The Impact of the Molecular Weight of Degradation Products with Silicon from Porous Chitosan–Siloxane Hybrids on Neuronal Cell Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Porous Hybrid Extractions
2.2. Biological Assessment
2.2.1. Preparation of Culture Medium with Extracted Degradation Products
2.2.2. RT4-D6P2T Cell Proliferation
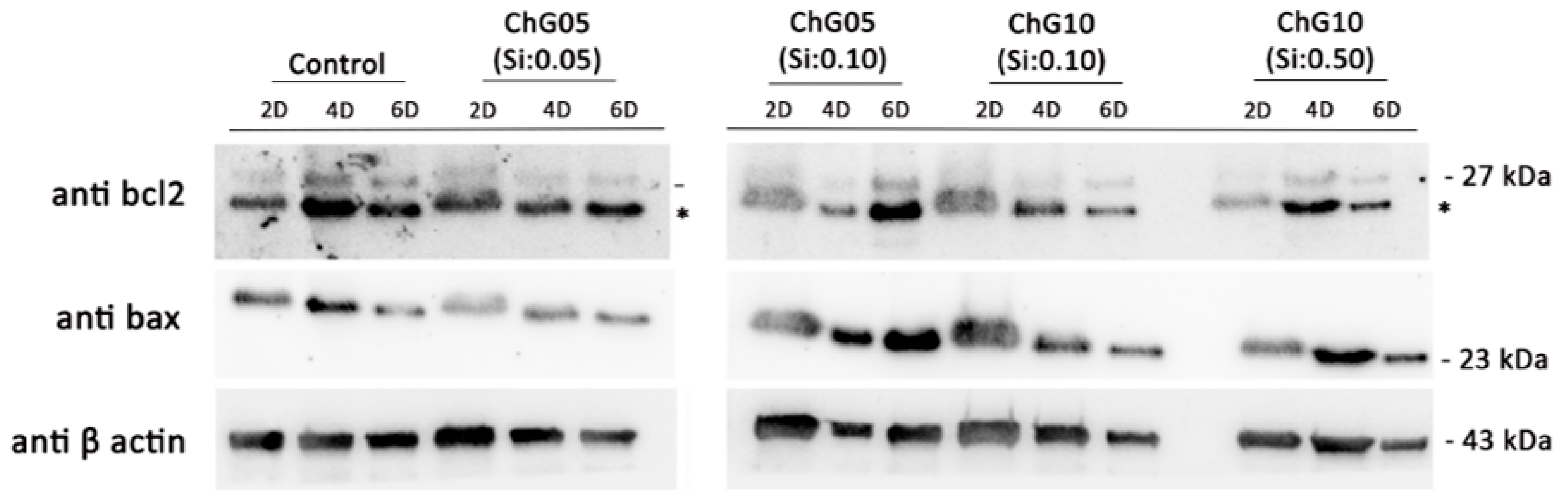
2.2.3. RT4-D6P2T Total Protein Extraction and Western Blot
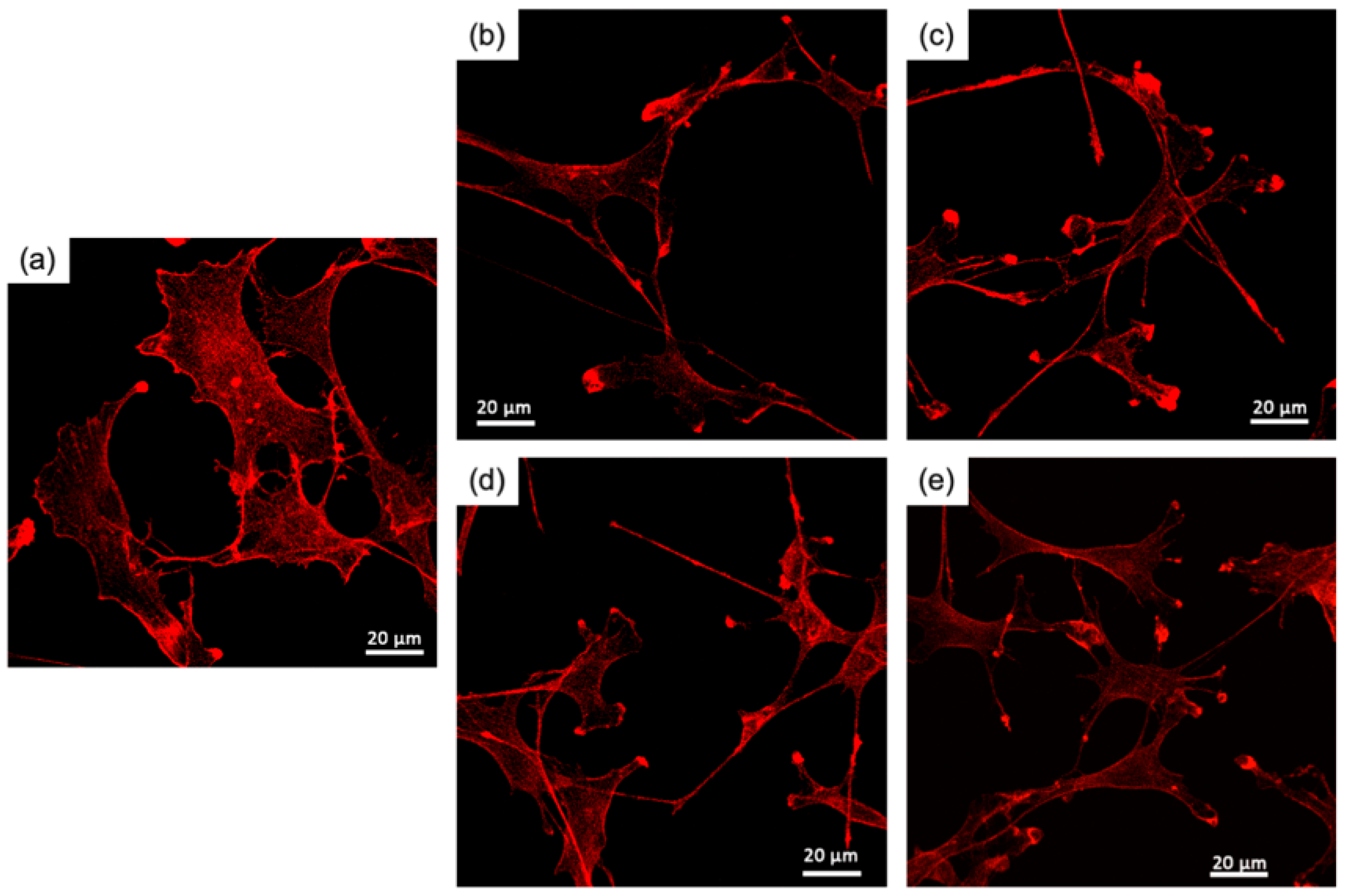
2.2.4. RT4-D6P2T Cell Morphology
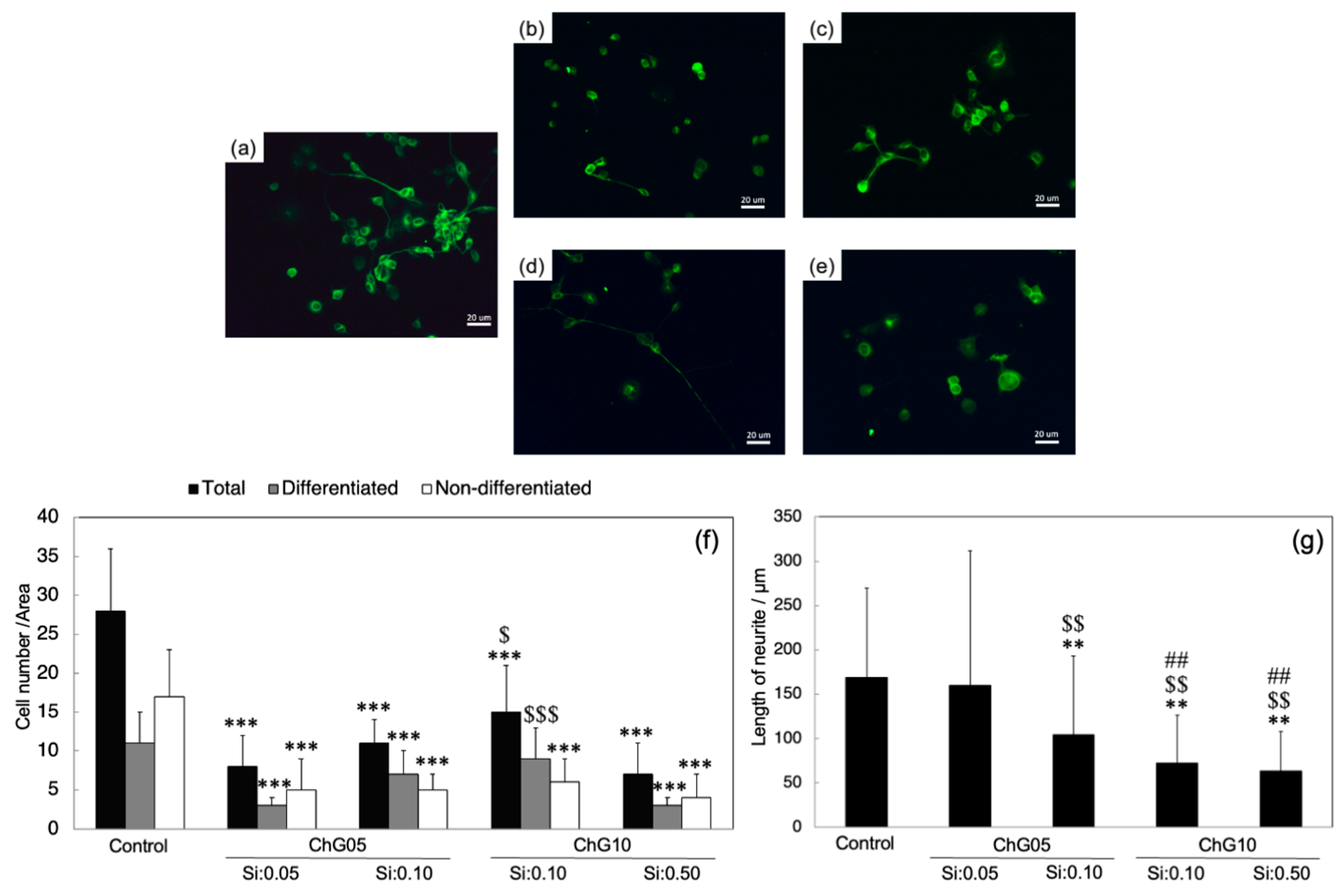
2.2.5. NSC-34 Cell Differentiation
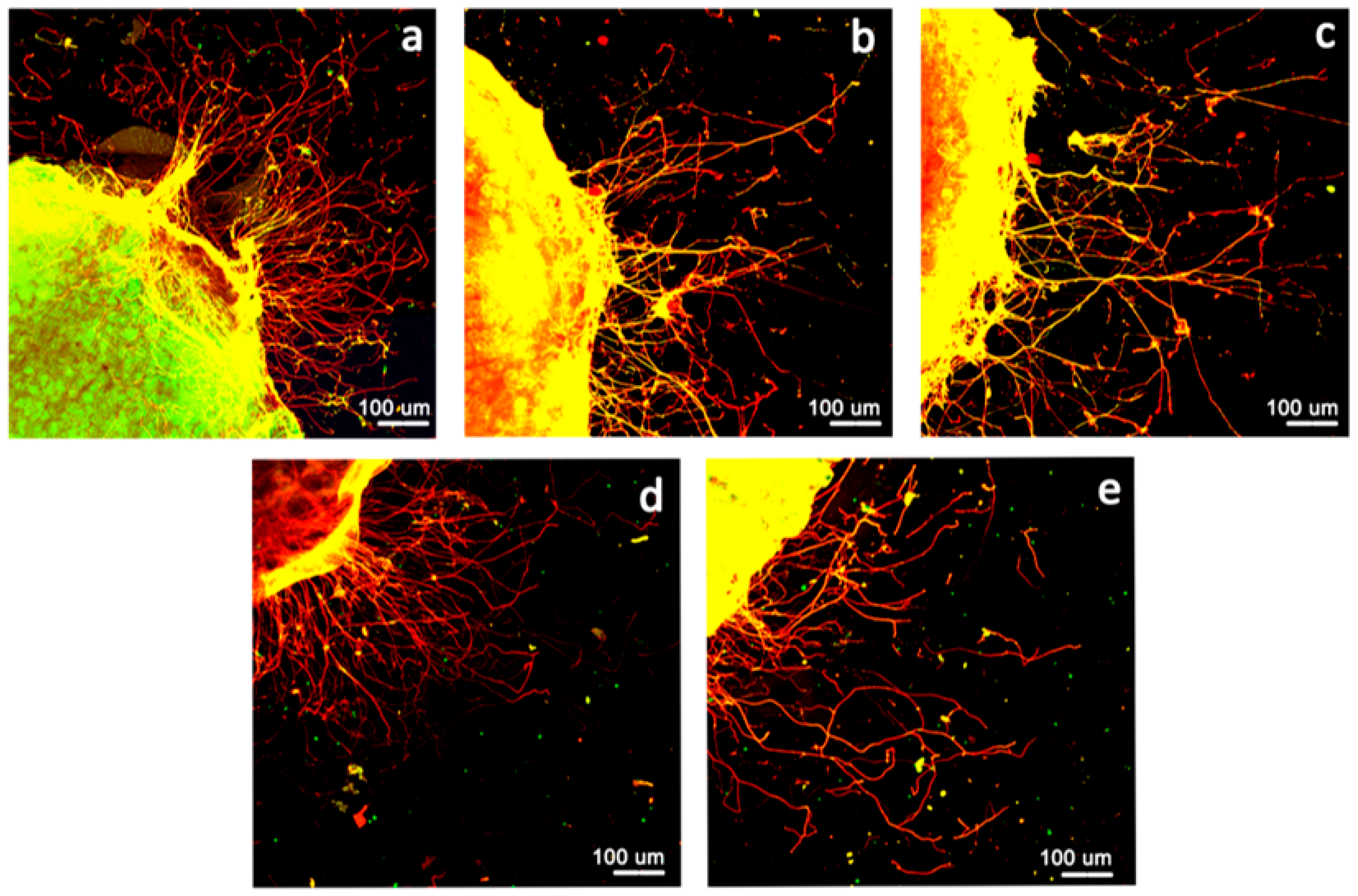
2.2.6. Neurite Outgrowth Assay Using Ex Vivo Models
Dorsal Root Ganglia (DRG) Dissection and Cultures
Immunofluorescence
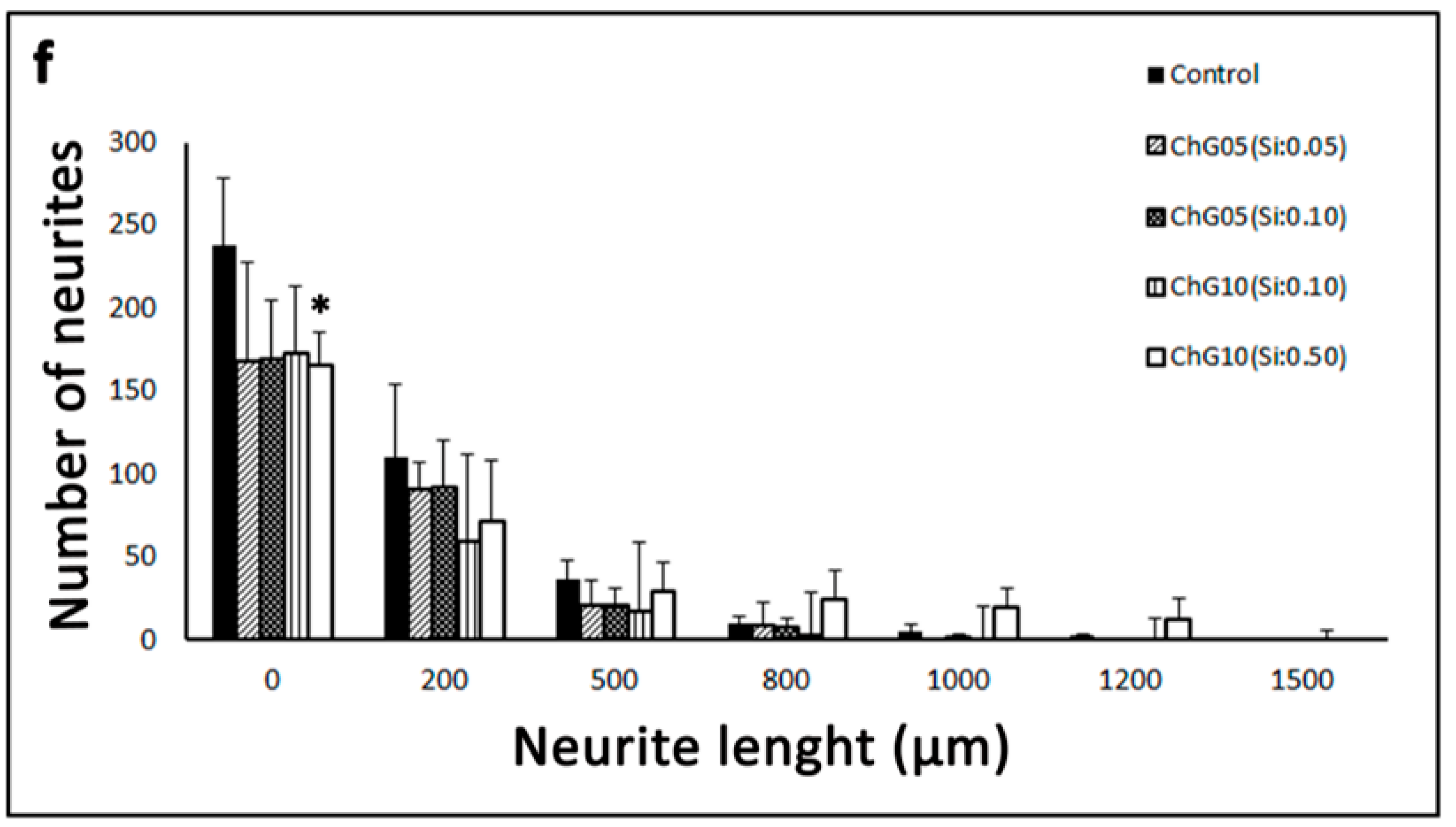
Quantification of Neurite Outgrowth
2.3. Statistical Analysis
3. Results and Discussion
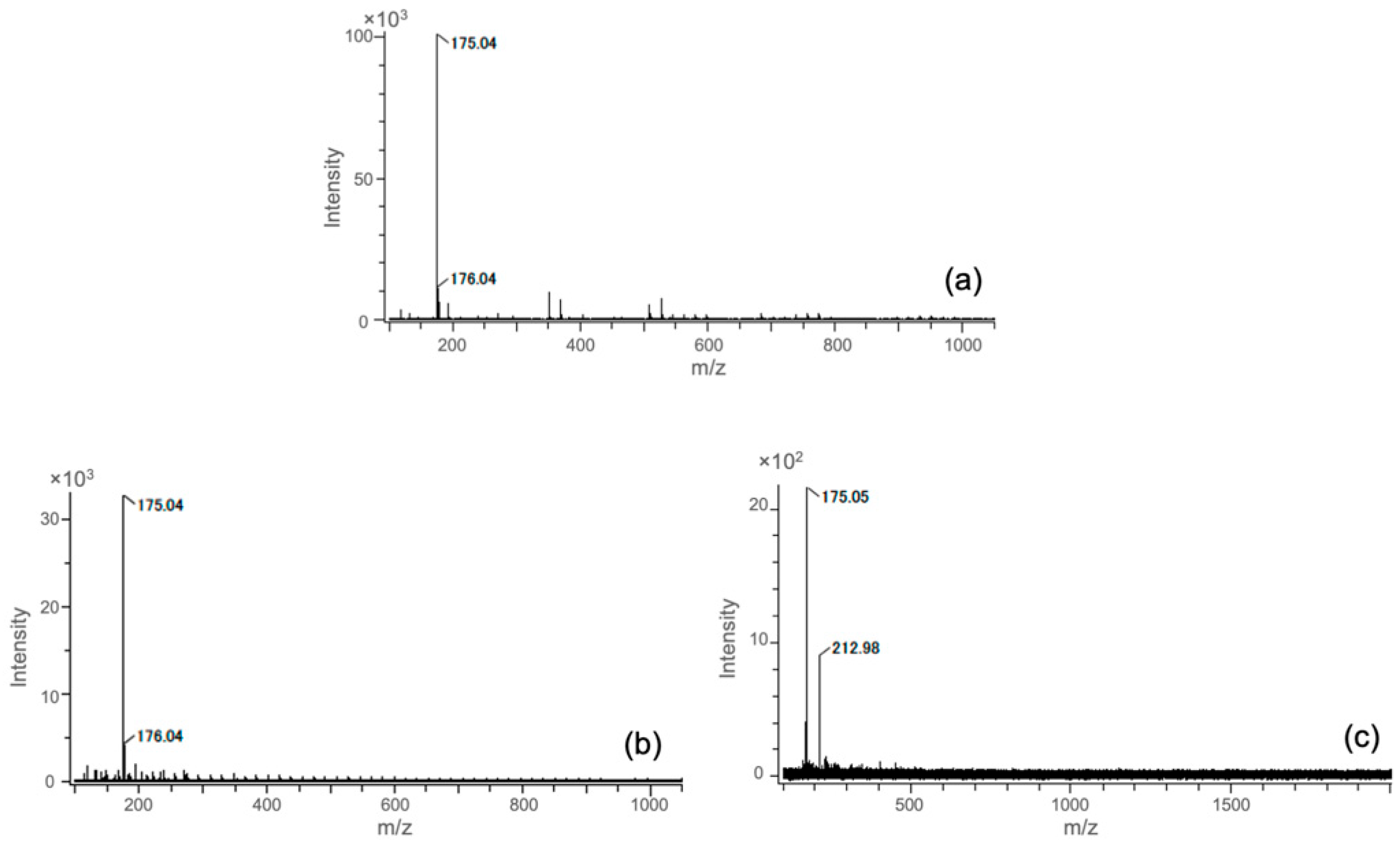
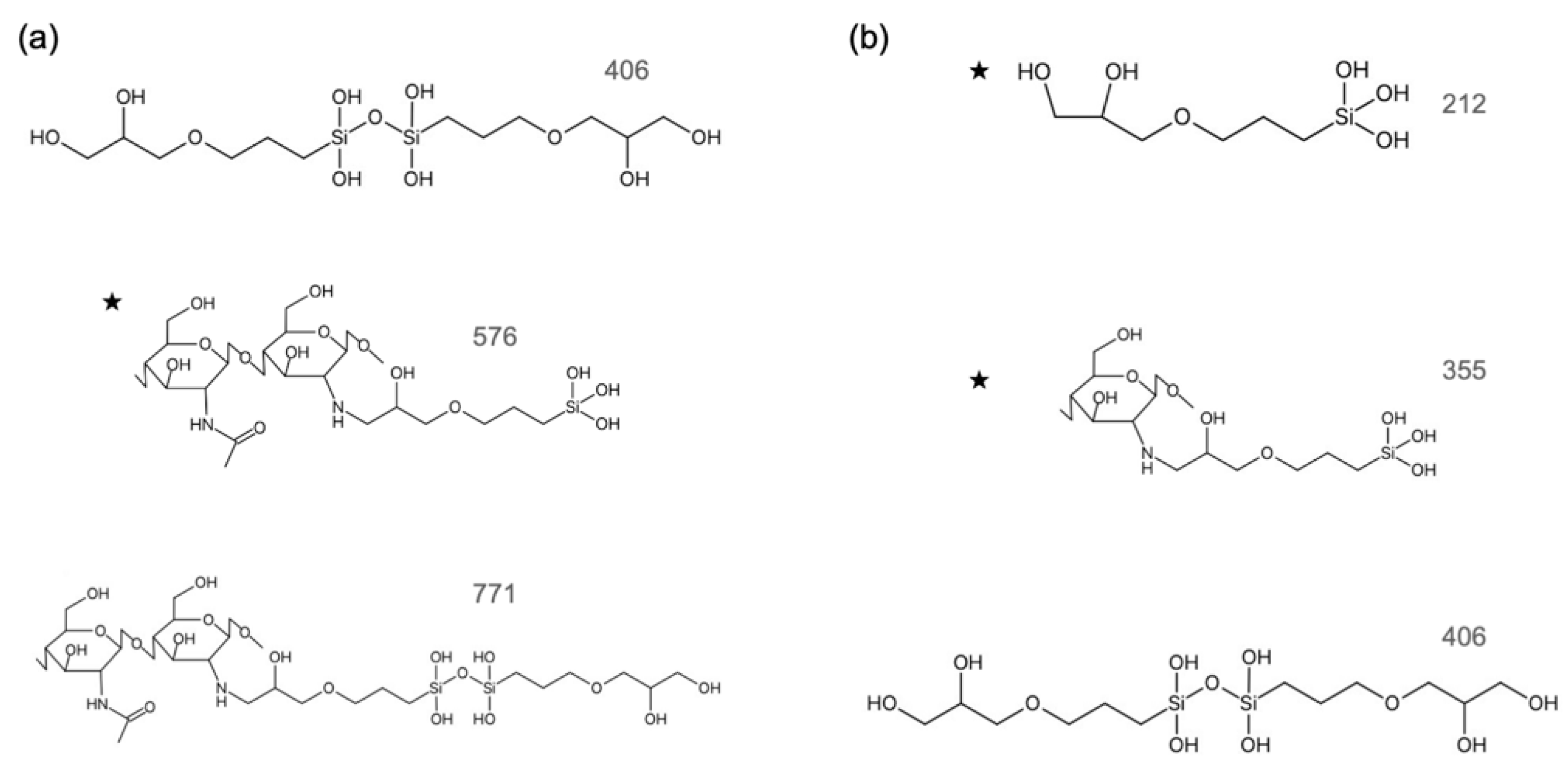
