Effect of Polyethylene Glycol Methyl Ether Methacrylate on the Biodegradability of Polyvinyl Alcohol/Starch Blend Films
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
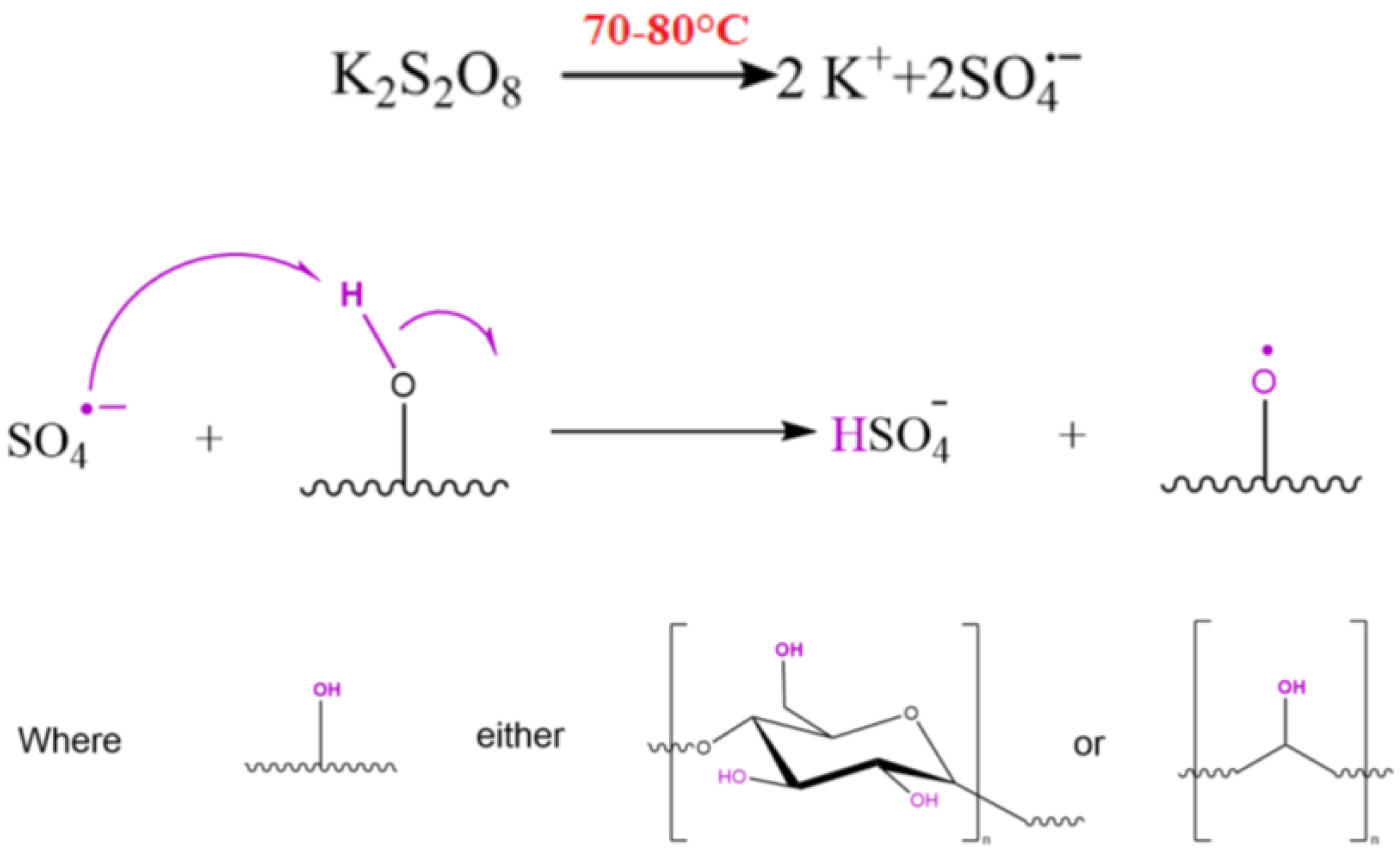
2.2.1. Synthesis of the Blend Copolymer
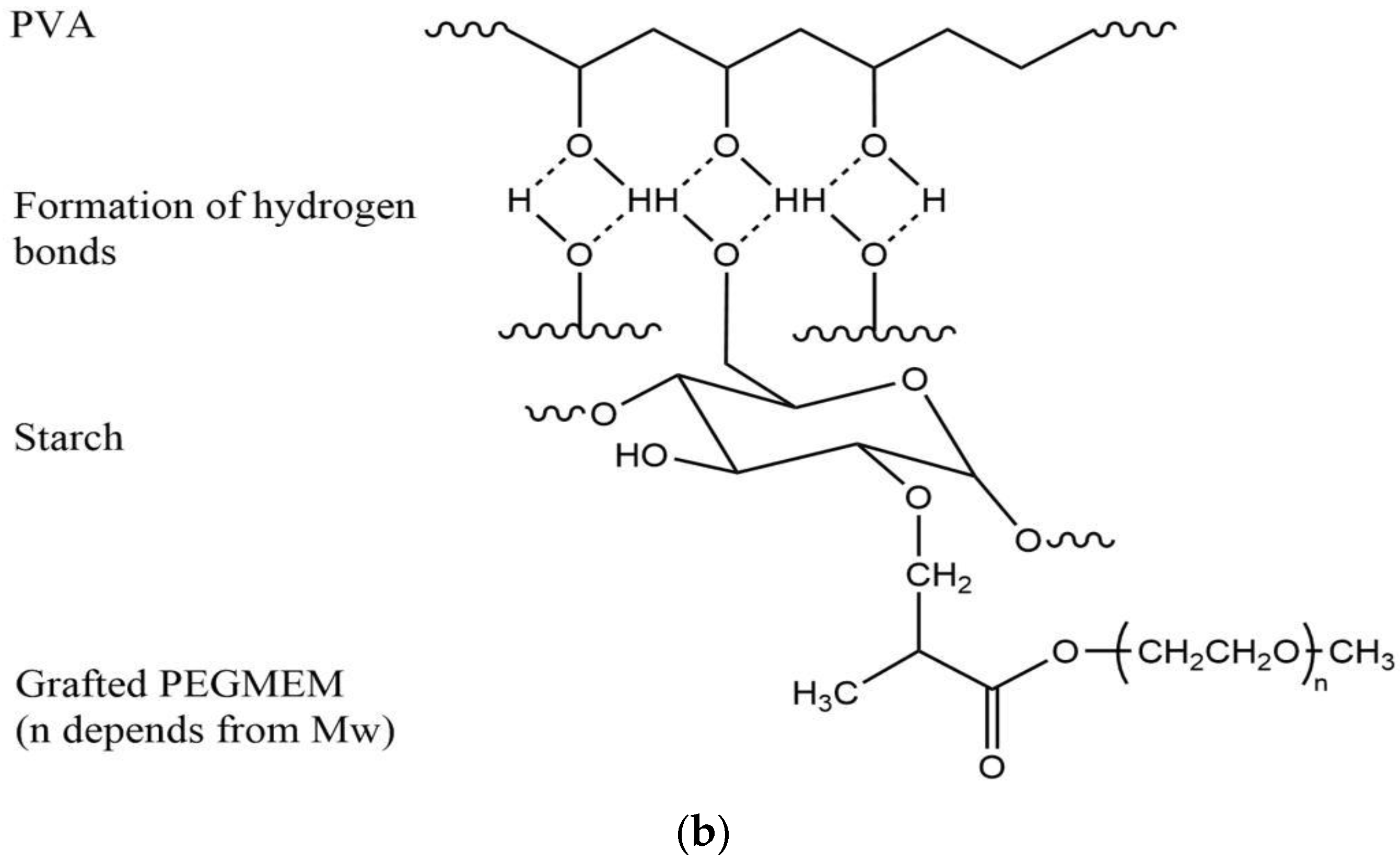
2.2.2. Synthesis of the Grafting Polymer
2.2.3. Preparation of the PVA/S/PEGMA Films
2.2.4. Tests
3. Results and Discussion
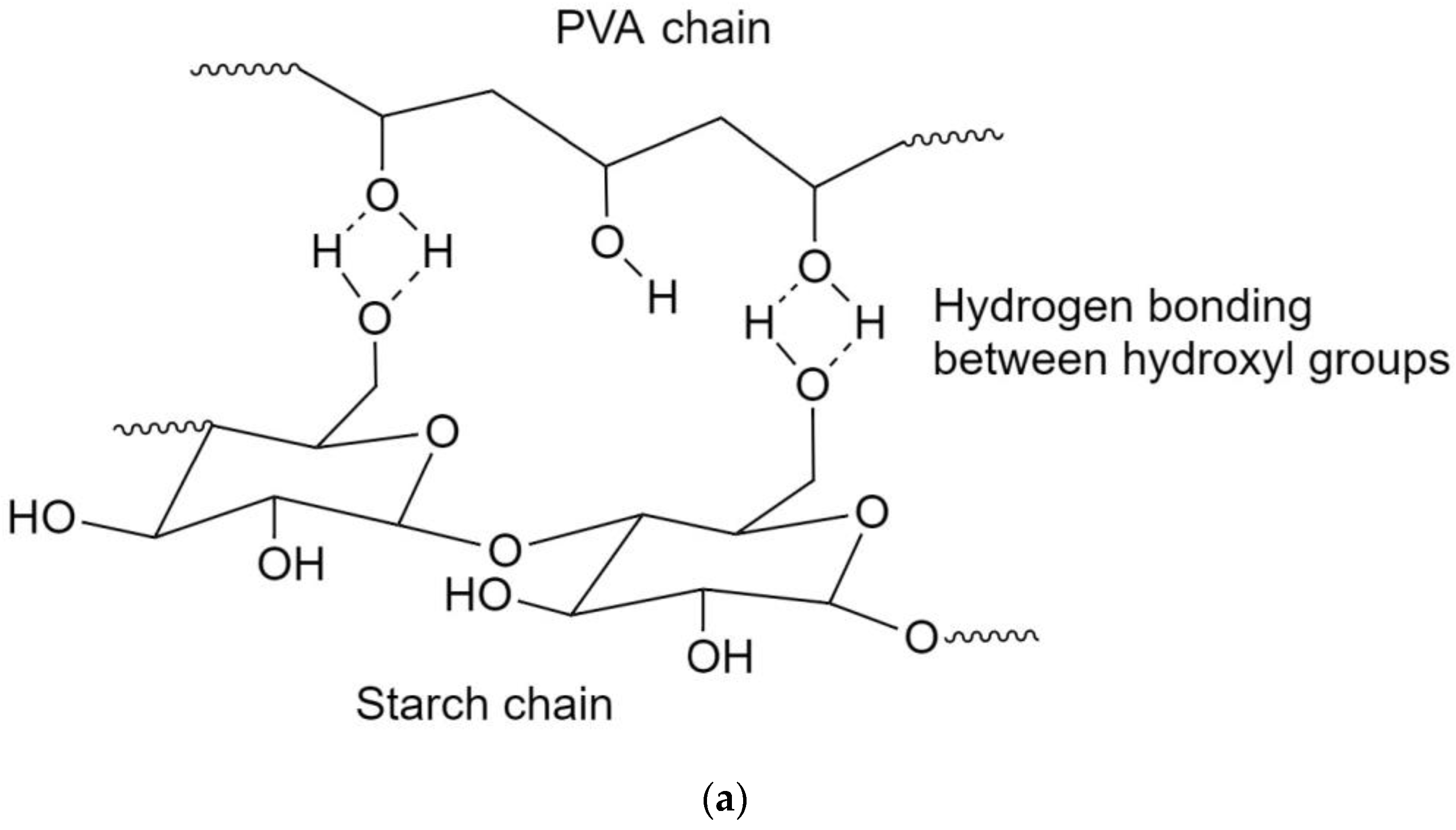
3.1. FTIR Spectra
3.2. Thermogravimetric Analysis (TGA)
3.3. Mechanical Properties
3.4. Biodegradability of the Films
3.5. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Tian, H.; Yan, J.; Rajulu, A.V.; Xiang, A.; Luo, X. Fabrication and properties of polyvinyl alcohol/starch blend films: Effect of composition and humidity. Int. J. Biolo. Macromol. 2017, 96, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymers materials. Exp. Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Obasi, H.; Igwe, I.; Ogbobe, O.; Madufor, I.; Egeolu, F. Analysis of the Mechanical and Degradation Performances of Selected Starch/Polypropylene Blends. Int. J. Appl. Basic. Med. Res. 2015, 7, 42–52. [Google Scholar]
- Sadanand, V.; Rajini, N.; Varada, R.A.; Satyanarayana, B. Preparation of cellulose composites with in situ generated copper nanoparticles using leaf extract and their properties. Carbohydr. Polym. 2016, 150, 32–39. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Akinyele, B.P.; Olusegun, S.J.; Oluwasina, O.O.; Mohallem, N.D.S. Evaluation of the effects of additives on the properties of starch-based bioplastic film. SN Appl. Sci. 2021, 3, 421. [Google Scholar] [CrossRef]
