Polydopamine-Coated Polycaprolactone Electrospun Nanofiber Membrane Loaded with Thrombin for Wound Hemostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PCL Fibrous Membranes
2.2. Coating PDA onto PCL Fibrous Membranes
2.3. Characterization of Membranes
2.4. Thrombin Loading
2.5. In Vitro Biosafety Evaluation
2.6. In Vivo Biosafety Evaluation
2.7. Blood Coagulation In Vitro
2.8. Red Blood Cell Adhesion
2.9. Platelet Adhesion
2.10. In Vivo Hemostatic Test
2.11. Statistical Analysis
3. Results
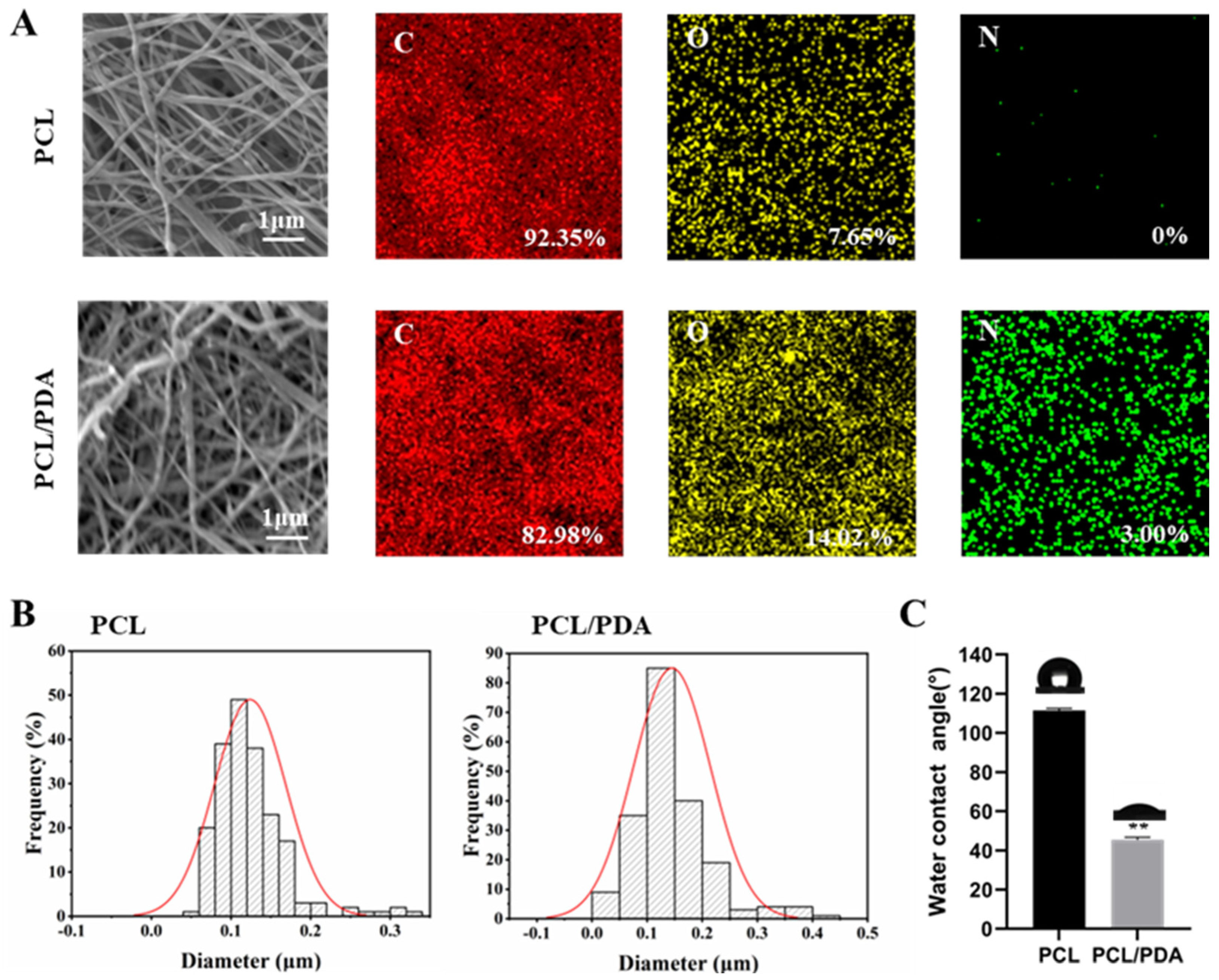
3.1. Characterization of Membranes
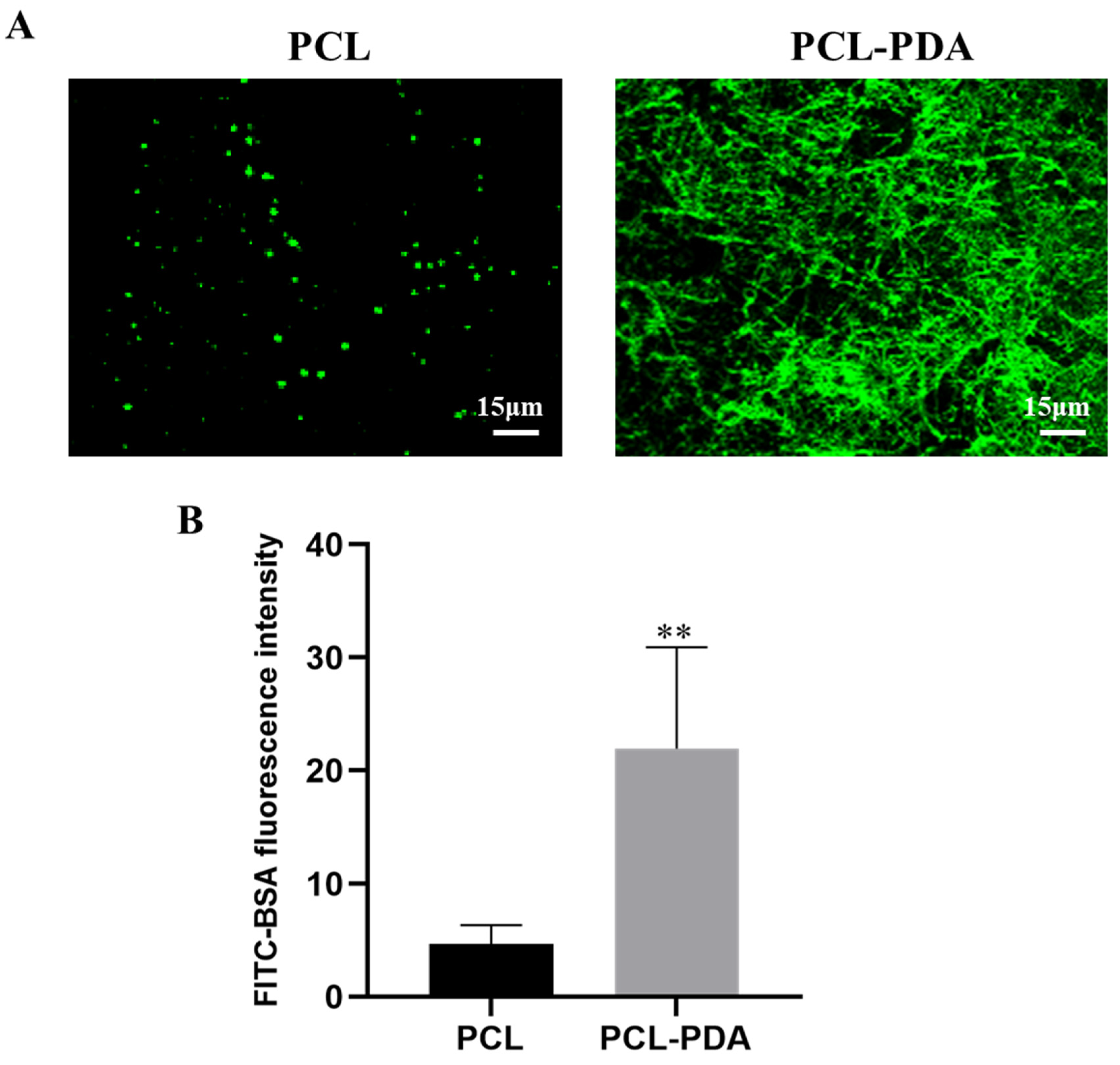
3.2. Loading of Thrombin
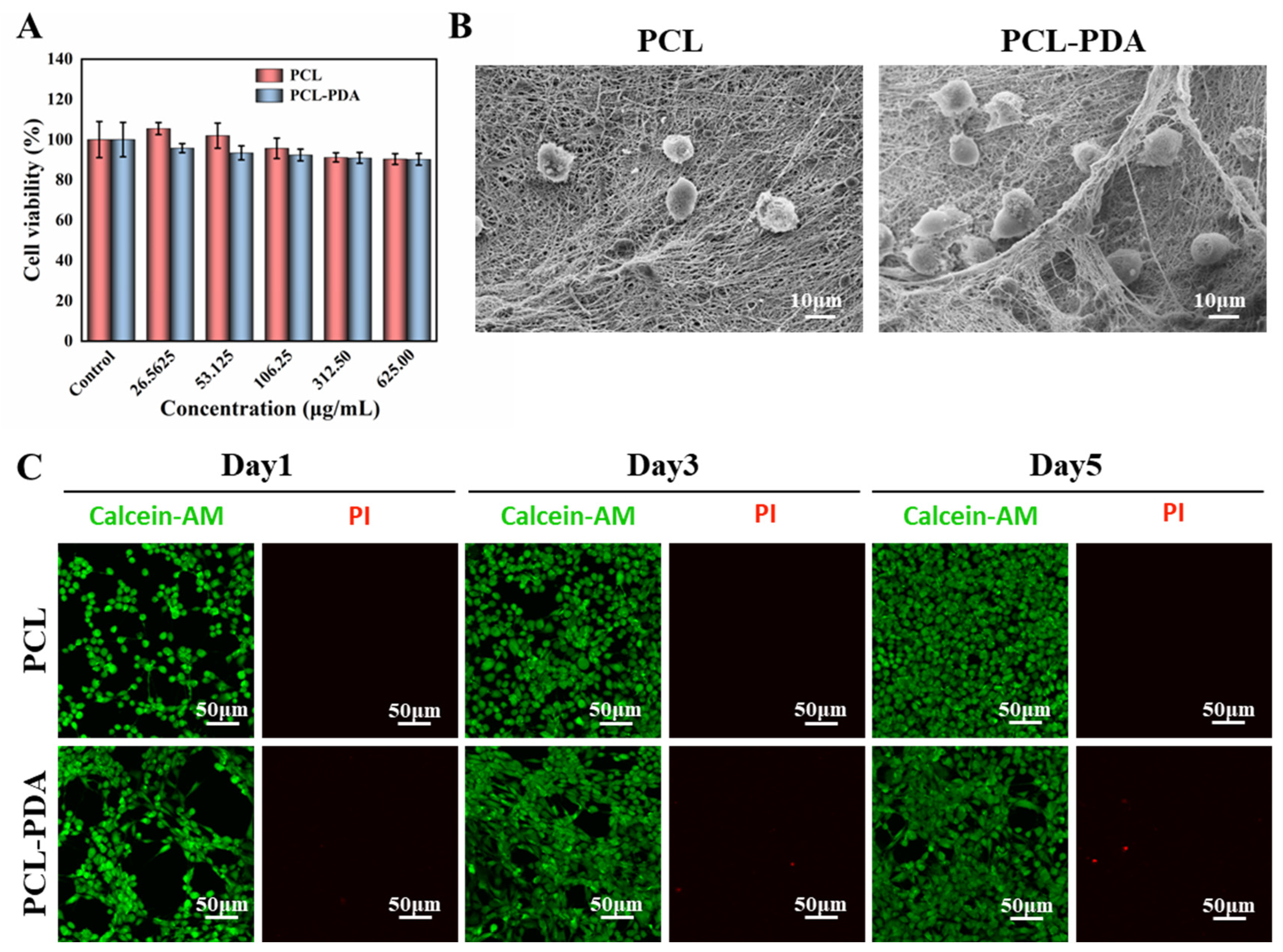
3.3. In Vitro Safety Evaluation of Membranes
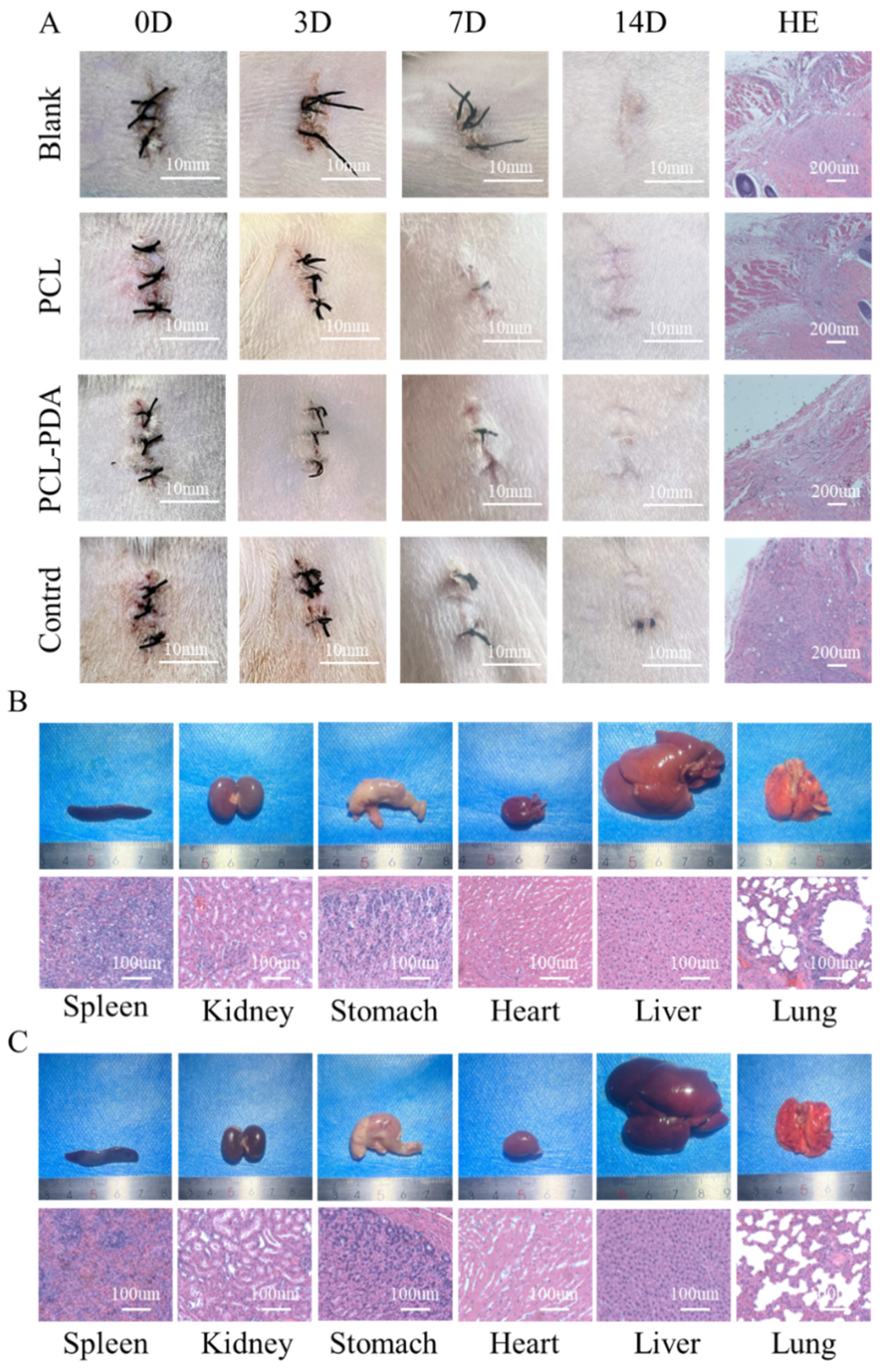
3.4. In Vivo Biosafety Assessment of Membranes
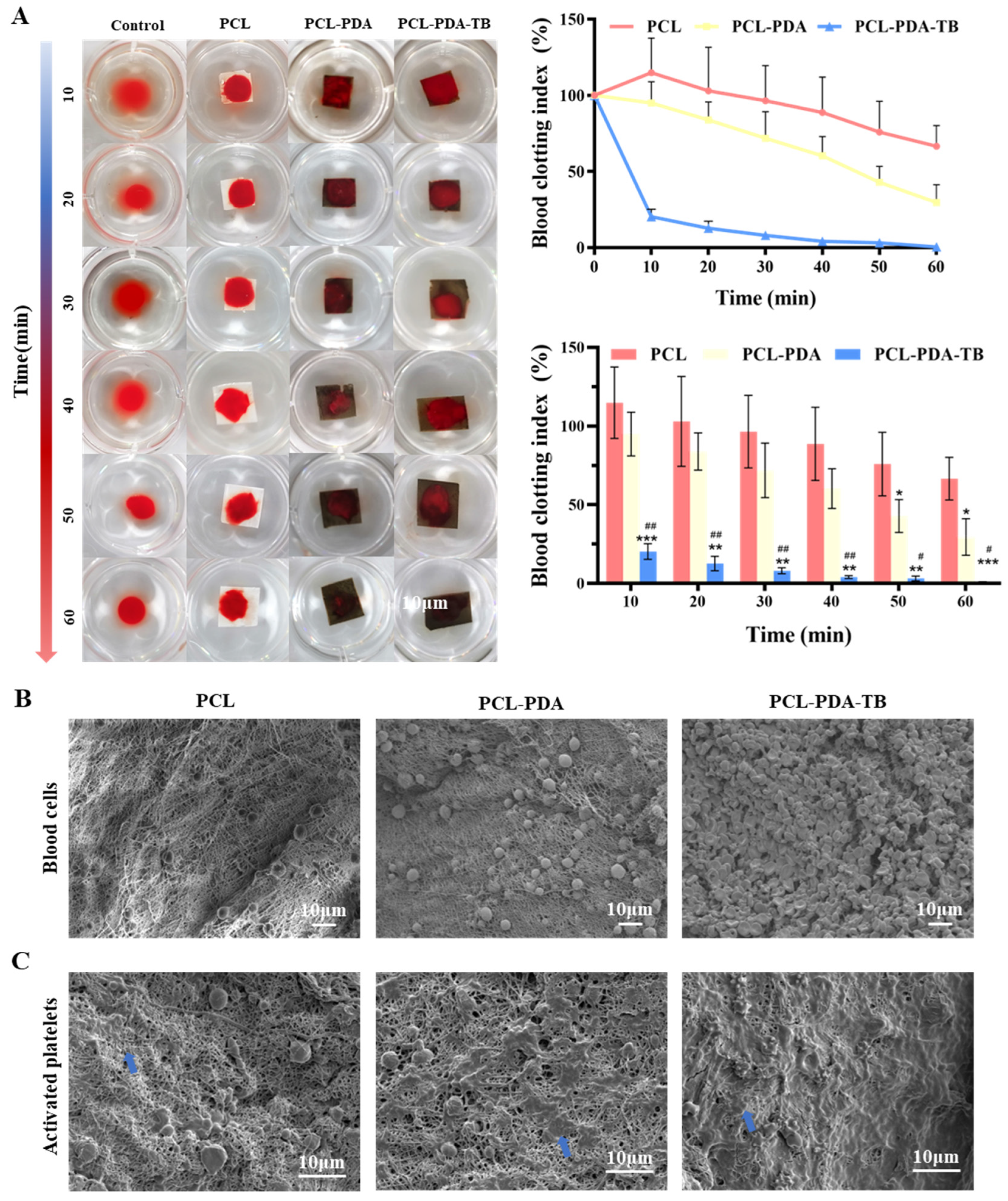
3.5. Evaluation of In Vitro Blood Clotting of Membranes
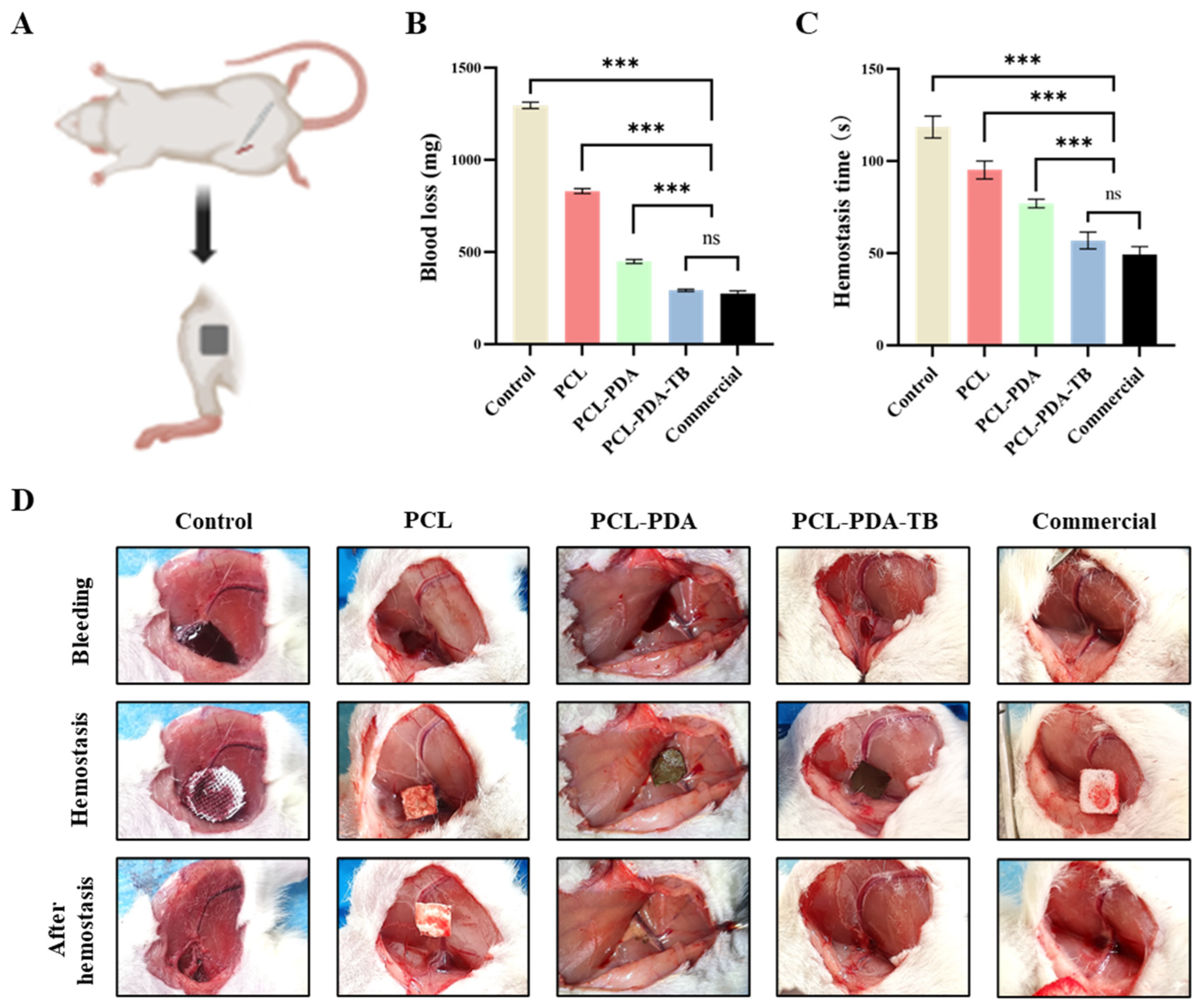
3.6. Evaluation of Hemostatic Performance In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Champion, H.R.; Bellamy, R.F.; Roberts, C.P.; Leppaniemi, A. A Profile of Combat Injury. J. Trauma Inj. Infect. Crit. Care 2003, 54, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.M.; Myers, J.G.; Dent, D.L.; Ermis, P.; Gray, G.A.; Villarreal, R.; Blow, O.; Woods, B.; McFarland, M.; Garavaglia, J.; et al. Seven Hundred Fifty-Three Consecutive Deaths in a Level I Trauma Center: The Argument for Injury Prevention. J. Trauma Inj. Infect. Crit. Care 2003, 54, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J. Trauma Inj. Infect. Crit. Care 2006, 60, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Muldowney, M.; Liu, Z.; Stansbury, L.G.; Vavilala, M.S.; Hess, J.R. Ultramassive Transfusion for Trauma in the Age of Hemostatic Resuscitation: A Retrospective Single-Center Cohort from a Large US Level-1 Trauma Center, 2011–2021. Obstet. Anesth. Dig. 2023, 136, 927–933. [Google Scholar] [CrossRef]
- Sperry, J.L.; Cotton, B.A.; Luther, J.F.; Cannon, J.W.; Schreiber, M.A.; Moore, E.E.; Namias, N.; Minei, J.P.; Wisniewski, S.R.; Guyette, F.X. Whole Blood Resuscitation and Association with Survival in Injured Patients with an Elevated Probability of Mortality. J. Am. Coll. Surg. 2023, 237, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Hu, P.; Wang, H.; Gan, L.; Kong, G.; Shi, Y.; Wang, T.; Jiang, B. China trauma treatment statistics 2019: A national retrospective study based on hospitalized cases. Front. Public Health 2023, 11, 1116828. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Xu, T.; Li, L.; Huo, M.; Li, X.; He, Y.; Lin, Q.; Mei, B.; Zhou, X.; et al. Epidemiological and clinical characteristics of 3327 cases of traffic trauma deaths in Beijing from 2008 to 2017: A retrospective analysis. Medicine 2020, 99, e18567. [Google Scholar] [CrossRef]
- Gu, H.; Li, H.; Wei, L.; Lu, J.; Wei, Q. Collagen-based injectable and self-healing hydrogel with multifunction for regenerative repairment of infected wounds. Regen. Biomater. 2023, 10, rbad018. [Google Scholar] [CrossRef]
- Ibne Mahbub, M.S.; Bae, S.H.; Gwon, J.G.; Lee, B.T. Decellularized liver extracellular matrix and thrombin loaded biodegradable TOCN/Chitosan nanocomposite for hemostasis and wound healing in rat liver hemorrhage model. Int. J. Biol. Macromol. 2023, 225, 1529–1542. [Google Scholar] [CrossRef]
