Advanced Mechanical Testing Technologies at the Cellular Level: The Mechanisms and Application in Tissue Engineering
Abstract
1. Introduction
2. Advanced Mechanical Testing Technologies and Their Mechanisms
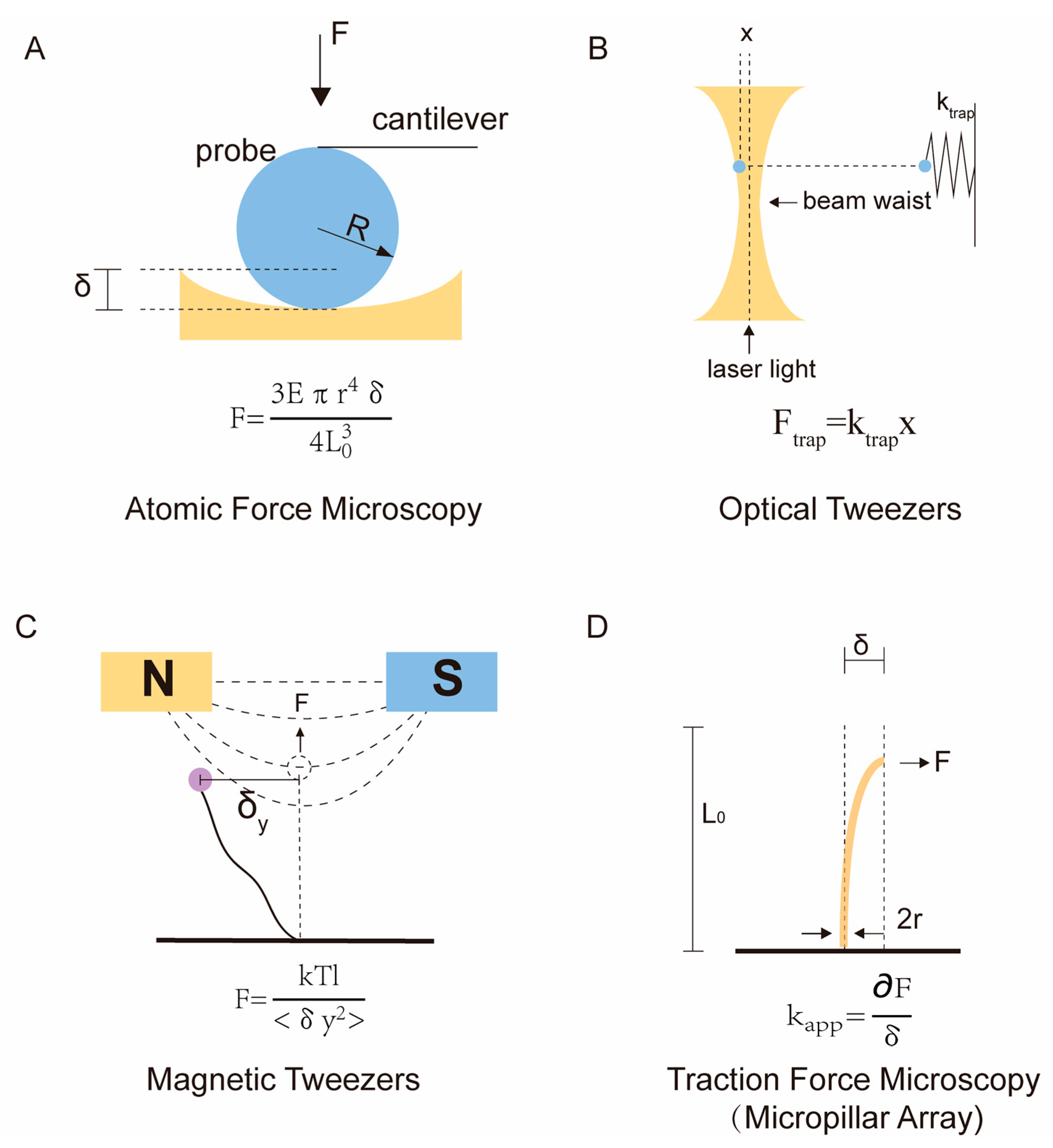
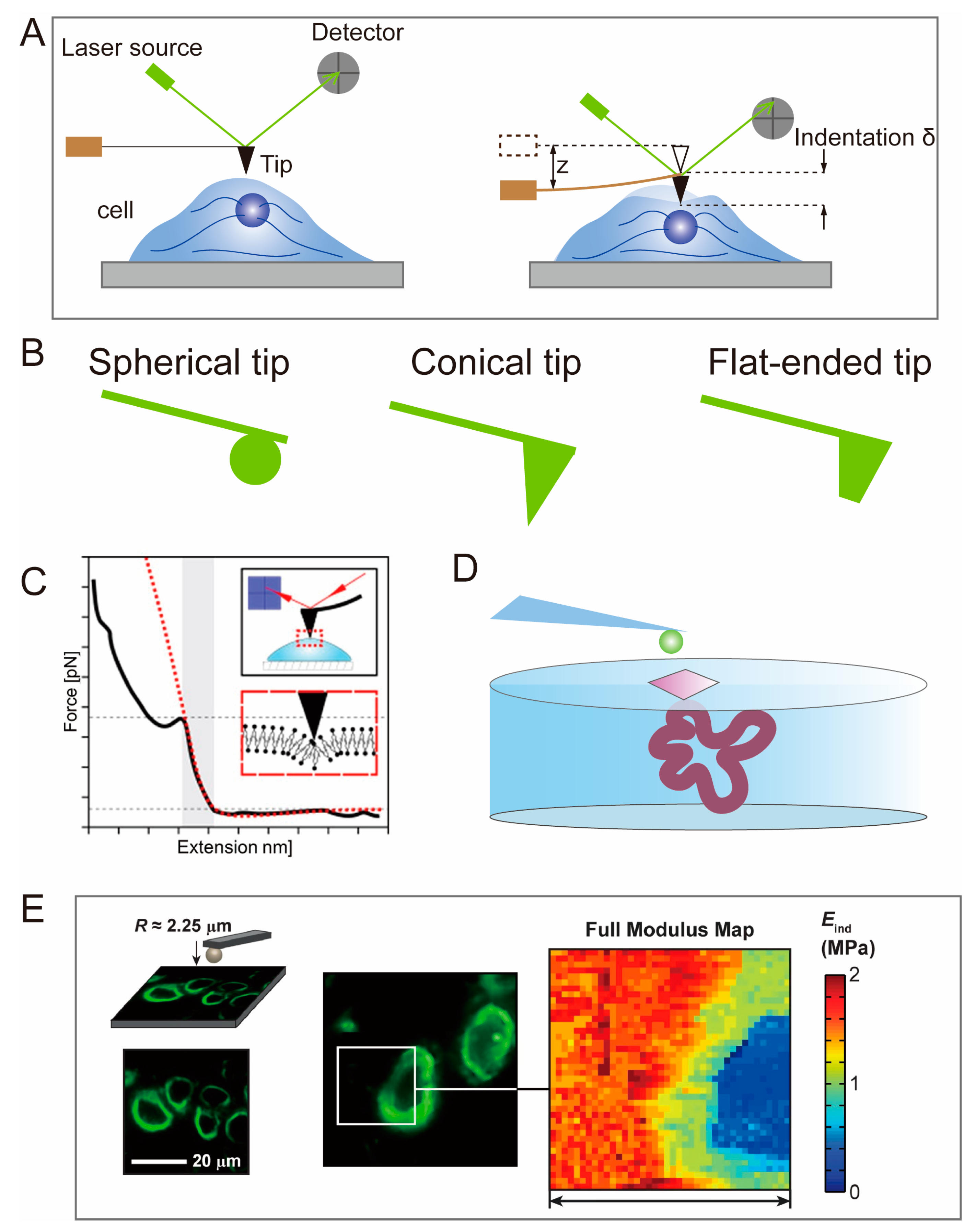
2.1. Atomic Force Microscopy
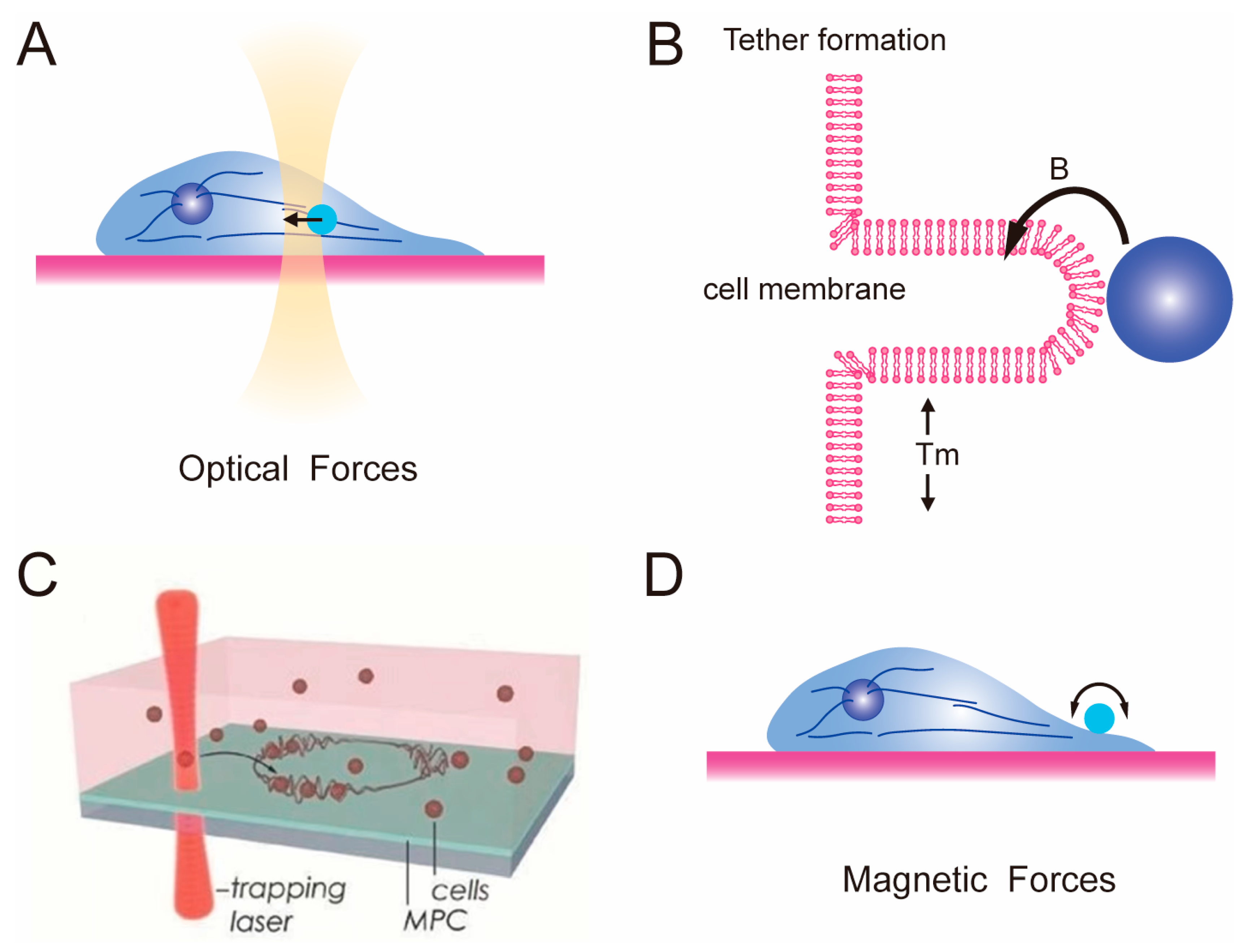
2.2. Optical Tweezer and Magnetic Tweezer
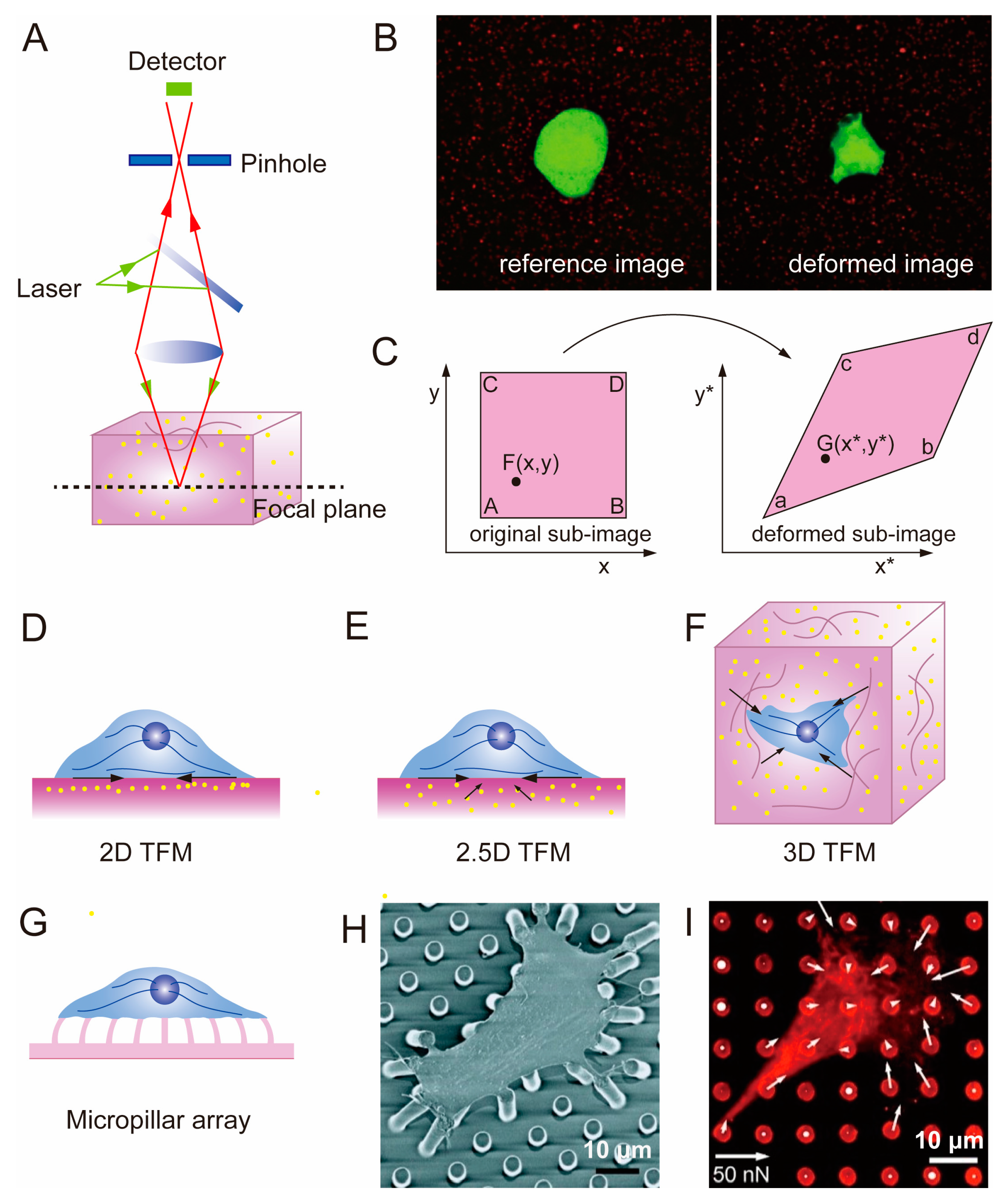
2.3. Traction Force Microscopy
2.4. Finite Element Analysis
3. Application of Advanced Mechanical Testing Technologies in Tissue Engineering
3.1. Hydrogel for 2D Cell Cultivation
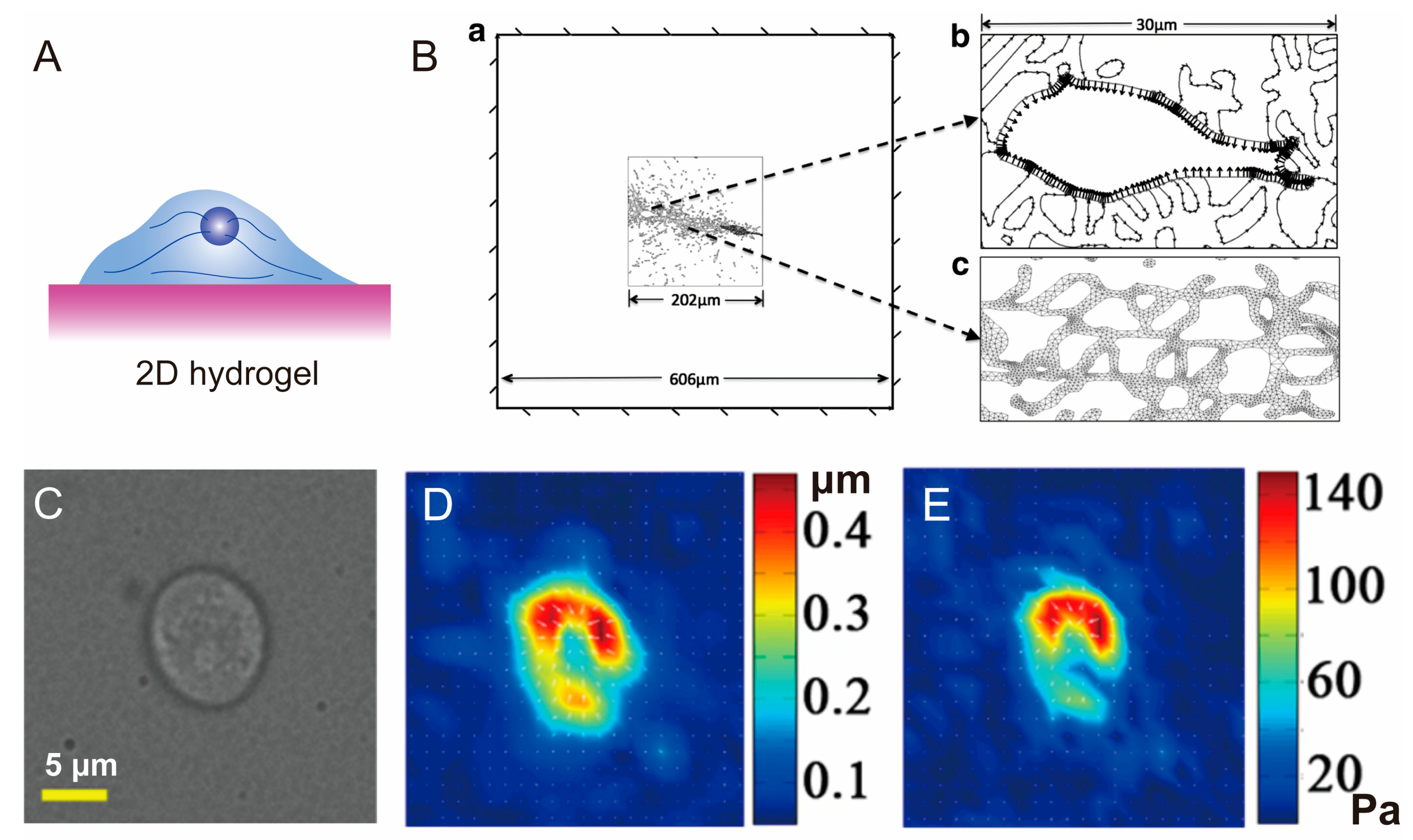
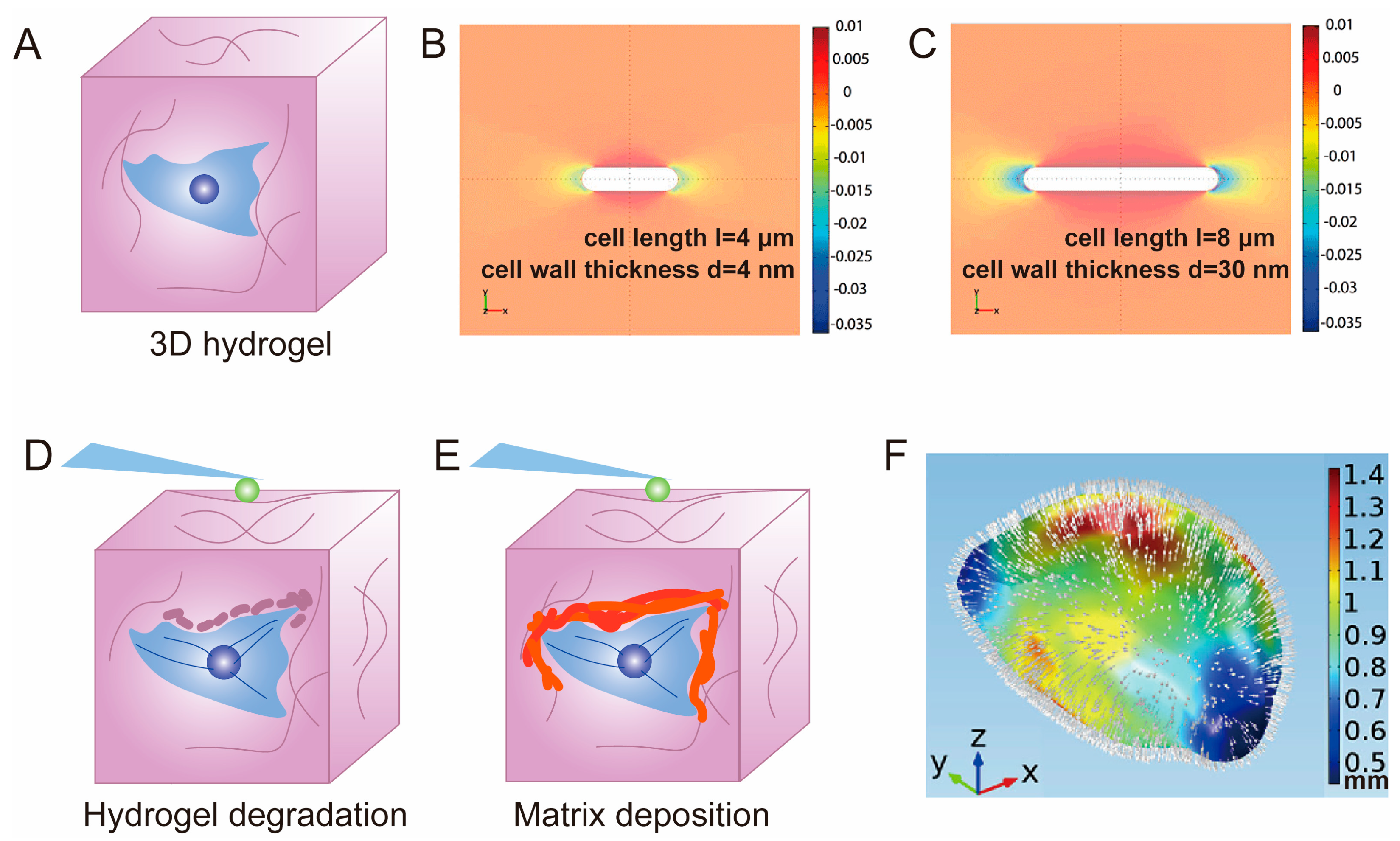
3.2. Hydrogel for 3D Cell Cultivation
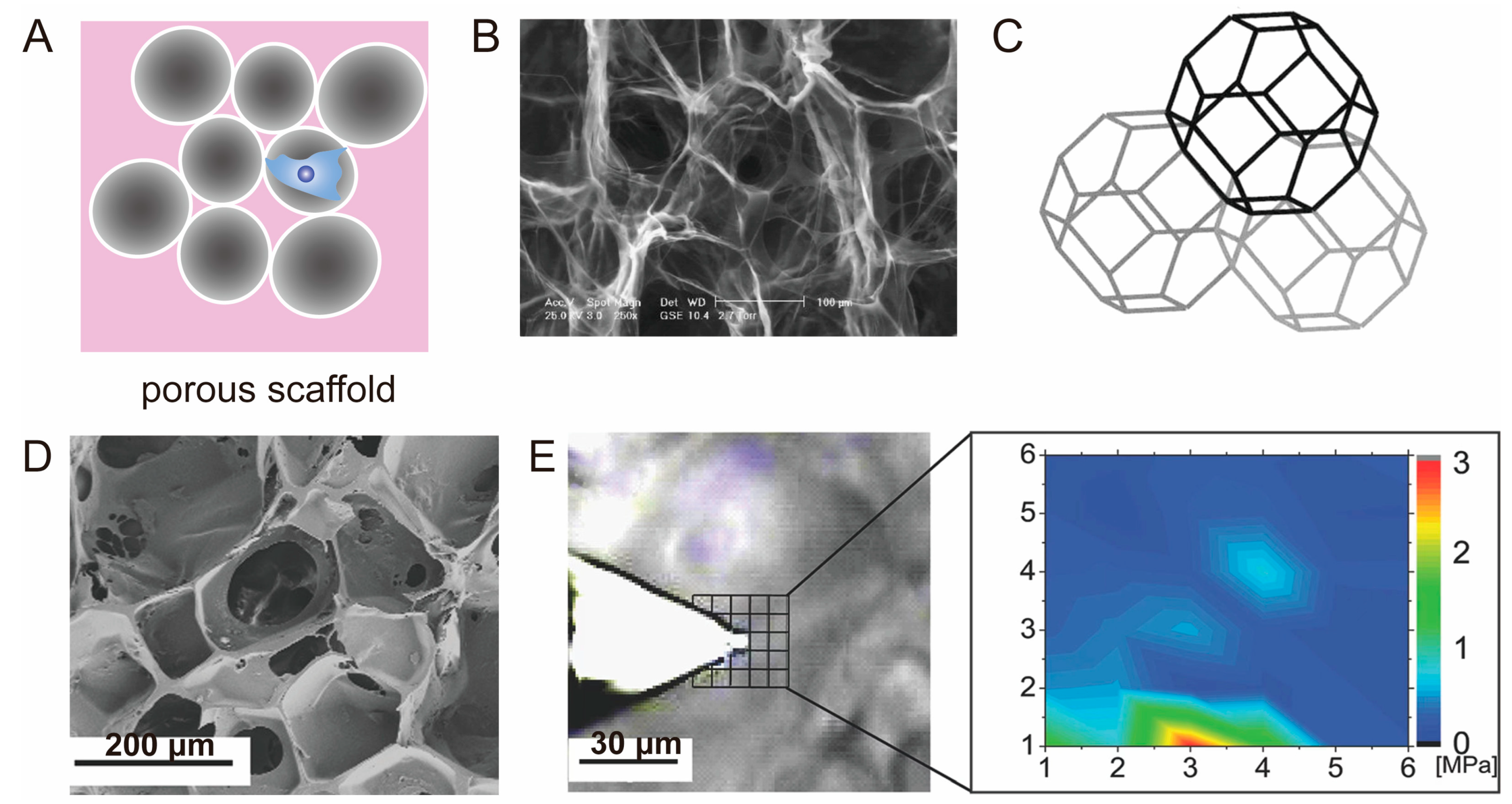
3.3. Porous Scaffold
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Ma, Y.; Han, T.; Yang, Q.; Wang, J.; Feng, B.; Jia, Y.; Wei, Z.; Xu, F. Viscoelastic cell microenvironment: Hydrogel-based strategy for recapitulating dynamic ECM mechanics. Adv. Funct. Mater. 2021, 31, 2100848. [Google Scholar] [CrossRef]
- Vining, K.H.; Stafford, A.; Mooney, D.J. Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials 2019, 188, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Heo, S.-J.; Driscoll, T.P.; Delucca, J.F.; McLeod, C.M.; Smith, L.J.; Duncan, R.L.; Mauck, R.L.; Elliott, D.M. Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage. Nat. Mater. 2016, 15, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef]
- Darling, E.M.; Zauscher, S.; Guilak, F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthr. Cartil. 2006, 14, 571–579. [Google Scholar] [CrossRef]
- Choi, J.B.; Youn, I.; Cao, L.; Leddy, H.A.; Gilchrist, C.L.; Setton, L.A.; Guilak, F. Zonal changes in the three-dimensional morphology of the chondron under compression: The relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J. Biomech. 2007, 40, 2596–2603. [Google Scholar] [CrossRef]
- Kroupa, K.R.; Gangi, L.R.; Zimmerman, B.K.; Hung, C.T.; Ateshian, G.A. Superficial zone chondrocytes can get compacted under physiological loading: A multiscale finite element analysis. Acta Biomater. 2023, 163, 248–258. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J. Cell. Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M.; O’Brien, F.J. Mechanosignalling in cartilage: An emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84. [Google Scholar] [CrossRef]
- Costa, J.B.; Park, J.; Jorgensen, A.M.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Atala, A.; Yoo, J.J.; Lee, S.J. 3D Bioprinted Highly Elastic Hybrid Constructs for Advanced Fibrocartilaginous Tissue Regeneration. Chem. Mater. 2020, 32, 8733–8746. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Martinac, B.; Nikolaev, Y.A.; Silvani, G.; Bavi, N.; Romanov, V.; Nakayama, Y.; Martinac, A.D.; Rohde, P.; Bavi, O.; Cox, C.D. Cell membrane mechanics and mechanosensory transduction. Curr. Top. Membr. 2020, 86, 83–141. [Google Scholar]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Masters, T.A.; Sheetz, M.P. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012, 22, 527–535. [Google Scholar] [CrossRef]
- Wickström, S.A.; Niessen, C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: Local cues for large scale organization. Curr. Opin. Cell Biol. 2018, 54, 89–97. [Google Scholar] [CrossRef]
- Zeevaert, K.; Elsafi Mabrouk, M.H.; Wagner, W.; Goetzke, R. Cell Mechanics in Embryoid Bodies. Cells 2020, 9, 2270. [Google Scholar] [CrossRef]
- Nouri-Goushki, M.; Angeloni, L.; Modaresifar, K.; Minneboo, M.; Boukany, P.E.; Mirzaali, M.J.; Ghatkesar, M.K.; Fratila-Apachitei, L.E.; Zadpoor, A.A. 3D-Printed Submicron Patterns Reveal the Interrelation between Cell Adhesion, Cell Mechanics, and Osteogenesis. ACS Appl. Mater. Interfaces 2021, 13, 33767–33781. [Google Scholar] [CrossRef] [PubMed]
- Abidine, Y.; Giannetti, A.; Revilloud, J.; Laurent, V.M.; Verdier, C. Viscoelastic properties in cancer: From cells to spheroids. Cells 2021, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, H.; Zhu, P.; Lu, H.; Tang, Q. Experiment study and FEM simulation on erythrocytes under linear stretching of optical micromanipulation. AIP Adv. 2017, 7, 085003. [Google Scholar] [CrossRef]
- Rodriguez, M.L.; McGarry, P.J.; Sniadecki, N.J. Review on Cell Mechanics: Experimental and Modeling Approaches. Appl. Mech. Rev. 2013, 65, 060801. [Google Scholar] [CrossRef]
- Kim, D.H.; Wong, P.K.; Park, J.; Levchenko, A.; Sun, Y. Microengineered platforms for cell mechanobiology. Annu. Rev. Biomed. Eng. 2009, 11, 203–233. [Google Scholar] [CrossRef]