- DeMoraes, J.O.; Scheibe, A.S.; Sereno, A.; Laurindo, J.B. Scale-up of the production of cassava starchbased films using tape-casting. J. Food Eng. 2013, 119, 800–808. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Metzger, C.; Briesen, H. Thermoplastic Starch Nanocomposites Reinforced with Cellulose Nanocrystal Suspensions Containing Residual Salt from Neutralization. Macromol. Mater. Eng. 2021, 306, 2100161. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Lendvai, L.; Karger-Kocsis, J.; Kmetty, Á.; Drakopoulos, S.X. Production and characterization of microfibrillated cellulose-reinforced thermoplastic starch composites. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Chaudhary, D.; Yusa, S.I.; Tadé, M.O. Glycerol/starch/Na+-montmorillonite nanocomposites: A XRD, FTIR, DSC and 1H NMR study. Carbohydr. Polym. 2011, 83, 1591–1597. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, P.; Zhao, F.; Ma, X. The composites based on plasticized starch and carbon nanotubes. Int. J. Biol. Macromol. 2013, 59, 13–19. [Google Scholar] [CrossRef]
- Ma, T.; Chang, P.R.; Zheng, P.; Ma, X. The composites based on plasticized starch and graphene oxide/reduced graphene oxide. Carbohydr. Polym. 2013, 94, 63–70. [Google Scholar] [CrossRef]
- Tian, H.; Wang, K.; Liu, D.; Yan, J.; Xiang, A.; Rajulu, A.V. Enhanced mechanical and thermal properties of poly (vinyl alcohol)/corn starch blends by nanoclay intercalation. Int. J. Biol. Macromol. 2017, 101, 314–320. [Google Scholar] [CrossRef]
- Halima, N.B. Poly (vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 46, 39823–39832. [Google Scholar] [CrossRef]
- Liu, D.; Bian, Q.; Li, Y.; Wang, Y.; Xiang, A.; Tian, H. Effect of oxidation degrees of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Compos. Sci. Technol. 2016, 129, 146–152. [Google Scholar] [CrossRef]