- Das, P.; Manna, S.; Roy, S.; Nandi, S.K.; Basak, P. Polymeric biomaterials-based tissue engineering for wound healing: A systemic review. Burn. Trauma 2023, 11, tkac058. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Yu, J.; Ding, B. Nanofibrous hemostatic materials: Structural design, fabrication methods, and hemostatic mechanisms. Acta Biomater. 2022, 154, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Mecwan, M.; Li, J.; Falcone, N.; Ermis, M.; Torres, E.; Morales, R.; Hassani, A.; Haghniaz, R.; Mandal, K.; Sharma, S.; et al. Recent advances in biopolymer-based hemostatic materials. Regen. Biomater. 2022, 9, rbac063. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wu, Z. A Narrative Review of Different Hemostatic Materials in Emergency Treatment of Trauma. Emerg. Med. Int. 2022, 2022, 6023261. [Google Scholar] [CrossRef]
- Zou, C.-Y.; Li, Q.-J.; Hu, J.-J.; Song, Y.-T.; Zhang, Q.-Y.; Nie, R.; Li-Ling, J.; Xie, H.-Q. Design of biopolymer-based hemostatic material: Starting from molecular structures and forms. Mater. Today Bio 2022, 17, 100468. [Google Scholar] [CrossRef]
- Valke, L.L.F.G.; Rijpma, S.; Meijer, D.; Schols, S.E.M.; van Heerde, W.L. Thrombin generation assays to personalize treatment in bleeding and thrombotic diseases. Front. Cardiovasc. Med. 2022, 9, 1033416. [Google Scholar] [CrossRef]
- Al-Amer, O.M. The role of thrombin in haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef]