3.1. Degradation Products Containing Si in the Extractions
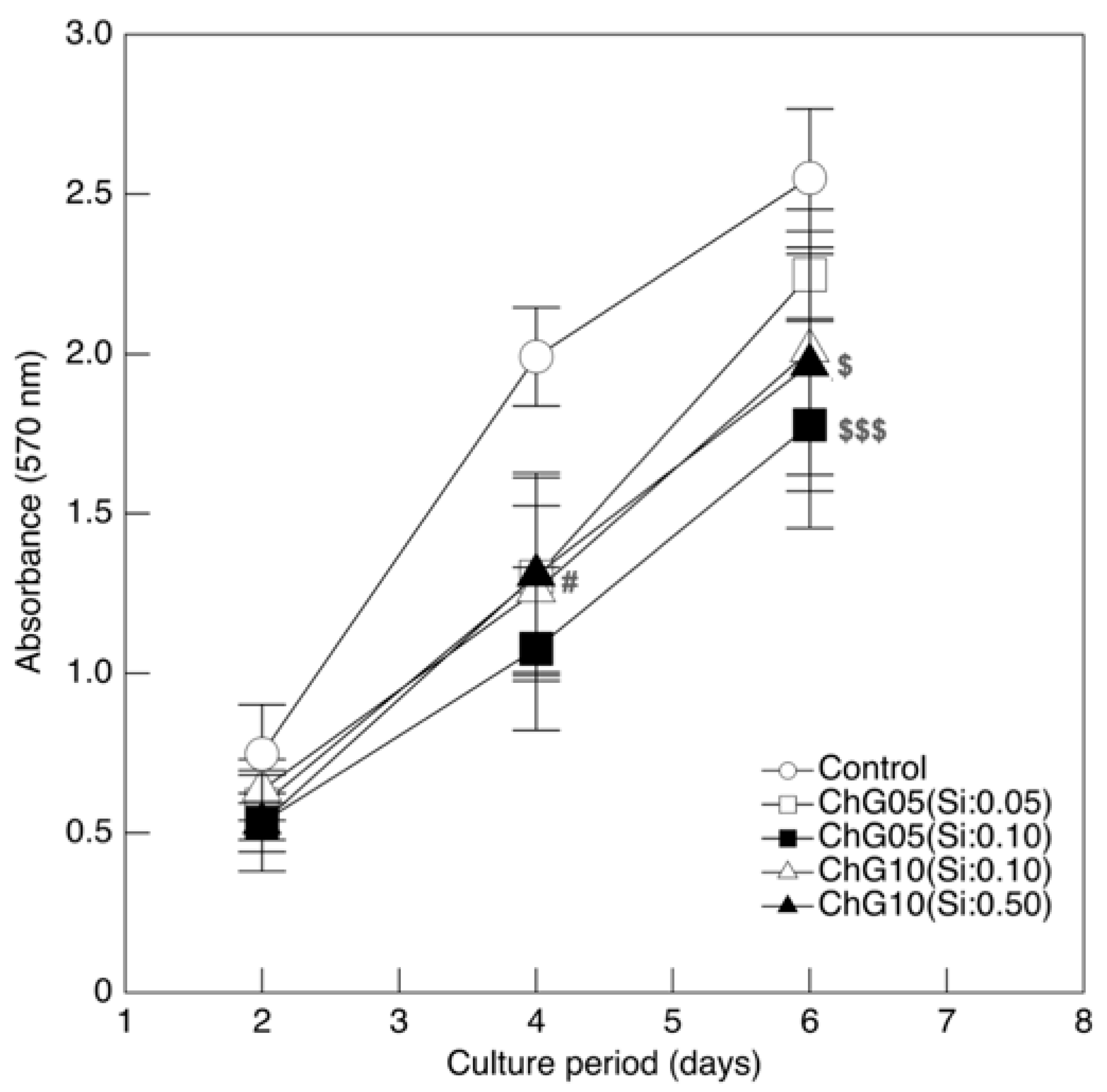
3.2. RT4-D6P2T Cytocompatibility
3.3. NSC-34 Differentiation
3.4. Neurite Outgrowth Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon as an essential element. Fed. Proc. 1974, 33, 1758–1766. [Google Scholar]
- Carlisle, E.M. Silicon as a trace nutrient. Sci. Total Environ. 1988, 73, 95–106. [Google Scholar] [CrossRef]
- Carlisle, E.M. In vitro requirement for silicon in articular cartilage and connective tissues formation in the chick. J. Nutr. 1976, 106, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jurkić, L.M.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metab. 2013, 10, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [PubMed]
- Gough, J.E.; Jones, J.R.; Hench, L.L. Nodule formation and mineralization of human primary osteoblasts cultured on a porous bioactive glass scaffolds. Biomaterials 2004, 25, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Greenspan, D. Interactions between bioactive glass and collagen: A review and new perspectives. J. Aust. Ceram. Soc. 2013, 49, 1–40. [Google Scholar]
- Zhou, X.; Moussa, F.M.; Mankoci, S.; Ustriyana, P.; Zhang, N.; Abdelmagid, S.; Molenda, J.; Murphy, W.L.; Safadi, F.F.; Sahai, N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016, 39, 192–202. [Google Scholar]
- Uribe, P.; Johansson, A.; Jugdaohsingh, R.; Powell, J.J.; Magnusson, C.; Davila, M.; Westerlund, A.; Ransjö, M. Soluble silica stimulates osteogenic differentiation and gap junction communication in human dental follicle cells. Sci. Rep. 2020, 10, 9923. [Google Scholar] [PubMed]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 2019, 11, 117–144. [Google Scholar] [PubMed]
- Yamada, S.; Ota, Y.; Obata, A.; Kasuga, T. Osteoblast-like cell responses to ion products released from magnesium- and silicate-containing calcium carbonates. Biomed. Mater. Eng. 2017, 28, 47–56. [Google Scholar] [PubMed]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Shirosaki, Y.; Tsuru, K.; Moribayashi, H.; Hayakawa, S.; Nakamura, Y.; Gibson, I.R.; Osaka, A. Preparation of osteocompatible Si(IV)-enriched chitosan–silicate hybrids. J. Ceram. Soc. Jpn. 2010, 118, 989–992. [Google Scholar]
- Shirosaki, Y.; Hirai, M.; Hayakawa, S.; Fujii, E.; Lopes, M.A.; Santos, J.D.; Osaka, A. Preparation and in vitro cytocompatibility of chitosan-siloxane hybrid hydrogels. J. Biomed. Mater. Res. Part A 2015, 103, 289–299. [Google Scholar]
- Amado, S.; Simões, M.J.; Silva, P.A.; Luís, A.L.; Shirosaki, Y.; Lopes, M.A.; Santos, J.D.; Fregnan, F.; Gambarotta, G.; Raimondo, S.; et al. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials 2008, 29, 4409–4419. [Google Scholar] [CrossRef]
- Parveen, A.; Rizvi, S.H.M.; Mahdi, F.; Tripathi, S.; Ahmad, I.; Shukla, R.K.; Khanna, V.K.; Singh, R.; Patel, D.K.; Mahdi, A.A. Silica nanoparticles mediated neuronal