ryan, E.; Turchanin, A.; Arava, L.M.R. Inhibition of lithium dendrite formation in lithium metal batteries via regulated cation transport through ultrathin sub-nanometer porous carbon nanomembranes. Adv. Energy Mater. 2021, 11, 2100666. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Dickey, M.D. Tough ionogels: Synthesis, toughening mechanisms, and mechanical properties-A perspective. JACS Au 2022, 2, 2645–2657. [Google Scholar] [CrossRef]
- Ne’ouze, M.-A.; Le Bideau, J.; Gaveau, P.; Bellayer, S.; Vioux, A. Ionogels, new materials arising from the confinement of ionic liquids within silica-derived networks. Chem. Mater. 2006, 18, 3931–3936. [Google Scholar] [CrossRef]
- Neouze, M.A.; Le Bideau, J.; Leroux, F.; Vioux, A. A route to heat resistant solid membranes with performances of liquid electrolytes. Chem. Commun. 2005, 8, 1082–1084. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Görller-Walrand, C.; Binnemans, K.; Bellayer, S.; Le Bideau, J.; Vioux, A. Luminescent ionogels based on europium-doped ionic liquids confined within silica-derived networks. Chem. Mater. 2006, 18, 5711–5715. [Google Scholar] [CrossRef]
- Ahmad, S.; Deepa, M. Ionogels encompassing ionic liquid with liquid like performance preferable for fast solid state electrochromic devices. Electrochem. Commun. 2007, 9, 1635–1638. [Google Scholar] [CrossRef]
- Kim, J.-D.; Mori, T.; Kudo, T.; Honma, I. Ionogel electrolytes at medium temperatures by composite of ionic liquids with proton conducting cesium hydrogen sulfate. Solid State Ion. 2008, 179, 1178–1181. [Google Scholar] [CrossRef]
- Mizumo, T. Rubbery Ionogels and Glassy Ionogels: Design and Ion Conduction. Kobunshi Ronbunshu 2008, 65, 516–524. [Google Scholar] [CrossRef]
- Echelmeyer, T.; Meyer, H.W.; van Wullen, L. Novel ternary composite electrolytes: Li ion conducting ionic liquids in silica glass. Chem. Mater. 2009, 21, 2280–2285. [Google Scholar] [CrossRef]
- Petit, D.; Korb, J.P.; Levitz, P.; LeBideau, J.; Brevet, D. Multiscale dynamics of 1H and 19F in confined ionogels for lithium batteries. Comptes Rendus Chim. 2010, 13, 409–411. [Google Scholar] [CrossRef]
- Gayet, F.; Viau, L.; Leroux, F.; Monge, S.; Robin, J.-J.; Vioux, A. Polymer nanocomposite ionogels, high-performance electrolyte membranes. J. Mater. Chem. 2010, 20, 9456. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Wu, F.; Tan, G.; Chen, R.; Li, L.; Xiang, J.; Zheng, Y. Novel Solid-State Li/LiFePO4 Battery Configuration with a Ternary Nanocomposite Electrolyte for Practical Applications. Adv. Mater. 2011, 23, 5081–5085. [Google Scholar] [CrossRef]
- Le Bideau, J.; Ducros, J.-B.; Soudan, P.; Guyomard, D. Solid-State Electrode Materials with Ionic-Liquid Properties for Energy Storage: The Lithium Solid-State Ionic-Liquid Concept. Adv. Funct. Mater. 2011, 21, 4073–4078. [Google Scholar] [CrossRef]