- Jiang, S.X.; Chahal, D.; Ali-Mohamad, N.; Kastrup, C.; Donnellan, F. Hemostatic powders for gastrointestinal bleeding: A review of old, new, and emerging agents in a rapidly advancing field. Endosc. Int. Open 2022, 10, E1136–E1146. [Google Scholar] [CrossRef]
- Sidonio, R.F.; Hoffman, M.; Kenet, G.; Dargaud, Y. Thrombin generation and implications for hemophilia therapies: A narrative review. Res. Pract. Thromb. Haemost. 2023, 7, 100018. [Google Scholar] [CrossRef]
- Shaw, J.R.; Castellucci, L.A.; Siegal, D.; Carrier, M. DOAC-associated bleeding, hemostatic strategies, and thrombin generation assays—A review of the literature. J. Thromb. Haemost. 2023, 21, 433–452. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Y.; Lei, L.; Hu, S. Moist-electric generators based on electrospun cellulose acetate nanofiber membranes with tree-like structure. J. Membr. Sci. 2022, 662, 120962. [Google Scholar] [CrossRef]
- Yang, C.; Topuz, F.; Park, S.H.; Szekely, G. Biobased thin-film composite membranes comprising priamine–genipin selective layer on nanofibrous biodegradable polylactic acid support for oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 5291–5303. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Bligh, S.W.A. A Review on Electrospun Poly(amino acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-loaded sandwich-like nanofibrous membrane prepared by electrospinning technology as wound dressing for accelerate wound healing. Mater. Sci. Eng. C 2021, 127, 112245. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Z.; Liu, J.; Dong, P.; Tian, F.; Li, F.; Meng, X. Electrospun kaolin-loaded chitosan/PEO nanofibers for rapid hemostasis and accelerated wound healing. Int. J. Biol. Macromol. 2022, 217, 998–1011. [Google Scholar] [CrossRef]