cell death in corpus striatum of rat brain: Implication of mitochondrial, endoplasmic reticulum and oxidative stress. J. Nanopart. Res. 2014, 16, 2664–2679. [Google Scholar] [CrossRef]
- Inoue, Y.; Ezure, H.; Ito, J.; Sawa, C.; Yamamoto, M.; Hata, H.; Moriyama, H.; Manome, Y.; Otsuka, N. Effect of Silica Nanoparticles on Cultured Central Nervous System Cells. World J. Neurosci. 2018, 8, 146–156. [Google Scholar]
- Hattori, K.; Hayakawa, S.; Shirosaki, Y. Effects of the silicon-containing chemical species dissolved from chitosan–siloxane hybrids on nerve cells. J. Sol-Gel Sci. Technol. 2022, 104, 606–616. [Google Scholar] [CrossRef]
- Leane, M.M.; Nankervis, R.; Smith, A.; Illum, L. Use of the ninhydrin assay to measure the release of chitosan from oral solid dosage forms. Int. J. Pharm. 2004, 271, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [PubMed]
- He, B.P.; Wen, W.; Strong, M. Activated microglia (BV-2) facilitation of TNF-α- mediated motor neuron death in vitro. J. Neuroimmunol. 2002, 128, 31–38. [Google Scholar] [CrossRef]