- Xie, Z.-L.; Xu, H.-B.; Geßner, A.; Kumke, M.U.; Priebe, M.; Fromm, K.M.; Taubert, A. A transparent, flexible, ion conductive, and luminescent PMMA ionogel based on a Pt/Eu bimetallic complex and the ionic liquid [Bmim][N(Tf)2]. J. Mater. Chem. 2012, 22, 8110. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Bayley, P.M.; Forsyth, M.; MacFarlane, D.R. Ionogels based on ionic liquids as potential highly conductive solid state electrolytes. Electrochim. Acta 2013, 91, 219–226. [Google Scholar] [CrossRef]
- Guyomard-Lack, A.; Delannoy, P.E.; Dupre, N.; Cerclier, C.V.; Humbert, B.; Le Bideau, J. Destructuring ionic liquids in ionogels: Enhanced fragility for solid devices. Phys. Chem. Chem. Phys. 2014, 16, 23639–23645. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.I.; Panzer, M.J. Poly(dimethylsiloxane)-Supported Ionogels with a High Ionic Liquid Loading. Angew. Chem. Int. Ed. Engl. 2014, 53, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, J.; Aguilera, L.; Matic, A. Effect of Lithium Salt on the Stability of Dispersions of Fumed Silica in the Ionic Liquid BMImBF4. Langmuir 2012, 28, 4080–4085. [Google Scholar] [CrossRef] [PubMed]
- Makita, K.; Akamatsu, Y.; Yamazaki, S.; Kai, Y.; Abe, Y. Surface Morphology of Silica Films Derived by the Sol-Gel Method and Its Application to a Water Repellent Glass. J. Ceram. Soc. Jpn. 1997, 105, 1012–1017. [Google Scholar] [CrossRef]
- Legrand, A.P.; Hommel, H.; Tuel, A.; Vidal, A.; Balard, H.; Papirer, E.; Levitz, P.; Czernichowski, M.; Erre, R.; Van Damme, H.; et al. Hydroxyls of silica powdersHydroxyles des silices divisees. Adv. Colloid Interface Sci. 1990, 33, 91–330. [Google Scholar] [CrossRef]
- Huber, P. Soft matter in hard confinement: Phase transition thermodynamics, structure, texture, diffusion and flow in nanoporous media. J. Phys. Condens. Matter 2015, 27, 103102. [Google Scholar] [CrossRef]
- Abdou, N.; Alonso, B.; Brun, N.; Devautour-Vinot, S.; Paillet, M.; Landois, P.; Mehdi, A.; Hesemann, P. Confinement effects on the ionic liquid dynamics in ionosilica ionogels: Impact of the ionosilica nature and the host/guest ratio. J. Phys. Chem. C 2022, 126, 20937–20945. [Google Scholar] [CrossRef]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of confinement on freezing and melting. J. Phys. Condens. Matter 2006, 18, R15–R68. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Su, X.; Suss, M.E.; Tian, H.; Guyes, E.N.; Shocron, A.N.; Conforti, K.M.; de Souza, J.P.; Kim, N.; Tedesco, M.; et al. Electrochemical Methods for Water Purification, Ion Separations, and Energy Conversion. Chem. Rev. 2022, 122, 13547–13635. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Ji, W.-F.; Ahmeda, M.M.M.; Bibi, A.; Lee, Y.-C.; Yeh, J.-M. Graphene-based PANI composite coatings with fine-controllable 3D hierarchical structures prepared from bio-inspired photo-/colloidal-lithography technique for flexible supercapacitor application. Electrochim. Acta 2021, 390, 138890. [Google Scholar] [CrossRef]