- Major, L.G.; Holle, A.W.; Young, J.L.; Hepburn, M.S.; Jeong, K.; Chin, I.L.; Sanderson, R.W.; Jeong, J.H.; Aman, Z.M.; Kennedy, B.F. Volume adaptation controls stem cell mechanotransduction. ACS Appl. Mater. Interfaces 2019, 11, 45520–45530. [Google Scholar] [CrossRef]
- Meli, V.S.; Atcha, H.; Veerasubramanian, P.K.; Nagalla, R.R.; Luu, T.U.; Chen, E.Y.; Guerrero-Juarez, C.F.; Yamaga, K.; Pandori, W.; Hsieh, J.Y. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020, 6, eabb8471. [Google Scholar] [CrossRef]
- Mammoto, T.; Mammoto, A.; Ingber, D.E. Mechanobiology and Developmental Control. Annu. Rev. Cell Dev. Biol. 2013, 29, 27–61. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Yui, Y.; Damiano, L.; Bainer, R.O.; Lakins, J.N.; Acerbi, I.; Ou, G.; Wijekoon, A.C.; Levental, K.R.; Gilbert, P.M.; et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 2014, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Huang, B.; Wang, N. Cytoskeletal prestress: The cellular hallmark in mechanobiology and mechanomedicine. Cytoskeleton 2021, 78, 249–276. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Chen, C.S. Measuring cell-generated forces: A guide to the available tools. Nat. Methods 2016, 13, 415–423. [Google Scholar] [CrossRef]
- Oates, A.C.; Gorfinkiel, N.; González-Gaitán, M.; Heisenberg, C.-P. Quantitative approaches in developmental biology. Nat. Rev. Genet. 2009, 10, 517–530. [Google Scholar] [CrossRef]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef]
- Roca-Cusachs, P.; Conte, V.; Trepat, X. Quantifying forces in cell biology. Nat. Cell Biol. 2017, 19, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Corin, K.A.; Gibson, L.J. Cell contraction forces in scaffolds with varying pore size and cell density. Biomaterials 2010, 31, 4835–4845. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Xiong, C.; Yuan, F. Elastic hydrogel as a sensor for detection of mechanical stress generated by single cells grown in three-dimensional environment. Biomaterials 2016, 98, 103–112. [Google Scholar] [CrossRef]
- Heller, I.; Hoekstra, T.P.; King, G.A.; Peterman, E.J.; Wuite, G.J. Optical tweezers analysis of DNA–protein complexes. Chem. Rev. 2014, 114, 3087–3119. [Google Scholar] [CrossRef]
- Ritchie, D.B.; Woodside, M.T. Probing the structural dynamics of proteins and nucleic acids with optical tweezers. Curr. Opin. Struct. Biol. 2015, 34, 43–51. [Google Scholar] [CrossRef]
- Xin, Q.; Li, P.; He, Y.; Shi, C.; Qiao, Y.; Bian, X.; Su, J.; Qiao, R.; Zhou, X.; Zhong, J. Magnetic tweezers for the mechanical research of DNA at the single molecule level. Anal. Methods 2017, 9, 5720–5730. [Google Scholar] [CrossRef]
- Erdemir, A.; Guess, T.M.; Halloran, J.; Tadepalli, S.C.; Morrison, T.M. Considerations for reporting finite element analysis studies in biomechanics. J. Biomech. 2012, 45, 625–633. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Hansma, P.K.; Elings, V.; Marti, O.; Bracker, C. Scanning tunneling microscopy and atomic force microscopy: Application to biology and technology. Science 1988, 242, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Markert, C.D.; Guo, X.; Skardal, A.; Wang, Z.; Bharadwaj, S.; Zhang, Y.; Bonin, K.; Guthold, M. Characterizing the micro-scale elastic modulus of hydrogels for use in regenerative medicine. J. Mech. Behav. Biomed. Mater. 2013, 27, 115–127. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Wang, W.-H.; Hardy, S.D.; Geahlen, R.L.; Raman, A. Measuring nanoscale viscoelastic parameters of cells directly from AFM force-displacement curves. Sci. Rep. 2017, 7, 1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Opdahl, A.; Marmo, C.; Somorjai, G.A. AFM and SFG studies of pHEMA-based hydrogel contact lens surfaces in saline solution: Adhesion, friction, and the presence of non-crosslinked polymer chains at the surface. Biomaterials 2002, 23, 1657–1666. [Google Scholar] [CrossRef]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Haga, H.; Sasaki, S.; Kawabata, K.; Ito, E.; Ushiki, T.; Sambongi, T. Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy 2000, 82, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, M.; Fritz, M.; Kacher, C.M.; Cleveland, J.P.; Hansma, P.K. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 1996, 70, 556–567. [Google Scholar] [CrossRef]
- Norman, M.D.A.; Ferreira, S.A.; Jowett, G.M.; Bozec, L.; Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nat. Protoc. 2021, 16, 2418–2449. [Google Scholar] [CrossRef]