- Sabbagh, F.; Khatir, N.M.; Kiarostami, K. Synthesis and Characterization of k-Carrageenan/PVA Nanocomposite Hydrogels in Combination with MgZnO Nanoparticles to Evaluate the Catechin Release. Polymers 2023, 15, 272. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Ex vivo transdermal delivery of nicotinamide mononucleotide using polyvinyl alcohol microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef]
- Jia, R.J.; Teng, K.Y.; Huang, J.Y.; Wei, X.; Qin, Z.Y. Hydrogen Bonding Crosslinking of Starch-Polyvinyl Alcohol Films Reinforced by Ultrasound-Assisted and Cellulose Nanofibers Dispersed Cellulose Nanocrystals. Starch 2022, 74, 2100227. [Google Scholar] [CrossRef]
- Fahmy, A.; Mohamed, T.A.; Abu-Saied, M.; Helaly, H.; El-Dossoki, F. Structure/property relationship of polyvinyl alcohol/dimethoxydimethylsilane composite membrane: Experimental and theoretical studies. Spectrochim Acta A Mol. Biomol. Spectrosc. 2020, 228, 117810. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.; Abou-Saied, M.; Helaly, H.; El-Dessoki, F.; Mohamed, T.A. Novel PVA/Methoxytrimethylsilane elastic composite membranes: Preparation, characterization and DFT computation. J. Mol. Struct. 2021, 1235, 130173. [Google Scholar] [CrossRef]
- Negim, E.; Rakhmetullayeva, R.; Yeligbayeva, G.; Urkimbaeva, P.; Primzharova, S.; Kaldybekov, D.; Khatib, J.; Mun, G. Improving biodegradability of polyvinyl alcohol/starch blend films for packaging applications. Int. J. Basic Appl. Sci. 2014, 3, 263–273. [Google Scholar] [CrossRef]
- Negim, E.M.; Bakytzhanuly, B.; Urkimbayeva, P.I.; Bekbayeva, L.; Othman, M.B.H.; Mohamad Ibrahim, M.N.; Mun, G.A. Effect of acrylic acid on the mechanical properties of PVA/starch blend films. Egypt. J. Chem. 2020, 63, 1911–1919. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International (ASTM): West Conshohocken, PA, USA, 2018.
- Sun, J.; Zheng, W.; Hu, G.; Liu, F.; Liu, S.; Yang, L.; Zhang, Z. Electrochemically assisted persulfate oxidation of organic pollutants in aqueous solution: Influences, mechanisms and feasibility. Catalysts 2023, 13, 135. [Google Scholar] [CrossRef]
