Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides
Abstract
1. Introduction
2. Materials and Experimental Methodology
2.1. Materials
2.2. Characterization Techniques
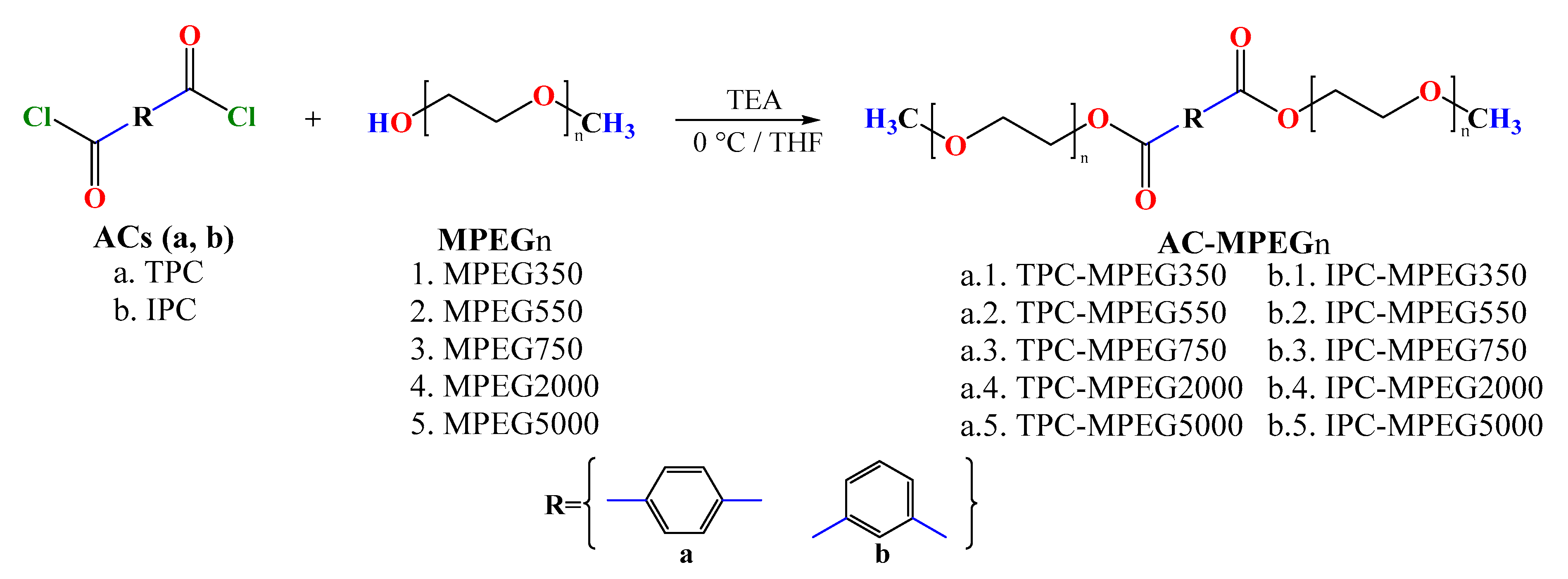
2.3. PCMs’ Synthesis
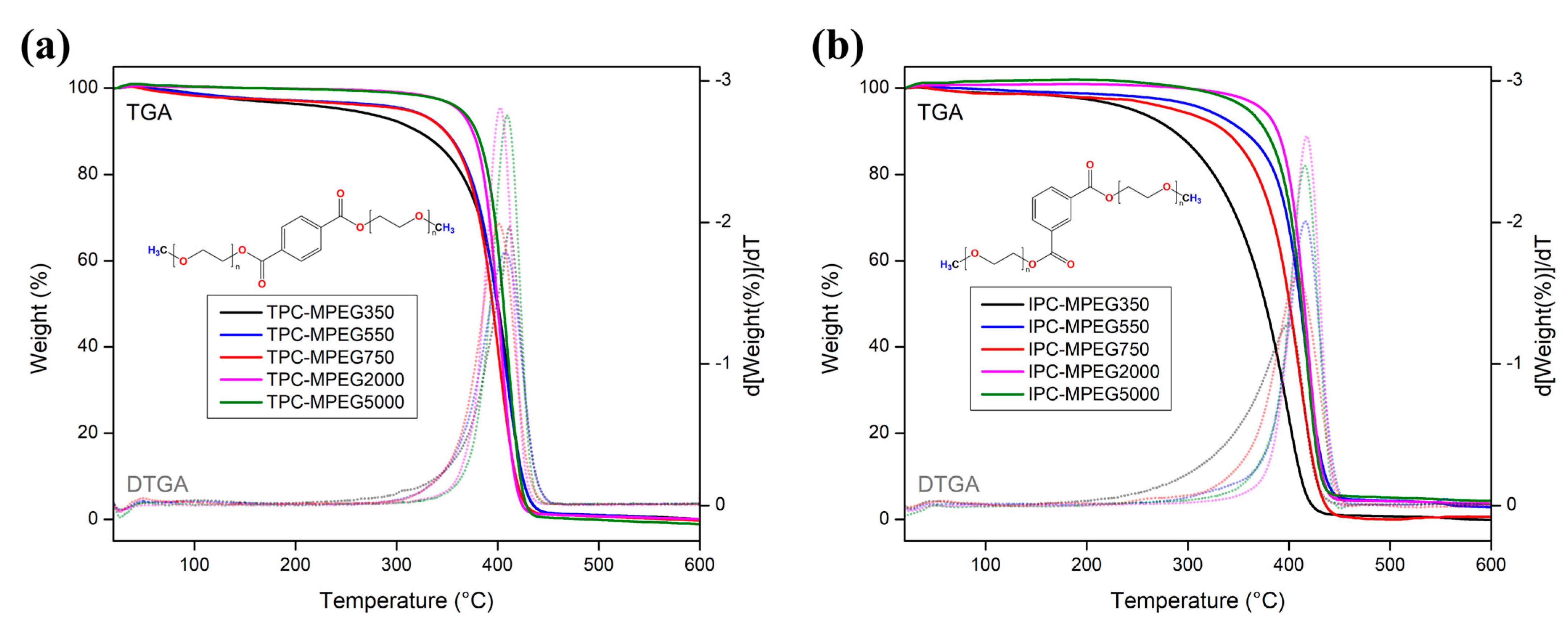
3. Results and Discussion
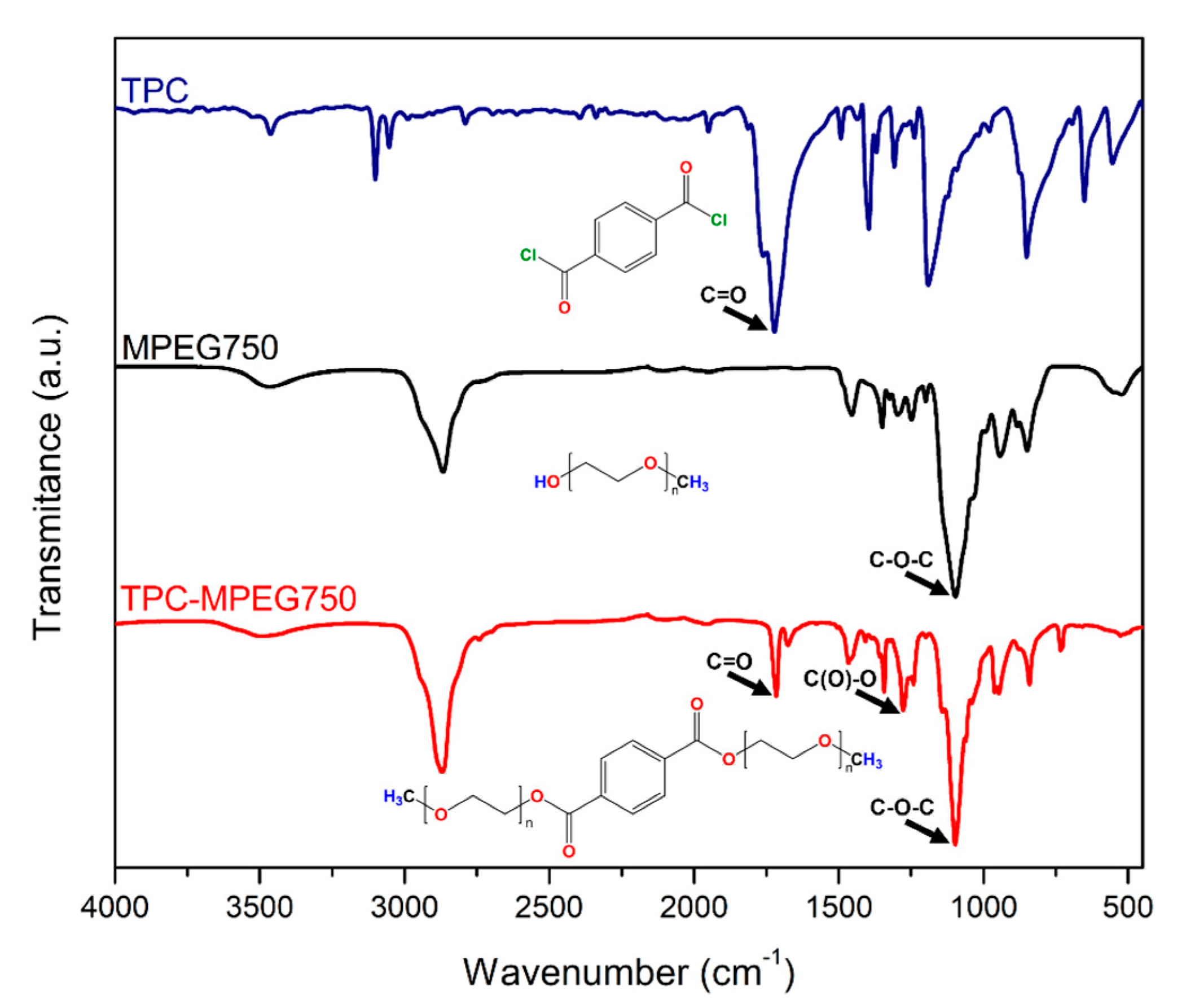
3.1. PCMs’ Synthesis and Spectroscopy Characterization
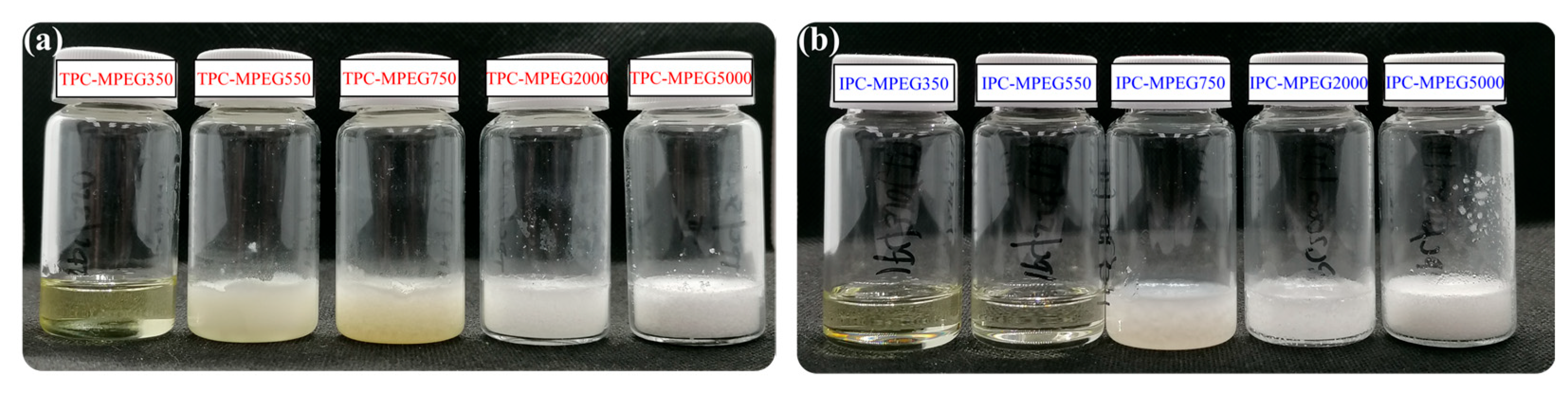
3.2. Solubility Tests
3.3. PCMs’ Thermal Properties
3.4. PCMs’ Crystal Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Yasiri, Q.; Szabó, M. Incorporation of phase change materials into building envelope for thermal comfort and energy saving: A comprehensive analysis. J. Build. Eng. 2021, 36, 102122. [Google Scholar] [CrossRef]
- Sharif, M.K.A.; Al-Abidi, A.A.; Mat, S.; Sopian, K.; Ruslan, M.H.; Sulaiman, M.Y.; Rosli, M.A.M. Review of the application of phase change material for heating and domestic hot water systems. Renew. Sustain. Energy Rev. 2015, 42, 557–568. [Google Scholar] [CrossRef]
- GABC. Global Roadmap Towards Low-GHG and Resilient Buildings; Global Alliance for Buildings and Construction, International Energy Agency, UN Environment: Paris, France, 2016. [Google Scholar]
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Majid, M.Z.A. A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- Avtar, R.; Tripathi, S.; Aggarwal, A.K.; Kumar, P. Population–Urbanization–Energy Nexus: A Review. Resources 2019, 8, 136. [Google Scholar] [CrossRef]
- Statistical Review of World Energy; Energy Economics In: BP Global. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 1 June 2023).