- Lachowski, D.; Matellan, C.; Gopal, S.; Cortes, E.; Robinson, B.K.; Saiani, A.; Miller, A.F.; Stevens, M.M.; del Río Hernández, A.E. Substrate Stiffness-Driven Membrane Tension Modulates Vesicular Trafficking via Caveolin-1. ACS Nano 2022, 16, 4322–4337. [Google Scholar] [CrossRef]
- Chery, D.R.; Han, B.; Li, Q.; Zhou, Y.; Heo, S.-J.; Kwok, B.; Chandrasekaran, P.; Wang, C.; Qin, L.; Lu, X.L.; et al. Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis. Acta Biomater. 2020, 111, 267–278. [Google Scholar] [CrossRef]
- Kontomaris, S.-V. The Hertz model in AFM nanoindentation experiments: Applications in biological samples and biomaterials. Micro Nanosyst. 2018, 10, 11–22. [Google Scholar] [CrossRef]
- Kopycinska-Müller, M.; Geiss, R.H.; Hurley, D.C. Contact mechanics and tip shape in AFM-based nanomechanical measurements. Ultramicroscopy 2006, 106, 466–474. [Google Scholar] [CrossRef]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Engel, A.; Schoenenberger, C.-A.; Müller, D.J. High resolution imaging of native biological sample surfaces using scanning probe microscopy. Curr. Opin. Struct. Biol. 1997, 7, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Cascione, M.; Leporatti, S.; Dituri, F.; Giannelli, G. Transforming Growth Factor-β Promotes Morphomechanical Effects Involved in Epithelial to Mesenchymal Transition in Living Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 20, 108. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Pellegrino, P.; Rizzello, L.; Rinaldi, R. Tailoring cell morphomechanical perturbations through metal oxide nanoparticles. Nanoscale Res. Lett. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Benoit, D.S.; Durney, A.R.; Anseth, K.S. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006, 12, 1663–1673. [Google Scholar] [CrossRef]
- Horton, E.R.; Vallmajo-Martin, Q.; Martin, I.; Snedeker, J.G.; Ehrbar, M.; Blache, U. Extracellular matrix production by mesenchymal stromal cells in hydrogels facilitates cell spreading and is inhibited by FGF-2. Adv. Healthc. Mater. 2020, 9, 1901669. [Google Scholar] [CrossRef] [PubMed]
- Jowett, G.M.; Norman, M.D.A.; Yu, T.T.L.; Rosell Arévalo, P.; Hoogland, D.; Lust, S.T.; Read, E.; Hamrud, E.; Walters, N.J.; Niazi, U.; et al. ILC1 drive intestinal epithelial and matrix remodelling. Nat. Mater. 2021, 20, 250–259. [Google Scholar] [CrossRef]
- Staunton, J.R.; Doss, B.L.; Lindsay, S.; Ros, R. Correlating confocal microscopy and atomic force indentation reveals metastatic cancer cells stiffen during invasion into collagen I matrices. Sci. Rep. 2016, 6, 19686. [Google Scholar] [CrossRef]
- Li, M.; Dang, D.; Xi, N.; Wang, Y.; Liu, L. Nanoscale imaging and force probing of biomolecular systems using atomic force microscopy: From single molecules to living cells. Nanoscale 2017, 9, 17643–17666. [Google Scholar] [CrossRef]
- Neffe, A.T.; Pierce, B.F.; Tronci, G.; Ma, N.; Pittermann, E.; Gebauer, T.; Frank, O.; Schossig, M.; Xu, X.; Willie, B.M.; et al. One Step Creation of Multifunctional 3D Architectured Hydrogels Inducing Bone Regeneration. Adv. Mater. 2015, 27, 1738–1744. [Google Scholar] [CrossRef]
- Zhang, X.; Flores, L.R.; Keeling, M.C.; Sliogeryte, K.; Gavara, N. Ezrin Phosphorylation at T567 Modulates Cell Migration, Mechanical Properties, and Cytoskeletal Organization. Int. J. Mol. Sci. 2020, 21, 435. [Google Scholar] [CrossRef]
- Ayala, Y.A.; Pontes, B.; Ether, D.S.; Pires, L.B.; Araujo, G.R.; Frases, S.; Romão, L.F.; Farina, M.; Moura-Neto, V.; Viana, N.B. Rheological properties of cells measured by optical tweezers. BMC Biophys. 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tassieri, M.; Gibson, G.M.; Evans, R.; Yao, A.M.; Warren, R.; Padgett, M.J.; Cooper, J.M. Measuring storage and loss moduli using optical tweezers: Broadband microrheology. Phys. Rev. E 2010, 81, 026308. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Acceleration and Trapping of Particles by Radiation Pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef]
- Arbore, C.; Perego, L.; Sergides, M.; Capitanio, M. Probing force in living cells with optical tweezers: From single-molecule mechanics to cell mechanotransduction. Biophys. Rev. 2019, 11, 765–782. [Google Scholar] [CrossRef]
- Nussenzveig, H.M. Cell membrane biophysics with optical tweezers. Eur. Biophys. J. 2018, 47, 499–514. [Google Scholar] [CrossRef]
- Dai, J.; Sheetz, M.P. Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys. J. 1995, 68, 988–996. [Google Scholar] [CrossRef]
- Jones, C.; Ahmed, W. Optical Tweezers for Force Measurement in Living Cells. Bull. Am. Phys. Soc. 2018, 63. [Google Scholar]