- Mirmajidi, T.; Chogan, F.; Rezayan, A.H.; Sharifi, A.M. In vitro and in vivo evaluation of a nanofiber wound dressing loaded with melatonin. Int. J. Pharm. 2021, 596, 120213. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ahmed, M.F.; Li, Y.; Bejoy, J.; Zeng, C. PCL-PDMS-PCL Copolymer-Based Microspheres Mediate Cardiovascular Differentiation from Embryonic Stem Cells. Tissue Eng. Part C Methods 2017, 23, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Waite, J.H.; Tanzer, M.L. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef]
- Fei, F.; Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Tailoring the Performance of Organic Solvent Nanofiltration Membranes with Biophenol Coatings. ACS Appl. Polym. Mater. 2019, 1, 452–460. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, L.; Zhu, Z.; Wong, W.Y. Strongly coupled interface facilitating charge separation to the improved visible light-driven hydrogen production on CdS@ polydopamine/NiS photocatalyst. J. Mater. Chem. A 2023, 11, 11840–11848. [Google Scholar] [CrossRef]
- Cai, S.; Zuo, X.; Zhao, H.; Yang, S.; Chen, R.; Chen, L.; Zhang, R.; Ding, D.; Cai, T. Evaluation of N-doped carbon for the peroxymonosulfate activation and removal of organic contaminants from livestock wastewater and groundwater. J. Mater. Chem. A 2022, 10, 9171–9183. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhou, S.; Chang, Q.; Lu, F.; Xing, M. Injectable Mussel-Inspired Immobilization of Platelet-Rich Plasma on Microspheres Bridging Adipose Micro-Tissues to Improve Autologous Fat Transplantation by Controlling Release of PDGF and VEGF, Angiogenesis, Stem Cell Migration. Adv. Health Mater. 