- Evin, D.; Ucar, A. Energy impact and eco-efficiency of the envelope insulation in residential buildings in Turkey. Appl. Therm. Eng. 2019, 154, 573–584. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Bozlar, M.; Liu, Z.; Guo, H.; Meggers, F. Active building envelope systems toward renewable and sustainable energy. Renew. Sustain. Energy Rev. 2019, 104, 470–491. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. An experimental evaluation of thermal behavior of the building envelope using macroencapsulated PCM for energy savings. Renew. Energy 2020, 149, 1300–1313. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Y.; Lu, W.; Cheng, M. Fabrication and energy efficiency of translucent concrete panel for building envelope. Energy 2022, 248, 123635. [Google Scholar] [CrossRef]
- Mesloub, A.; Ghosh, A.; Touahmia, M.; Albaqawy, G.A.; Alsolami, B.M.; Ahriz, A. Assessment of the overall energy performance of an SPD smart window in a hot desert climate. Energy 2022, 252, 124073. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Zhao, M. Synthesis and characterization of PEG/ZSM-5 composite phase change materials for latent heat storage. Renew. Energy 2018, 121, 45–52. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Kośny, J. PCM-Enhanced Building Components: An Application of Phase Change Materials in Building Envelopes and Internal Structures; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Zhang, H.; Gao, X.; Chen, C.; Xu, T.; Fang, Y.; Zhang, Z. A capric–palmitic–stearic acid ternary eutectic mixture/expanded graphite composite phase change material for thermal energy storage. Compos. Part A Appl. Sci. Manuf. 2016, 87, 138–145. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Yang, G.; Wang, Y.; Zhao, L.; Mao, Z.; Wang, B.; Feng, X.; Sui, X. Shape-stabilized hydrated salt/paraffin composite phase change materials for advanced thermal energy storage and management. Chem. Eng. J. 2020, 385, 123958. [Google Scholar] [CrossRef]
- Agarwal, A.; Sarviya, R. Characterization of Commercial Grade Paraffin wax as Latent Heat Storage material for Solar dryers. Mater. Today Proc. 2017, 4, 779–789. [Google Scholar] [CrossRef]
- Reyes, A.; Henríquez-Vargas, L.; Vásquez, J.; Pailahueque, N.; Aguilar, G. Analysis of a laboratory scale thermal energy accumulator using two-phases heterogeneous paraffin wax-water mixtures. Renew. Energy 2020, 145, 41–51. [Google Scholar] [CrossRef]
- Gulfam, R.; Zhang, P.; Meng, Z. Advanced thermal systems driven by paraffin-based phase change materials—A review. Appl. Energy 2019, 238, 582–611. [Google Scholar] [CrossRef]
- Xie, N.; Luo, J.; Li, Z.; Huang, Z.; Gao, X.; Fang, Y.; Zhang, Z. Salt hydrate/expanded vermiculite composite as a form-stable phase change material for building energy storage. Sol. Energy Mater. Sol. Cells 2019, 189, 33–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, J.; Sun, L.; Xie, T.; Yang, K.; Li, Z. Preparation and characterization of high efficiency microencapsulated phase change material based on paraffin wax core and SiO2 shell derived from sodium silicate precursor. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126905. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Zhang, S.; Zhou, Y.; Arıcı, M. Characteristics of PCMs-filled double glazing unit under fire: A detailed thermal structural analysis. J. Build. Eng. 2023, 65, 105672. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, L.; Zou, B.; Qiao, G.; Zhang, T.; Cong, L.; Jiang, F.; Li, C.; Huang, Y.; Ding, Y. Expanded graphite—Paraffin composite phase change materials: Effect of particle size on the composite structure and properties. Appl. Therm. Eng. 2020, 171, 115015. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Bhavsar, R.R.; Singh, V.K. Recent developments in thermo-physical property enhancement and applications of solid solid phase change materials. J. Therm. Anal. Calorim. 2020, 139, 3023–3049. [Google Scholar] [CrossRef]
- Xiong, Q.; Alshehri, H.M.; Monfaredi, R.; Tayebi, T.; Majdoub, F.; Hajjar, A.; Delpisheh, M.; Izadi, M. Application of phase change material in improving trombe wall efficiency: An up-to-date and comprehensive overview. Energy Build. 2022, 258, 111824. [Google Scholar] [CrossRef]