- Guo, M.; Ehrlicher, A.J.; Mahammad, S.; Fabich, H.; Jensen, M.H.; Moore, J.R.; Fredberg, J.J.; Goldman, R.D.; Weitz, D.A. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys. J. 2013, 105, 1562–1568. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Serey, X.; Sarkar, R.; Chen, P.; Erickson, D. Controlled photonic manipulation of proteins and other nanomaterials. Nano Lett. 2012, 12, 1633–1637. [Google Scholar] [CrossRef]
- Hao, T.; Feng, N.; Gong, F.; Yu, Y.; Liu, J.; Ren, Y.-X. Complexin-1 regulated assembly of single neuronal SNARE complex revealed by single-molecule optical tweezers. Commun. Biol. 2023, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.R.; Takeuchi, S.; Paul, O. Multicellular Biohybrid Materials: Probing the Interplay of Cells of Different Types Precisely Positioned and Constrained on 3D Wireframe-like Microstructures. Adv. Healthc. Mater. 2017, 6, 1601053. [Google Scholar] [CrossRef]
- Sheetz, M.P. Cell control by membrane–cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2001, 2, 392–396. [Google Scholar] [CrossRef]
- Hochmuth, F.; Shao, J.-Y.; Dai, J.; Sheetz, M.P. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J. 1996, 70, 358–369. [Google Scholar] [CrossRef]
- De Belly, H.; Stubb, A.; Yanagida, A.; Labouesse, C.; Jones, P.H.; Paluch, E.K.; Chalut, K.J. Membrane Tension Gates ERK-Mediated Regulation of Pluripotent Cell Fate. Cell Stem Cell 2021, 28, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Hetmanski, J.H.; de Belly, H.; Busnelli, I.; Waring, T.; Nair, R.V.; Sokleva, V.; Dobre, O.; Cameron, A.; Gauthier, N.; Lamaze, C. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell 2019, 51, 460–475.e410. [Google Scholar] [CrossRef]
- Bergert, M.; Lembo, S.; Sharma, S.; Russo, L.; Milovanović, D.; Gretarsson, K.H.; Börmel, M.; Neveu, P.A.; Hackett, J.A.; Petsalaki, E.; et al. Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation. Cell Stem Cell 2021, 28, 209–216.e204. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Kong, C.-W.; Chen, S.; Cheng, S.H.; Li, R.A.; Sun, D. Probing the mechanobiological properties of human embryonic stem cells in cardiac differentiation by optical tweezers. J. Biomech. 2012, 45, 123–128. [Google Scholar] [CrossRef]
- Titushkin, I.; Cho, M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys. J. 2007, 93, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Muncie, J.M.; Weaver, V.M. Membrane Tension Locks In Pluripotency. Cell Stem Cell 2021, 28, 175–176. [Google Scholar] [CrossRef]
- Tsujita, K.; Satow, R.; Asada, S.; Nakamura, Y.; Arnes, L.; Sako, K.; Fujita, Y.; Fukami, K.; Itoh, T. Homeostatic membrane tension constrains cancer cell dissemination by counteracting BAR protein assembly. Nat. Commun. 2021, 12, 5930. [Google Scholar] [CrossRef]
- Schneider, D.; Baronsky, T.; Pietuch, A.; Rother, J.; Oelkers, M.; Fichtner, D.; Wedlich, D.; Janshoff, A. Tension monitoring during epithelial-to-mesenchymal transition links the switch of phenotype to expression of moesin and cadherins in NMuMG cells. PLoS ONE 2013, 8, e80068. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Y.C.; Zhang, Y.; Xiao, Y.F.; Li, B. Optical forces: From fundamental to biological applications. Adv. Mater. 2020, 32, 2001994. [Google Scholar] [CrossRef]
- Kirkham, G.R.; Britchford, E.; Upton, T.; Ware, J.; Gibson, G.M.; Devaud, Y.; Ehrbar, M.; Padgett, M.; Allen, S.; Buttery, L.D.; et al. Precision Assembly of Complex Cellular Microenvironments using Holographic Optical Tweezers. Sci. Rep. 2015, 5, 8577. [Google Scholar] [CrossRef]
- Mierke, C.T.; Puder, S.; Aermes, C.; Fischer, T.; Kunschmann, T. Effect of PAK inhibition on cell mechanics depends on Rac1. Front. Cell Dev. Biol. 2020, 8, 13. [Google Scholar] [CrossRef]
- Mierke, C.T. The role of the optical stretcher is crucial in the investigation of cell mechanics regulating cell adhesion and motility. Front. Cell Dev. Biol. 2019, 7, 184. [Google Scholar] [CrossRef]
- Park, S.; Joo, Y.K.; Chen, Y. Versatile and high-throughput force measurement platform for dorsal cell mechanics. Sci. Rep. 2019, 9, 13286. [Google Scholar] [CrossRef]
- Tseng, P.; Judy, J.W.; Di Carlo, D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat. Methods 2012, 9, 1113–1119. [Google Scholar] [CrossRef]
- Aermes, C.; Hayn, A.; Fischer, T.; Mierke, C.T. Environmentally controlled magnetic nano-tweezer for living cells and extracellular matrices. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bausch, A.R.; Möller, W.; Sackmann, E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 1999, 76, 573–579. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.H.; Krenn, B.E.; van Driel, R.; Kanger, J.S. Micro magnetic tweezers for nanomanipulation inside live cells. Biophys. J. 2005, 88, 2137–2144. [Google Scholar] [CrossRef]