- Taha, I.M.; Zaghlool, A.; Nasr, A.; Nagib, A.; El Azab, I.H.; Mersal, G.A.M.; Ibrahim, M.M.; Fahmy, A. Impact of starch coating embedded with silver nanoparticles on strawberry storage time. Polymers 2022, 14, 1439. [Google Scholar] [CrossRef]
- Awada, H.; Daneault, C. Chemical modification of poly (vinyl alcohol) in water. Appl. Sci. 2015, 4, 840–850. [Google Scholar] [CrossRef]
- Fahmy, A.; Anis, B.; Szymoniak, P.; Altmann, K.; Schönhals, A. Graphene Oxide/Polyvinyl Alcohol–Formaldehyde Composite Loaded by Pb Ions: Structure and Electrochemical Performance. Polymers 2022, 14, 2303. [Google Scholar] [CrossRef]
- Mahmoud, A.; Fahmy, A.; Naser, A.; Abu Saied, M. Novel sulfonated poly (vinyl alcohol)/carboxy methyl cellulose/acrylamide-based hybrid polyelectrolyte membranes. Sci. Rep. 2022, 12, 22017. [Google Scholar] [CrossRef]
- Mahmoud, A.; Abu Saied, M.; Naser, A.; Fahmy, A. Synthesis and Characterization of Nylon 6, 6-Polyvinyl Alcohol-Based Polyelectrolytic Membrane. Arab. J. Sci. Eng. 2022, 48, 8941–8956. [Google Scholar] [CrossRef]
- Fahmy, A.; Saeed, A.M.; Dawood, U.; Abdelbary, H.; Altmann, K.; Schönhals, A. Nano-MnO2/xanthan gum composite films for NO2 gas sensing. Mater. Chem. Phys. 2023, 296, 127277. [Google Scholar] [CrossRef]
- Al Mamun, S.M.; Rahman, M.; Roland, S.; Mahmood, R. Impact of Molecular Weight on the Thermal Stability and the Miscibility of Poly(ε-caprolactone)/Polystyrene Binary Blends. J. Polym. Environ. 2018, 26, 3511–3519. [Google Scholar] [CrossRef]