- Jia, M.; Sha, A.; Jiang, W.; Wang, W.; Li, J.; Dai, J.; Lu, Z. Laboratory evaluation of poly(ethylene glycol) for cooling of asphalt pavements. Constr. Build. Mater. 2021, 273, 121774. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Versatility of polyethylene glycol (PEG) in designing solid–solid phase change materials (PCMs) for thermal management and their application to innovative technologies. J. Mater. Chem. A 2017, 5, 18379–18396. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Du, X.; Wang, H.; Wu, Y.; Du, Z.; Cheng, X. Solid-solid phase-change materials based on hyperbranched polyurethane for thermal energy storage. J. Appl. Polym. Sci. 2017, 134, 45014. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, T.; Karrar, E.; Xie, D.; Zheng, L.; Jin, J.; Wang, X.; Jin, Q. Highly efficient synthesis of 4,4-dimethylsterol oleates using acyl chloride method through esterification. Food Chem. 2021, 364, 130140. [Google Scholar] [CrossRef]
- Ghaffari, A.; Pooresmaeil, M.; Namazi, H.; Entezami, A.A. New polymer systems based on polyethylene glycol: Synthesis, characterization, and study of the solubility behavior. Polym. Bull. 2020, 77, 5663–5680. [Google Scholar] [CrossRef]
- Gök, Ö. Didodecyl terephthalate, ditetradecyl terephthalate, and dioctadecyl terephthalate compounds as novel phase change materials for medium temperature applications. J. Energy Storage 2022, 56, 105859. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Fischer, L.J.; Worlitschek, J.; Stamatiou, A. Assessment of the Thermal Properties of Aromatic Esters as Novel Phase Change Materials. Crystals 2020, 10, 919. [Google Scholar] [CrossRef]
- Sęk, D. Relationship between the structure and thermal properties of certain polymers with aromatic units in the chain. Acta Polym. 1984, 35, 626–633. [Google Scholar] [CrossRef]
- Mary, Y.S.; Panicker, C.Y.; Sapnakumari, M.; Narayana, B.; Sarojini, B.; Al-Saadi, A.A.; Van Alsenoy, C.; War, J.A.; Fun, H. Infrared spectrum, structural and optical properties and molecular docking study of 3-(4-fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazole-1-carbaldehyde. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 529–538. [Google Scholar] [CrossRef]
- Crépy, L.; Chaveriat, L.; Banoub, J.; Martin, P.; Joly, N. Synthesis of Cellulose Fatty Esters as Plastics-Influence of the Degree of Substitution and the Fatty Chain Length on Mechanical Properties. Chemsuschem 2009, 2, 165–170. [Google Scholar] [CrossRef]
- More, A.P.; Kokate, S.R.; Rane, P.C.; Mhaske, S.T. Studies of different techniques of aminolysis of poly(ethylene terephthalate) with ethylenediamine. Polym. Bull. 2016, 74, 3269–3282. [Google Scholar] [CrossRef]
- Khairuddin; Pramono, E.; Utomo, S.B.; Wulandari, V.; Zahrotul, A.W.; Clegg, F. FTIR studies on the effect of concentration of polyethylene glycol on polimerization of Shellac. J. Physics Conf. Ser. 2016, 776, 12053. [Google Scholar] [CrossRef]
- Zagonel, G.F.; Peralta-Zamora, P.; Ramos, L.P. Multivariate monitoring of soybean oil ethanolysis by FTIR. Talanta 2004, 63, 1021–1025. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Analysis of Bio-Based Fatty Esters PCM’s Thermal Properties and Investigation of Trends in Relation to Chemical Structures. Appl. Sci. 2019, 9, 225. [Google Scholar] [CrossRef]
- Yang, C.-H.; Chen, S.-H.; Pan, Y.-W.; Chuang, C.-N.; Chao, W.-C.; Young, T.-H.; Chiu, W.-Y.; Wang, C.-K.; Hsieh, K.-H. Preparation and characterization of methoxy-poly(ethylene glycol) side chain grafted onto chitosan as a wound dressing film. J. Appl. Polym. Sci. 2015, 132, 42340. [Google Scholar] [CrossRef]
- Han, N.; Li, Z.; Zhang, X.; Yu, W.; Chen, X.; Wang, D.; Li, J. Synthesis and characterization of cellulose-g-polyoxyethylene (2) hexadecyl ether solid–solid phase change materials. Cellulose 2016, 23, 1663–1674. [Google Scholar] [CrossRef]
- Du, X.; Wang, H.; Cheng, X.; Du, Z. Synthesis and thermal energy storage properties of a solid–solid phase change material with a novel comb-polyurethane block copolymer structure. RSC Adv. 2016, 6, 42643–42648. [Google Scholar] [CrossRef]
- Methoxypolyethylene Glycol 350 Average mol wt 350 9004-74-4. Available online: http://www.sigmaaldrich.com/ (accessed on 10 July 2023).
- Poly(ethylene glycol) Methyl Ether Average Mn 550|9004-74-4. Available online: http://www.sigmaaldrich.com/ (accessed on 10 July 2023).
- Poly(ethylene glycol) Methyl Ether Average Mn 750|9004-74-4. Available online: http://www.sigmaaldrich.com/ (accessed on 10 July 2023).
- Poly(ethylene glycol) Methyl Ether Average Mn ~2,000|9004-74-4. Available online: http://www.sigmaaldrich.com/ (accessed on 10 July 2023).