2017, 6, 1700131. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.E.; Nah, H.; Seok, J.M.; Jeong, M.H.; Park, K.; Kwon, I.K.; Lee, J.S.; A Park, S. Vascular endothelial growth factor immobilized on mussel-inspired three-dimensional bilayered scaffold for artificial vascular graft application: In vitro and in vivo evaluations. J. Colloid Interface Sci. 2019, 537, 333–344. [Google Scholar] [CrossRef]
- Pi, W.; Zhang, Y.; Li, L.; Li, C.; Zhang, M.; Zhang, W.; Cai, Q.; Zhang, P. Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration. Biofabrication 2022, 14, 035006. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Pendharkar, S.; Jian, G. Biodegradable Hemostatic Wound Dressings. U.S. Patent US2004241212A1, 2 December 2004. [Google Scholar]
- Mısırlıoğlu, S.; Türkgeldi, E.; Yagmur, H.; Urman, B.; Ata, B. Use of a gelatin-thrombin hemostatic matrix in obstetrics and gynecological surgery. J. Turk. Soc. Obstet. Gynecol. 2018, 15, 193–199. [Google Scholar] [CrossRef]
- Wasilko, S.M.; Quinlan, N.J.; Shafritz, A.B. Topical Hemostatic Agents and Their Role in Upper Extremity Surgery. J. Hand Surg. 2015, 40, 602–604. [Google Scholar] [CrossRef]
- Lawson, J.H. The Clinical Use and Immunologic Impact of Thrombin in Surgery. Semin. Thromb. Hemost. 2006, 32, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K. Role of increase in permeability and circulatory failure in the development of organ dysfunction in severe acute pancreatitis. Nihon Rinsho. Jpn. J. Clin. Med. 2004, 62, 1999–2004. [Google Scholar]