- Oliver, T.; Dembo, M.; Jacobson, K. Traction forces in locomoting cells. Cell Motil. Cytoskelet. 1995, 31, 225–240. [Google Scholar] [CrossRef]
- Dembo, M.; Wang, Y.L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999, 76, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, S.V.; Sabass, B.; Schwarz, U.S.; Waterman, C.M. High-resolution traction force microscopy. Methods Cell Biol. 2014, 123, 367–394. [Google Scholar]
- Del Alamo, J.C.; Meili, R.; Álvarez-González, B.; Alonso-Latorre, B.; Bastounis, E.; Firtel, R.; Lasheras, J.C. Three-dimensional quantification of cellular traction forces and mechanosensing of thin substrata by fourier traction force microscopy. PLoS ONE 2013, 8, e69850. [Google Scholar]
- Liu, K.; Yuan, Y.; Huang, J.; Wei, Q.; Pang, M.; Xiong, C.; Fang, J. Improved-throughput traction microscopy based on fluorescence micropattern for manual microscopy. PLoS ONE 2013, 8, e70122. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Su, Y.; Xu, X.; Liu, H.; Mei, K.; Lan, S.; Zhang, S.; Wu, X.; Cao, Y.; et al. Quantifying 3D cell-matrix interactions during mitosis and the effect of anticancer drugs on the interactions. Nano Res. 2021, 14, 4163–4172. [Google Scholar] [CrossRef]
- Legant, W.R.; Choi, C.K.; Miller, J.S.; Shao, L.; Gao, L.; Betzig, E.; Chen, C.S. Multidimensional traction force microscopy reveals out-of-plane rotational moments about focal adhesions. Proc. Natl. Acad. Sci. USA 2013, 110, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Colin-York, H.; Barbieri, L.; Javanmardi, Y.; Guo, Y.; Korobchevskaya, K.; Moeendarbary, E.; Li, D.; Fritzsche, M. Astigmatic traction force microscopy (aTFM). Nat. Commun. 2021, 12, 2168. [Google Scholar] [CrossRef] [PubMed]
- Holenstein, C.N.; Silvan, U.; Snedeker, J.G. High-resolution traction force microscopy on small focal adhesions-improved accuracy through optimal marker distribution and optical flow tracking. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Holley, M.T.; YekrangSafakar, A.; Maziveyi, M.; Alahari, S.K.; Park, K. Measurement of cell traction force with a thin film PDMS cantilever. Biomed. Microdevices 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Zheng, Q.; Peng, M.; Liu, Z.; Li, S.; Han, R.; Ouyang, H.; Fan, Y.; Pan, C.; Hu, W.; Zhai, J. Dynamic real-time imaging of living cell traction force by piezo-phototronic light nano-antenna array. Sci. Adv. 2021, 7, eabe7738. [Google Scholar] [CrossRef]
- Li, Z.; Persson, H.; Adolfsson, K.; Abariute, L.; Borgström, M.T.; Hessman, D.; Åström, K.; Oredsson, S.; Prinz, C.N. Cellular traction forces: A useful parameter in cancer research. Nanoscale 2017, 9, 19039–19044. [Google Scholar] [CrossRef] [PubMed]
- Hippler, M.; Weißenbruch, K.; Richler, K.; Lemma, E.D.; Nakahata, M.; Richter, B.; Barner-Kowollik, C.; Takashima, Y.; Harada, A.; Blasco, E. Mechanical stimulation of single cells by reversible host-guest interactions in 3D microscaffolds. Sci. Adv. 2020, 6, eabc2648. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef]
- Franck, C.; Hong, S.; Maskarinec, S.A.; Tirrell, D.A.; Ravichandran, G. Three-dimensional Full-field Measurements of Large Deformations in Soft Materials Using Confocal Microscopy and Digital Volume Correlation. Exp. Mech. 2007, 47, 427–438. [Google Scholar] [CrossRef]
- Mulligan, J.A.; Bordeleau, F.; Reinhart-King, C.A.; Adie, S.G. Traction force microscopy for noninvasive imaging of cell forces. Biomech. Oncol. 2018, 1092, 319–349. [Google Scholar]
- Du, Y.; Herath, S.; Wang, Q.; Asada, H.; Chen, P. Determination of Green’s function for three-dimensional traction force reconstruction based on geometry and boundary conditions of cell culture matrices. Acta Biomater. 2018, 67, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pan, X.; Li, S.; Peng, X.; Xiong, C.; Fang, J. A Digital Volume Correlation Technique for 3-D Deformation Measurements of Soft Gels. Int. J. Appl. Mech. 2011, 03, 335–354. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Notbohm, J.; Kim, J.-H.; Franck, C.; Maskarinec, S.; Tirrell, D.; Asthagiri, A.; Ravichandran, G. Three-dimensional traction force microscopy for studying cellular interactions with biomaterials. Procedia Iutam 2012, 4, 144–150. [Google Scholar] [CrossRef]
- Legant, W.R.; Miller, J.S.; Blakely, B.L.; Cohen, D.M.; Genin, G.M.; Chen, C.S. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 2010, 7, 969–971. [Google Scholar] [CrossRef]