- Poly(ethylene glycol) Methyl Ether Average Mn 5000|9004-74-4. Available online: http://www.sigmaaldrich.com/ (accessed on 10 July 2023).
- Hu, X.; Wu, H.; Liu, S.; Gong, S.; Du, Y.; Li, X.; Lu, X.; Qu, J. Fabrication of Organic Shape-stabilized Phase Change Material and Its Energy Storage Applications. Eng. Sci. 2021, 17, 1–27. [Google Scholar] [CrossRef]
- Hartshorn, S.R. Structural Adhesives: Chemistry and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zahir, H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, J.; Lin, L.; Shi, J. A Review of Composite Phase Change Materials Based on Biomass Materials. Polymers 2022, 14, 4089. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Niu, X.; Ling, X. Preparation, Characterization, and Thermal Properties of Microencapsulated Phase Change Material for Low-Temperature Thermal Energy Storage. Energy Fuels 2019, 33, 1631–1636. [Google Scholar] [CrossRef]
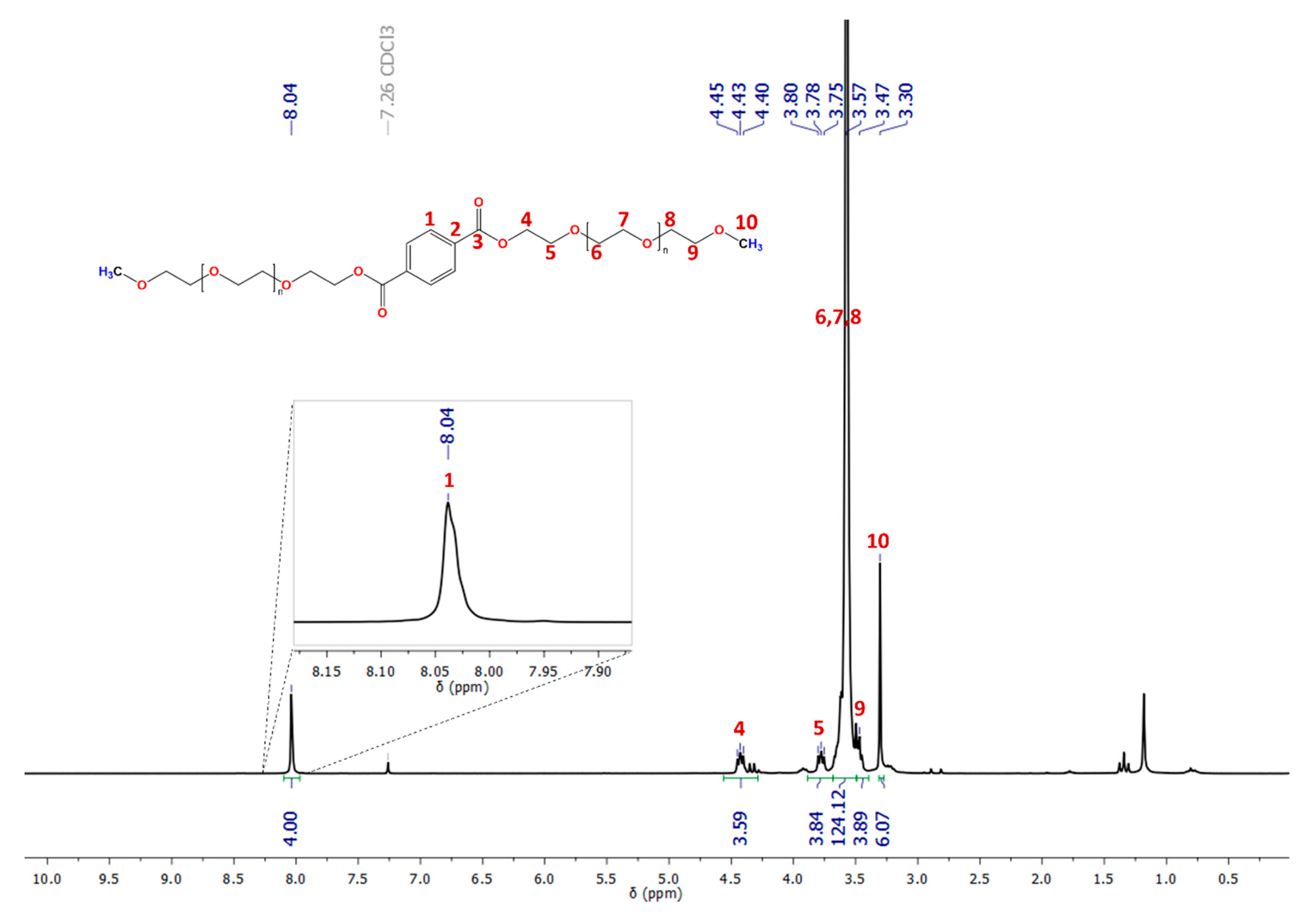




| Signal Number | δ (ppm) and Integral (I) | PCMs | ||||
|---|---|---|---|---|---|---|
| TPC- MPEG350 | TPC- MPEG550 | TPC- MPEG750 | TPC- MPEG2000 | TPC- MPEG5000 | ||
| 1 | δ | 7.90 | 7.95 | 8.04 | 8.10 | 8.04 |
| I | 4 | 4 | 4 | 4 | 4 | |
| 4 | δ | 4.29 | 4.34 | 4.43 | 4.49 | 4.44 |
| I | 4 | 4 | 4 | 4 | 4 | |
| 5 | δ | 3.64 | 3.69 | 3.78 | 3.99 | 4.12 |
| I | 4 | 4 | 4 | 4 | 4 | |
| 6,7,8 | δ | 3.43 | 3.49 | 3.57 | 3.64 | 3.58 |
| I | 46 | 85 | 124 | 532 | 1400 | |
| 9 | δ | 3.32 | 3.38 | 3.47 | - a | - a |
| I | 4 | 4 | 4 | - a | - a | |
| 10 | δ | 3.15 | 3.21 | 3.30 | 3.37 | 3.32 |
| I | 6 | 6 | 6 | 6 | 6 | |
| Integral | 58 | 97 | 136 | 540 | 1408 | |
| It | 58 | 94 | 130 | 357 | 902 | |
| Solvents | a IPC- | ||||
|---|---|---|---|---|---|
| MPEG350 | MPEG550 | MPEG750 | MPEG2000 | MPEG5000 | |
| H2O | +/− | +/− | +/− | +/− | +/− |
| MeOH | + | + | + | + | + |
| n-hexane | − | − | − | − | − |
| CHCl3 | + | + | + | + | + |
| THF | + | + | + | − | − |
| THF/CHCl3 | + | + | + | + | + |
| Solvents | aTPC- | ||||
| MPEG350 | MPEG550 | MPEG750 | MPEG2000 | MPEG5000 | |
| H2O | +/− | +/− | +/− | +/− | +/− |