- Sánchez-Machado, D.I.; Maldonado-Cabrera, A.; López-Cervantes, J.; Maldonado-Cabrera, B.; Chávez-Almanza, A.F. Therapeutic effects of electrospun chitosan nanofibers on animal skin wounds: A systematic review and meta-analysis. Int. J. Pharm. X 2023, 5, 100175. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Petroni, S.; Tagliaro, I.; Antonini, C.; D’arienzo, M.; Orsini, S.F.; Mano, J.F.; Brancato, V.; Borges, J.; Cipolla, L. Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Mar. Drugs 2023, 21, 147. [Google Scholar] [CrossRef]
- Erdi, M.; Sandler, A.; Kofinas, P. Polymer nanomaterials for use as adjuvant surgical tools. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1889. [Google Scholar] [CrossRef]
- Zhao, H.; Waite, J.H. Linking Adhesive and Structural Proteins in the Attachment Plaque of Mytilus californianus. J. Biol. Chem. 2006, 281, 26150–26158. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.-B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-Inspired Encapsulation and Functionalization of Individual Yeast Cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Tang, L.; Zhong, S.; Yang, Z.; Wang, J.; Weng, Y.; Tu, Q.; Jiang, C.; Huang, N. In Vitro Investigation of Enhanced Hemocompatibility and Endothelial Cell Proliferation Associated with Quinone-Rich Polydopamine Coating. ACS Appl. Mater. Interfaces 2013, 5, 1704–1714. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, J.S.; Park, C.B. Spatial Control of Cell Adhesion and Patterning through Mussel-Inspired Surface Modification by Polydopamine. Langmuir 2010, 26, 15104–15108. [Google Scholar] [CrossRef]