- Watson, P.A. Function follows form: Generation of intracellular signals by cell deformation. FASEB J. 1991, 5, 2013–2019. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nelson, C.M. Mapping of mechanical strains and stresses around quiescent engineered three-dimensional epithelial tissues. Biophys. J. 2012, 103, 152–162. [Google Scholar] [CrossRef]
- du Roure, O.; Saez, A.; Buguin, A.; Austin, R.H.; Chavrier, P.; Silberzan, P.; Ladoux, B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2390–2395. [Google Scholar] [CrossRef]
- Ghassemi, S.; Meacci, G.; Liu, S.; Gondarenko, A.A.; Mathur, A.; Roca-Cusachs, P.; Sheetz, M.P.; Hone, J. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc. Natl. Acad. Sci. USA 2012, 109, 5328–5333. [Google Scholar] [CrossRef]
- Ghibaudo, M.; Saez, A.; Trichet, L.; Xayaphoummine, A.; Browaeys, J.; Silberzan, P.; Buguin, A.; Ladoux, B. Traction forces and rigidity sensing regulate cell functions. Soft Matter 2008, 4, 1836–1843. [Google Scholar] [CrossRef]
- Trickey, W.R.; Lee, G.M.; Guilak, F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J. Orthop. Res. 2000, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.R.; Ting-Beall, H.P.; Lee, G.M.; Kelley, S.S.; Hochmuth, R.M.; Guilak, F. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 1999, 32, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, L.G.; Haider, M.A.; Vail, T.P.; Guilak, F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J. Biomech. Eng. 2003, 125, 323–333. [Google Scholar] [CrossRef]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef]
- Shin, D.; Athanasiou, K. Cytoindentation for obtaining cell biomechanical properties. J. Orthop. Res. 1999, 17, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Herzog, W.; Epstein, M. Modelling of location- and time-dependent deformation of chondrocytes during cartilage loading. J. Biomech. 1999, 32, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kirmizis, D.; Logothetidis, S. Atomic force microscopy probing in the measurement of cell mechanics. Int. J. Nanomed. 2010, 5, 137–145. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Ofek, G.; Athanasiou, K. Micromechanical properties of chondrocytes and chondrons: Relevance to articular cartilage tissue engineering. J. Mech. Mater. Struct. 2007, 2, 1059–1086. [Google Scholar] [CrossRef][Green Version]
- Schwarz, U.S.; Soiné, J.R. Traction force microscopy on soft elastic substrates: A guide to recent computational advances. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness sensing by cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef] [PubMed]
- Zemel, A.; De, R.; Safran, S.A. Mechanical consequences of cellular force generation. Curr. Opin. Solid State Mater. Sci. 2011, 15, 169–176. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen-and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Nie, K.; Han, S.; Yang, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Enzyme-crosslinked electrospun fibrous gelatin hydrogel for potential soft tissue engineering. Polymers 2020, 12, 1977. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Devereux, B.; Wallace, D. Injectable collagen as a pH-sensitive hydrogel. Biomaterials 1994, 15, 985–995. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.S.; Guan, J.; Wu, S.J. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021, 125, 57–71. [Google Scholar] [CrossRef]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the conformation and mechanical properties of silk fibroin hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, H.; Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Lee, J.S.; Lee, O.J.; Lee, H.; Park, H.S.; Choi, K.Y. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 2021, 16, 5484–5532. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Q.; Li, Q.; Li, X. Fabrication of Silk Hydrogel Scaffolds with Aligned Porous Structures and Tunable Mechanical Properties. Gels 2023, 9, 181. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Tan, S.; Fang, J.Y.; Yang, Z.; Nimni, M.E.; Han, B. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials 2014, 35, 5294–5306. [Google Scholar] [CrossRef]
- Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef]