- Maier, O.; Böhm, J.; Dahm, M.; Brück, S.; Beyer, C.; Johann, S. Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem. Int. 2013, 62, 1029–1038. [Google Scholar] [CrossRef]
- Fueshko, S.; Wray, S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: An in vitro model for neurophilic neuronal migration. Dev. Biol. 1994, 166, 331–348. [Google Scholar] [CrossRef]
- Morano, M.; Wrobel, S.; Fregnan, F.; Ziv-Polat, O.; Shahar, A.; Ratzka, A.; Grothe, C.; Geuna, S.; Haastert-Talini, K. Nanotechnology versus stem cell engineering: In vitro comparison of neurite inductive potentials. Int. J. Nanomed. 2014, 9, 5289–5306. [Google Scholar]
- Cashman, N.R.; Durham, H.D.; Blusztajn, J.K.; Oda, K.; Tabira, T.; Shaw, I.T.; Dahrouge, S.; Antel, J.P. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 1992, 194, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Dillon, G.P.; Yu, X.; Bellamkonda, R.V. The polarity and magnitude of ambient charge influences three-dimensional neurite extension from DRGs. J. Biomed. Mater. Res. 2000, 51, 510–519. [Google Scholar] [PubMed]

| Antibodies for Western Blot Analysis | ||||
|---|---|---|---|---|
| Code | Dilution | Host | Source | |
| Primary antibodies | ||||
| Bcl2 | sc-492 | 1:300 | rabbit | Santa Cruz Biotechnology Inc |
| BAX | sc-23959 | 1:500 | mouse | Santa Cruz Biotechnology Inc |
| β-actin | A5316 | 1:4000 | mouse | Sigma |
| Secondary antibodies | ||||
| HRP-conjugated anti-rabbit | 7074 | 1:15,000 | goat | Cell signaling |
| HRP-conjugated anti-mouse | 7076 | 1:15,000 | goat | Cell signaling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirosaki, Y.; Fregnan, F.; Muratori, L.; Yasutomi, S.; Geuna, S.; Raimondo, S. The Impact of the Molecular Weight of Degradation Products with Silicon from Porous Chitosan–Siloxane Hybrids on Neuronal Cell Behavior. Polymers 2023, 15, 3272. https://doi.org/10.3390/polym15153272
Shirosaki Y, Fregnan F, Muratori L, Yasutomi S, Geuna S, Raimondo S. The Impact of the Molecular Weight of Degradation Products with Silicon from Porous Chitosan–Siloxane Hybrids on Neuronal Cell Behavior. Polymers. 2023; 15(15):3272. https://doi.org/10.3390/polym15153272
Chicago/Turabian StyleShirosaki, Yuki, Federica Fregnan, Luisa Muratori, Saki Yasutomi, Stefano Geuna, and Stefania Raimondo. 2023. "The Impact of the Molecular Weight of Degradation Products with Silicon from Porous Chitosan–Siloxane Hybrids on Neuronal Cell Behavior" Polymers 15, no. 15: 3272. https://doi.org/10.3390/polym15153272
APA StyleShirosaki, Y., Fregnan, F., Muratori, L., Yasutomi, S., Geuna, S., & Raimondo, S. (2023). The Impact of the Molecular Weight of Degradation Products with Silicon from Porous Chitosan–Siloxane Hybrids on Neuronal Cell Behavior. Polymers, 15(15), 3272. https://doi.org/10.3390/polym15153272