| MeOH | + | + | + | + | + |
| n-hexane | − | − | − | − | − |
| CHCl3 | + | + | + | + | + |
| THF | + | + | + | − | − |
| THF/CHCl3 | + | + | + | + | + |
| PCMs | Ti | Tonset | Td | Td5% | Td10% | R |
|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | (°C) | (%) | |
| MPEG350 | 179.0 | 258.6 | 337.9 | 204.5 | 236.2 | 0.0 |
| MPEG550 | 231.5 | 339.4 | 385.7 | 243.2 | 297.5 | 0.0 |
| MPEG750 | 267.3 | 370.7 | 401.4 | 171.8 | 269.4 | 0.0 |
| MPEG2000 | 329.0 | 389.1 | 405.1 | 380.9 | 394.4 | 0.9 |
| MPEG5000 | 334.7 | 392.6 | 406.3 | 392.5 | 400.4 | 1.2 |
| TPC-MPEG350 | 238.0 | 365.3 | 399.2 | 250.8 | 321.4 | 0.1 |
| TPC-MPEG550 | 285.0 | 363.3 | 398.1 | 311.6 | 348.4 | 0.0 |
| TPC-MPEG750 | 270.0 | 361.1 | 392.4 | 309.2 | 347.9 | 0.0 |
| TPC-MPEG2000 | 292.0 | 376.5 | 398.1 | 361.6 | 375.1 | 0.1 |
| TPC-MPEG5000 | 294.0 | 376.7 | 398.7 | 363.5 | 378.8 | 0.0 |
| IPC-MPEG350 | 183.0 | 328.7 | 387.0 | 245.5 | 286.5 | 0.0 |
| IPC-MPEG550 | 252.0 | 373.2 | 403.9 | 318.0 | 355.3 | 2.9 |
| IPC-MPEG750 | 248.0 | 359.5 | 396.1 | 287.8 | 337.1 | 0.6 |
| IPC-MPEG2000 | 2630 | 382.0 | 406.2 | 374.3 | 388.8 | 3.7 |
| IPC-MPEG5000 | 250.0 | 377.5 | 404.1 | 359.8 | 377.4 | 4.3 |
| PCM | Mn (g/mol) | Ar (%) | Melting Process | Crystallization Process | Supercooling | ||
|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | ΔT (°C) | |||
| TPC-MPEG350 | 830 | 9.2 | 10.0 | 79.8 | −39.9 | 64.6 | 49.9 |
| TPC-MPEG550 | 1230 | 6.2 | 24.8 | 100.6 | 2.7 | 75.8 | 22.1 |
| TPC-MPEG750 | 1630 | 4.7 | 24.1 | 111.2 | 20.7 | 109.6 | 3.4 |
| TPC-MPEG2000 | 4130 | 1.8 | 51.9 | 185.8 | 35.1 | 183.7 | 16.8 |
| TPC-MPEG5000 | 10,130 | 0.8 | 60.1 | 164.0 | 41.9 | 157.6 | 18.2 |
| IPC-MPEG350 | 830 | 9.2 | −23.7 | 57.9 | −32.2 | 48.6 | 8.5 |
| IPC-MPEG550 | 1230 | 6.2 | 10.9 | 98.4 | 2.7 | 81.0 | 8.2 |
| IPC-MPEG750 | 1630 | 4.7 | 21.5 | 99.1 | 20.3 | 87.0 | 1.2 |
| IPC-MPEG2000 | 4130 | 1.8 | 52.4 | 198.8 | 33.5 | 195.6 | 18.9 |
| IPC-MPEG5000 | 10,130 | 0.8 | 60.2 | 169.8 | 45.9 | 171.5 | 14.3 |
| Cycle | TPC-MPEG750 Thermal Properties | ||||