- Burdick, J.A.; Murphy, W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.-C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Ma, X.; Schickel, M.E.; Stevenson, M.D.; Sarang-Sieminski, A.L.; Gooch, K.J.; Ghadiali, S.N.; Hart, R.T. Fibers in the extracellular matrix enable long-range stress transmission between cells. Biophys. J. 2013, 104, 1410–1418. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Wan, Z.; Sun, X.; Lu, Y.; Huang, J.; Wang, F.; Zeng, Y.; Chen, Y.H.; Shi, Y.; et al. Profiling the origin, dynamics, and function of traction force in B cell activation. Sci. Signal. 2018, 11, eaai9192. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, Y.; Duan, X.; Fang, X.; Zhang, Q.; Zhang, Y.; Wang, P.; Huang, J. Spontaneous formation and spatial self-organization of mechanically induced mesenchymal-like cells within geometrically confined cancer cell monolayers. Biomaterials 2022, 281, 121337. [Google Scholar] [CrossRef]
- Ross, T.D.; Coon, B.G.; Yun, S.; Baeyens, N.; Tanaka, K.; Ouyang, M.; Schwartz, M.A. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 2013, 25, 613–618. [Google Scholar] [CrossRef]
- Li, X.; Klausen, L.H.; Zhang, W.; Jahed, Z.; Tsai, C.-T.; Li, T.L.; Cui, B. Nanoscale Surface Topography Reduces Focal Adhesions and Cell Stiffness by Enhancing Integrin Endocytosis. Nano Lett. 2021, 21, 8518–8526. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Meschewski, R.; Watson, E.; Valentine, M.T. Portable magnetic tweezers device enables visualization of the three-dimensional microscale deformation of soft biological materials. Biotechniques 2011, 51, 29–34. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- DeVolder, R.; Kong, H.J. Hydrogels for in vivo-like three-dimensional cellular studies. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 351–365. [Google Scholar] [CrossRef]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef]
- Matellan, C.; del Río Hernández, A.E. Engineering the cellular mechanical microenvironment—From bulk mechanics to the nanoscale. J. Cell Sci. 2019, 132, jcs229013. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Beningo, K.A.; Dembo, M.; Wang, Y.-L. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 18024–18029. [Google Scholar] [CrossRef]
- Tuson, H.H.; Auer, G.K.; Renner, L.D.; Hasebe, M.; Tropini, C.; Salick, M.; Crone, W.C.; Gopinathan, A.; Huang, K.C.; Weibel, D.B. Measuring the stiffness of bacterial cells from growth rates in hydrogels of tunable elasticity. Mol. Microbiol. 2012, 84, 874–891. [Google Scholar] [CrossRef]
- Darling, E.M.; Wilusz, R.E.; Bolognesi, M.P.; Zauscher, S.; Guilak, F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys. J. 2010, 98, 2848–2856. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Motwani, M.S.; Faull, P.A.; Seymour, A.J.; Yu, T.T.L.; Enayati, M.; Taheem, D.K.; Salzlechner, C.; Haghighi, T.; Kania, E.M.; et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nat. Commun. 2018, 9, 4049. [Google Scholar] [CrossRef]
- Hall, M.S.; Long, R.; Feng, X.; Huang, Y.; Hui, C.-Y.; Wu, M. Toward single cell traction microscopy within 3D collagen matrices. Exp. Cell Res. 2013, 319, 2396–2408. [Google Scholar] [CrossRef]
- Wei, Q.; Holle, A.; Li, J.; Posa, F.; Biagioni, F.; Croci, O.; Benk, A.S.; Young, J.; Noureddine, F.; Deng, J. BMP-2 signaling and mechanotransduction synergize to drive osteogenic differentiation via YAP/TAZ. Adv. Sci. 2020, 7, 1902931. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Chen, G.; Kawazoe, N. Porous scaffolds for regeneration of cartilage, bone and osteochondral tissue. Osteochondr. Tissue Eng. Nanotechnol. Scaffolding-Relat. Dev. Transl. 2018, 1058, 171–191. [Google Scholar]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Wen, Y.; Xun, S.; Haoye, M.; Baichuan, S.; Peng, C.; Xuejian, L.; Kaihong, Z.; Xuan, Y.; Jiang, P.; Shibi, L. 3D printed porous ceramic scaffolds for bone tissue engineering: A review. Biomater. Sci. 2017, 5, 1690–1698. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Robert, D.; Fayol, D.; Le Visage, C.; Frasca, G.; Brulé, S.; Ménager, C.; Gazeau, F.; Letourneur, D.; Wilhelm, C. Magnetic micro-manipulations to probe the local physical properties of porous scaffolds and to confine stem cells. Biomaterials 2010, 31, 1586–1595. [Google Scholar] [CrossRef]
- Vichare, S.; Sen, S.; Inamdar, M.M. Cellular mechanoadaptation to substrate mechanical properties: Contributions of substrate stiffness and thickness to cell stiffness measurements using AFM. Soft Matter 2014, 10, 1174–1181. [Google Scholar] [CrossRef]
- Ti, C.; Thomas, G.M.; Wen, Q.; Liu, Y. Fiber Optical Tweezers for Simultaneous Force Exertion and Measurements in a 3D Hydrogel Compartment. In Proceedings of the CLEO, San Jose, CA, USA, 10 May 2015; Optica Publishing Group: San Jose, CA, USA, 2015. [Google Scholar]