- Ge, H.; Yang, F.; Hao, Y.; Wu, G.; Zhang, H.; Dong, L. Thermal, mechanical and rheological properties of plasticized poly (L-lactic acid). J. Appl. Polym. Sci. 2013, 127, 2832–2839. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Copinet, A.; Tighzert, L.; Coma, V. Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J. Polym. Environ. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Thermoplastic PVA/PLA blends with improved processability and hydrophobicity. Ind. Eng. Chem. Res. 2014, 53, 17355–17361. [Google Scholar] [CrossRef]
- Derkowski, A.; Kuligiewicz, A. Thermal Analysis and Thermal Reactions of Smectites: A Review of Methodology, Mechanisms, and Kinetics. Clays Clay Miner. 2022, 70, 946–972. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Meneghello, G.; Parker, D.J.; Ainsworth, B.J.; Perera, S.P.; Chaudhuri, J.B.; Ellis, M.J.; De Bank, P.A. Fabrication and characterization of poly (lactic-co-glycolic acid)/polyvinyl alcohol blended hollow fibre membranes for tissue engineering applications. J. Membr. Sci. 2009, 344, 55–61. [Google Scholar] [CrossRef]
- Lei, Y.; Peng, H.; Wang, Y.; Liu, Y.; Han, F.; Xiao, Y.; Gao, Y. Preferential and rapid degradation of raw rice starch by an α-amylase of glycoside hydrolase subfamily GH13_37. Appl. Microbiol. Biotechnol. 2012, 94, 1577–1584. [Google Scholar] [CrossRef]
- EN 13432:2000; Packaging: Requirements for Packaging Recoverable through Composting and Biodegradation. Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. BSI Group—British Standards Institution (BSI): London, UK, 2000.
- ASTM 6400; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ASTM International (ASTM): West Conshohocken, PA, USA, 2021.
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.; Kubo, M. Degradation of Bioplas tics in Soil and Their Degradation Effects on Environmental Microorganisms. J. Agric. Chem. Environ. 2016, 5, 23–34. [Google Scholar] [CrossRef]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Congdon, T.; Shaw, P.; Gibson, M.I. Thermoresponsive, well-defined, poly (vinyl alcohol) co-polymers. Polym. Chem. 2015, 6, 4749–4757. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.Z.; Chu, C.C. Effect of the molecular weight of polyethylene glycol (PEG) on the properties of chitosan-PEG-poly (N-isopropylacrylamide) hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 2865–2872. [Google Scholar] [CrossRef]
- Singh, M.; Verma, S.K.; Biswas, I.; Mehta, R. Effect of molecular weight of polyethylene glycol on the rheological properties of fumed silica-polyethylene glycol shear thickening fluid. Mater. Res. Express 2018, 5, 055704. [Google Scholar] [CrossRef]


| [PVA-b-S] (Proportions) | [PVA-b-S] (mass, g) | |
|---|---|---|
| M1 | 1:1 | 5:5 |
| M2 | 1.5:1 | 7:5 |
| M3 | 2:1 | 10:5 |
| M4 | 3:1 | 15:5 |
| M5 | 1:2 | 5:10 |
| M6 | 3:2 | 15:10 |
| M7 | 3:1 | 15:5 |
| M8 | 3:1 | 15:5 |
| [PVA-b-S] | [PVA-b-S]-g-PEGMA300 | [PVA-b-S]-g PEGMA500 | [PVA-b-S]-g PEGMA950 | |
|---|---|---|---|---|
| M1 | 1:1 | 1:1:1 | 1:1:1 | 1:1:1 |
| M2 | 1.5:1 | 1.5:1:1 | 1.5:1:1 | 1.5:1:1 |
| M3 | 2:1 | 2:1:1 | 2:1:1 | 2:1:1 |
| M4 | 3:1 | 3:1:1 | 3:1:1 | 3:1:1 |
| M5 | 1:2 | 3:1.5:1 | 3:1.5:1 | 3:1.5:1 |
| M6 | 3:2 | 3:2:1 | 3:2:1 | 3:2:1 |
| M7 | 3:1 | 3:1:1.5 | 3:1:1.5 | 3:1:1.5 |
| M8 | 3:1 | 3:1:2 | 3:1:2 | 3:1:2 |
| Copolymer | Temperature Range, °C | Weight Lost % | Residue % | IDT a °C | PDTmax a °C | Tg b °C | Tm b °C |
|---|---|---|---|---|---|---|---|
| M1 300 | 29.7–386.5 | 87.4 | 14.2 | 210 | 48.4 | 190.9 | |
| 386.5–531.6 | 4.40 | 9.60 | |||||
| 531.6–896.9 | 14.6 | 4.90 | |||||
| M1 500 | 30.7–121.8 | 8.40 | 90.9 | 220 | 310 | 49.4 | 195.7 |
| 121.8–182.8 | 10.6 | 81.3 | |||||
| 182.8–261.1 | 25.8 | 55.4 | |||||
| 261.1–381.5 | 25.3 | 30.1 | |||||
| 381.5–524.3 | 4.80 | 25.3 | |||||
| M1 950 | 29.8–219.1 | 14.7 | 85.4 | 380 | 500 | 84.8 | 209.3 |
| 219.1–275.7 | 4.50 | 80.9 | |||||
| 275.7–333.3 | 5.60 | 75.3 | |||||