- Chang, C.-M.; Weng, C.-J.; Chien, C.-M.; Chuang, T.-L.; Lee, T.-Y.; Yeh, J.-M.; Wei, Y. Polyaniline/carbon nanotube nanocomposite electrodes with biomimetic hierarchical structure for supercapacitors. J. Mater. Chem. A 2013, 1, 14719. [Google Scholar] [CrossRef]
- Hwang, J.-J.; Peng, H.-H.; Yeh, J.-M. α-Al2O3 Improves the Properties of Gel Polyacrylonitrile Nanocomposite Electrolytes Used as Electrolyte Materials in Rechargeable Lithium Batteries. J. Appl. Polym. Sci. 2011, 120, 2041–2047. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]





| Sample Code | Ratio of Ion–Gel Electrolyte Formulation | ||||
|---|---|---|---|---|---|
| LiClO4 (Mole) | BMIM-ClO4 (Mole) | MTES/TEOS (Mole) | Ratio | HCOOH (Mole) | |
| SIEM0 | 0.5 | 2.5 | 0/1.0 | 0 | 6 |
| SIEM2 | 0.5 | 2.5 | 0.167/0.833 | 0.2 | 6 |
| SIEM4 | 0.5 | 2.5 | 0.286/0.714 | 0.4 | 6 |
| SIEM6 | 0.5 | 2.5 | 0.375/0.625 | 0.6 | 6 |
| SIEM8 | 0.5 | 2.5 | 0.445/0.555 | 0.8 | 6 |
| SIEM10 | 0.5 | 2.5 | 0.5/0.5 | 1.0 | 6 |
| Sample | Tg (°C) | Tc (°C) |
|---|---|---|
| BMIM-ClO4 | −72.2 | −40.5 |
| BMIM-ClO4 + LiClO4 | −64.2 | -- |
| SIEM0 | −62.8 | -- |
| SIEM2 | −80.8 | -- |
| SIEM4 | −63.3 | -- |
| SIEM6 | −63.5 | -- |
| SIEM8 | −64.3 | -- |
| SIEM10 | −62.8 | -- |
| Sample | T2 (%) | T3 (%) | Q2 (%) | Q3 (%) | Q4 (%) | DC (%) |
|---|---|---|---|---|---|---|
| SIEM2 | 4.1 | 22.7 | 5.5 | 55.1 | 12.7 | 82.21 |
| SIEM4 | 13.7 | 25.9 | 5.5 | 44.2 | 10.6 | 81.53 |
| SIEM6 | 14.8 | 33.3 | 5.1 | 33.3 | 13.5 | 84.19 |
| SIEM8 | 21.7 | 33.2 | 2.7 | 32.7 | 9.7 | 83.24 |
| SIEM10 | 20.7 | 38.8 | 2.2 | 27.8 | 10.5 | 85.05 |
| Sample | Ionic Conductivity (S/cm) | Ea (kJ/mol) | Rb (Ω) | Ri (Ω) |
|---|---|---|---|---|
| BMIM-ClO4 | 1.46 × 10−3 | 28.9 | -- | -- |
| SIEM2 | 9.26 × 10−4 | 17.8 | 17.1 | 185.84 |
| SIEM4 | 1.65 × 10−3 | 24.1 | 22.2 | 253.64 |
| SIEM6 | 6.76 × 10−4 | 26.0 | 23.4 | 436.25 |
| SIEM8 | 7.24 × 10−4 | 31.4 | 39.6 | 901.87 |
| SIEM10 | 1.11 × 10−3 | 27.8 | 25.6 | 905.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-C.; Chen-Yang, Y.W.; Hwang, J.-J. Preparation and Characterization of Silica-Based Ionogel Electrolytes and Their Application in Solid-State Lithium Batteries. Polymers 2023, 15, 3505. https://doi.org/10.3390/polym15173505
Huang J-C, Chen-Yang YW, Hwang J-J. Preparation and Characterization of Silica-Based Ionogel Electrolytes and Their Application in Solid-State Lithium Batteries. Polymers. 2023; 15(17):3505. https://doi.org/10.3390/polym15173505
Chicago/Turabian StyleHuang, Ji-Cong, Yui Whei Chen-Yang, and Jiunn-Jer Hwang. 2023. "Preparation and Characterization of Silica-Based Ionogel Electrolytes and Their Application in Solid-State Lithium Batteries" Polymers 15, no. 17: 3505. https://doi.org/10.3390/polym15173505
APA StyleHuang, J.-C., Chen-Yang, Y. W., & Hwang, J.-J. (2023). Preparation and Characterization of Silica-Based Ionogel Electrolytes and Their Application in Solid-State Lithium Batteries. Polymers, 15(17), 3505. https://doi.org/10.3390/polym15173505