- Long, H.; Vos, B.E.; Betz, T.; Baker, B.M.; Trappmann, B. Nonswelling and Hydrolytically Stable Hydrogels Uncover Cellular Mechanosensing in 3D. Adv. Sci. 2022, 9, 2105325. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zhao, Y.-P. Kinetic behaviour of the cells touching substrate: The interfacial stiffness guides cell spreading. Sci. Rep. 2014, 4, 3910. [Google Scholar] [CrossRef]
- Eng, G.; Lee, B.W.; Parsa, H.; Chin, C.D.; Schneider, J.; Linkov, G.; Sia, S.K.; Vunjak-Novakovic, G. Assembly of complex cell microenvironments using geometrically docked hydrogel shapes. Proc. Natl. Acad. Sci. USA 2013, 110, 4551–4556. [Google Scholar] [CrossRef]
- Mohagheghian, E.; Luo, J.; Yavitt, F.M.; Wei, F.; Bhala, P.; Amar, K.; Rashid, F.; Wang, Y.; Liu, X.; Ji, C.; et al. Quantifying stiffness and forces of tumor colonies and embryos using a magnetic microrobot. Sci. Robot. 2023, 8, eadc9800. [Google Scholar] [CrossRef]
- Elsayad, K.; Werner, S.; Gallemí, M.; Kong, J.; Sánchez Guajardo, E.R.; Zhang, L.; Jaillais, Y.; Greb, T.; Belkhadir, Y. Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission–Brillouin imaging. Sci. Signal. 2016, 9, rs5. [Google Scholar] [CrossRef]
- Liu, A.P. Biophysical tools for cellular and subcellular mechanical actuation of cell signaling. Biophys. J. 2016, 111, 1112–1118. [Google Scholar] [CrossRef]
- Antonacci, G.; Braakman, S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 2016, 6, 37217. [Google Scholar] [CrossRef]
- Cheng, Y.-R.; Jiang, B.-Y.; Chen, C.-C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Rahil, Z.; Li, I.T.; Chowdhury, F.; Leckband, D.E.; Chemla, Y.R.; Ha, T. Constructing modular and universal single molecule tension sensor using protein G to study mechano-sensitive receptors. Sci. Rep. 2016, 6, 21584. [Google Scholar] [CrossRef]
- Muraoka, T.; Umetsu, K.; Tabata, K.V.; Hamada, T.; Noji, H.; Yamashita, T.; Kinbara, K. Mechano-sensitive synthetic ion channels. J. Am. Chem. Soc. 2017, 139, 18016–18023. [Google Scholar] [CrossRef]
- Liu, R.; Ding, J. Chromosomal Repositioning and Gene Regulation of Cells on a Micropillar Array. ACS Appl. Mater. Interfaces 2020, 12, 35799–35812. [Google Scholar] [CrossRef]
- Yang, X.; Lin, C.; Chen, X.; Li, S.; Li, X.; Xiao, B. Structure deformation and curvature sensing of PIEZO1 in lipid membranes. Nature 2022, 604, 377–383. [Google Scholar] [CrossRef]
- Nourse, J.L.; Pathak, M.M. How cells channel their stress: Interplay between Piezo1 and the cytoskeleton. Semin. Cell Dev. Biol. 2017, 71, 3–12. [Google Scholar] [CrossRef]
| Technique | Test Parameters | Target Parameters | Application | Advantages | Limitations |
|---|---|---|---|---|---|
| AFM | Cantilever deflection | Modulus of cell or substrates | 2D hydrogel [162,183] 3D hydrogel [59,173,174] Porous scaffold [62] | Force measurements in real-time | Surface contact |
| OT | Optical beads displacement | Modulus of cell or substrates | 3D hydrogel [184,185] | High resolution | Heating; photodamage |
| MT | Magnetic beads displacement | Modulus of cell or substrates | 2D hydrogel [163] 3D hydrogel [186] Porous scaffold [182] | No photodamage | Limited sensitivity |
| TFM | Displacement of fluorescent beads | Strain, stress | 2D hydrogel [159,160] 3D hydrogel [35,115] | Non-invasively. Wide applicability | High sensitivity to noise. Dependency on software |
| FEM | / | Strain, stress | 2D hydrogel [158,183,187] 3D hydrogel [172,188] Porous scaffold [34] | Qualitative modeling | Low accuracy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhang, M.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Advanced Mechanical Testing Technologies at the Cellular Level: The Mechanisms and Application in Tissue Engineering. Polymers 2023, 15, 3255. https://doi.org/10.3390/polym15153255
Zhu Y, Zhang M, Sun Q, Wang X, Li X, Li Q. Advanced Mechanical Testing Technologies at the Cellular Level: The Mechanisms and Application in Tissue Engineering. Polymers. 2023; 15(15):3255. https://doi.org/10.3390/polym15153255
Chicago/Turabian StyleZhu, Yingxuan, Mengqi Zhang, Qingqing Sun, Xiaofeng Wang, Xiaomeng Li, and Qian Li. 2023. "Advanced Mechanical Testing Technologies at the Cellular Level: The Mechanisms and Application in Tissue Engineering" Polymers 15, no. 15: 3255. https://doi.org/10.3390/polym15153255
APA StyleZhu, Y., Zhang, M., Sun, Q., Wang, X., Li, X., & Li, Q. (2023). Advanced Mechanical Testing Technologies at the Cellular Level: The Mechanisms and Application in Tissue Engineering. Polymers, 15(15), 3255. https://doi.org/10.3390/polym15153255