| 333.3–438.7 | 14.2 | 61.1 | |||||
| 438.7–899.2 | 20.3 | 40.9 |
| Samples | [PVA-b-S]-g-PEGMA | 300 | 500 | 950 |
|---|---|---|---|---|
| Tensile, Mpa | ||||
| M1 | 1:1:1 | 17.5 | 24.6 | 34.2 |
| M2 | 1.5:1:1 | 18.5 | 25.7 | 35.7 |
| M3 | 2:1:1 | 20 | 29.3 | 37.2 |
| M4 | 3:1:1 | 23 | 33.9 | 38.9 |
| M5 | 3:1.5:1 | 19.5 | 28.5 | 37.8 |
| M6 | 3:2:1 | 18 | 25 | 34.5 |
| M7 | 3:1:1.5 | 25.5 | 30.2 | 40.1 |
| M8 | 3:1:2 | 28 | 35.7 | 46.2 |
| Samples | [PVA-b-S]-g-PEGMA | 300 | 500 | 950 |
|---|---|---|---|---|
| Elongation, % | ||||
| M1 | 1:1:1 | 150 | 145 | 134 |
| M2 | 1.5:1:1 | 141 | 138.6 | 129.3 |
| M3 | 2:1:1 | 132 | 126.77 | 121.7 |
| M4 | 3:1:1 | 128 | 118.6 | 110.1 |
| M5 | 3:1.5:1 | 145 | 115.3 | 110.8 |
| M6 | 3:2:1 | 139.5 | 105.7 | 101.9 |
| M7 | 3:1:1.5 | 120.5 | 93.8 | 78.9 |
| M8 | 3:1:2 | 105.9 | 81.6 | 67.5 |
| Samples | [PVA-b-S]-g-PEGMA | 300 | 500 | 950 |
|---|---|---|---|---|
| Time of Films Weight Loss until 90% (Days) | ||||
| M1 | 1:1:1 | 18 | 18 | 24 |
| M2 | 1.5:1:1 | 17 | 17 | 22 |
| M3 | 2:1:1 | 15 | 16 | 20 |
| M4 | 3:1:1 | 12 | 14 | 18 |
| M5 | 3:1.5:1 | 15 | 16 | 18 |
| M6 | 3:2:1 | 16 | 16 | 20 |
| M7 | 3:1:1.5 | 96 | 100 | 120 |
| M8 | 3:1:2 | 96 | 100 | 120 |
| Samples | [PVA-b-S]-g-PEGMA | 300 | 950 | 500 |
|---|---|---|---|---|
| Time of Films Weight Loss until 90% (Days) | ||||
| M1 | 1:1:1 | 25 | 30 | 35 |
| M2 | 1.5:1:1 | 25 | 25 | 28 |
| M3 | 2:1:1 | 30 | 28 | 35 |
| M4 | 3:1:1 | 18 | 20 | 25 |
| M5 | 3:1.5:1 | 20 | 25 | 28 |
| M6 | 3:2:1 | 28 | 30 | 36 |
| M7 | 3:1:1.5 | 118 | 120 | 120 |
| M8 | 3:1:2 | 118 | 120 | 120 |
| Samples | [PVA-b-S]-g-PEGMA | 300 | 500 | 950 |
|---|---|---|---|---|
| Time of Films Weight Loss until 90% (Days) | ||||
| M1 | 1:1:1 | 22 | 26 | 30 |
| M2 | 1.5:1:1 | 25 | 25 | 31 |
| M3 | 2:1:1 | 28 | 30 | 35 |
| M4 | 3:1:1 | 30 | 30 | 37 |
| M5 | 3:1.5:1 | 32 | 36 | 43 |