- Wu, R.; Gao, G.; Xu, Y. Electrospun Fibers Immobilized with BMP-2 Mediated by Polydopamine Combined with Autogenous Tendon to Repair Developmental Dysplasia of the Hip in a Porcine Model. Int. J. Nanomed. 2020, 15, 6563–6577. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Antoniou, E.; Karidis, N.P.; Kontzoglou, K.; Kouraklis, G. Surgical control of life-threatening post-ERCP bleeding with a gelatin matrix-thrombin hemostatic agent. Int. J. Surg. Case Rep. 2012, 3, 471–473. [Google Scholar] [CrossRef]
- Coughlin, S.R. Protease-activated receptors and platelet function. Thromb. Haemost. 1999, 82, 353–356. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, D.; Li, M.; Zhang, P.; Rao, F.; Huang, W.; Wang, C.; Guo, W.; Wang, T. Polydopamine-Coated Polycaprolactone Electrospun Nanofiber Membrane Loaded with Thrombin for Wound Hemostasis. Polymers 2023, 15, 3122. https://doi.org/10.3390/polym15143122
Cui D, Li M, Zhang P, Rao F, Huang W, Wang C, Guo W, Wang T. Polydopamine-Coated Polycaprolactone Electrospun Nanofiber Membrane Loaded with Thrombin for Wound Hemostasis. Polymers. 2023; 15(14):3122. https://doi.org/10.3390/polym15143122
Chicago/Turabian StyleCui, Dapeng, Ming Li, Peng Zhang, Feng Rao, Wei Huang, Chuanlin Wang, Wei Guo, and Tianbing Wang. 2023. "Polydopamine-Coated Polycaprolactone Electrospun Nanofiber Membrane Loaded with Thrombin for Wound Hemostasis" Polymers 15, no. 14: 3122. https://doi.org/10.3390/polym15143122
APA StyleCui, D., Li, M., Zhang, P., Rao, F., Huang, W., Wang, C., Guo, W., & Wang, T. (2023). Polydopamine-Coated Polycaprolactone Electrospun Nanofiber Membrane Loaded with Thrombin for Wound Hemostasis. Polymers, 15(14), 3122. https://doi.org/10.3390/polym15143122