| M6 | 3:2:1 | 50 | 50 | 56 |
| M7 | 3:1:1.5 | 100 | 120 | 120 |
| M8 | 3:1:2 | 100 | 120 | 120 |
| Samples | [PVA-b-S]-g-PEGMA | 300 | 500 | 950 |
|---|---|---|---|---|
| Time of Films Weight Loss until 90% (Days) | ||||
| M1 | 1:1:1 | 15 | 16 | 18 |
| M2 | 1.5:1:1 | 16 | 18 | 20 |
| M3 | 2:1:1 | 16 | 15 | 20 |
| M4 | 3:1:1 | 8 | 10 | 12 |
| M5 | 3:1.5:1 | 10 | 12 | 15 |
| M6 | 3:2:1 | 18 | 18 | 22 |
| M7 | 3:1:1.5 | 100 | 120 | 120 |
| M8 | 3:1:2 | 100 | 120 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iskalieva, A.; Yesmurat, M.; Al Azzam, K.M.; Ainakulova, D.; Yerbolat, Y.; Negim, E.-S.; Ibrahim, M.N.M.; Gulzhakhan, Y. Effect of Polyethylene Glycol Methyl Ether Methacrylate on the Biodegradability of Polyvinyl Alcohol/Starch Blend Films. Polymers 2023, 15, 3165. https://doi.org/10.3390/polym15153165
Iskalieva A, Yesmurat M, Al Azzam KM, Ainakulova D, Yerbolat Y, Negim E-S, Ibrahim MNM, Gulzhakhan Y. Effect of Polyethylene Glycol Methyl Ether Methacrylate on the Biodegradability of Polyvinyl Alcohol/Starch Blend Films. Polymers. 2023; 15(15):3165. https://doi.org/10.3390/polym15153165
Chicago/Turabian StyleIskalieva, Asylzat, Mateyev Yesmurat, Khaldun M. Al Azzam, Dana Ainakulova, Yerzhanov Yerbolat, El-Sayed Negim, Mohamad Nasir Mohamad Ibrahim, and Yeligbayeva Gulzhakhan. 2023. "Effect of Polyethylene Glycol Methyl Ether Methacrylate on the Biodegradability of Polyvinyl Alcohol/Starch Blend Films" Polymers 15, no. 15: 3165. https://doi.org/10.3390/polym15153165
APA StyleIskalieva, A., Yesmurat, M., Al Azzam, K. M., Ainakulova, D., Yerbolat, Y., Negim, E.-S., Ibrahim, M. N. M., & Gulzhakhan, Y. (2023). Effect of Polyethylene Glycol Methyl Ether Methacrylate on the Biodegradability of Polyvinyl Alcohol/Starch Blend Films. Polymers, 15(15), 3165. https://doi.org/10.3390/polym15153165