|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | ΔT (°C) | |
| 1 | 23.8 | 113.3 | 20.2 | 108.9 | 3.5 |
| 2 | 23.9 | 101.6 | 22.5 | 119.3 | 1.4 |
| 3 | 24.0 | 107.0 | 22.5 | 104.6 | 1.5 |
| 4 | 24.1 | 119.0 | 20.1 | 103.9 | 4.0 |
| 5 | 24.2 | 120.5 | 20.1 | 113.2 | 4.0 |
| 6 | 24.2 | 117.2 | 20.2 | 110.4 | 4.0 |
| 7 | 24.2 | 119.0 | 20.2 | 101.1 | 3.9 |
| 8 | 24.2 | 111.4 | 20.2 | 105.3 | 4.0 |
| 9 | 24.3 | 102.0 | 20.3 | 115.8 | 4.0 |
| 10 | 24.3 | 101.4 | 20.3 | 113.1 | 4.1 |
| Cycle | IPC-MPEG750 Thermal Properties | ||||
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | ΔT (°C) | |
| 1 | 21.4 | 100.6 | 20.3 | 88.3 | 1.1 |
| 2 | 21.5 | 99.4 | 20.3 | 86.6 | 1.1 |
| 3 | 21.5 | 100.9 | 20.3 | 89.0 | 1.2 |
| 4 | 21.5 | 100.8 | 20.3 | 89.3 | 1.2 |
| 5 | 21.5 | 99.7 | 20.3 | 88.4 | 1.2 |
| 6 | 21.5 | 99.0 | 20.3 | 87.0 | 1.2 |
| 7 | 21.4 | 98.1 | 20.3 | 85.1 | 1.2 |
| 8 | 21.4 | 98.0 | 20.3 | 84.6 | 1.2 |
| 9 | 21.4 | 96.5 | 20.3 | 84.6 | 1.1 |
| 10 | 21.4 | 98.4 | 20.3 | 87.4 | 1.2 |
| PCM | Quantity | Mean Size (mm) | σ (mm) |
|---|---|---|---|
| MPEG2000 | 8 | 2.5 | 0.4 |
| TPC-MPEG2000 | 7 | 2.6 | 0.6 |
| IPC-MPEG2000 | 4 | 3.8 | 0.7 |
| MPEG5000 | 14 | 2.0 | 0.3 |
| TPC-MPEG5000 | 5 | 3.2 | 0.6 |
| IPC-MPEG5000 | 4 | 3.3 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angel-López, A.; Norambuena, Á.; Arriaza-Echanes, C.; Terraza, C.A.; Tundidor-Camba, A.; Coll, D.; Ortiz, P.A. Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides. Polymers 2023, 15, 3069. https://doi.org/10.3390/polym15143069
Angel-López A, Norambuena Á, Arriaza-Echanes C, Terraza CA, Tundidor-Camba A, Coll D, Ortiz PA. Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides. Polymers. 2023; 15(14):3069. https://doi.org/10.3390/polym15143069
Chicago/Turabian StyleAngel-López, Alejandro, Ángel Norambuena, C. Arriaza-Echanes, Claudio A. Terraza, Alain Tundidor-Camba, Deysma Coll, and Pablo A. Ortiz. 2023. "Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides" Polymers 15, no. 14: 3069. https://doi.org/10.3390/polym15143069
APA StyleAngel-López, A., Norambuena, Á., Arriaza-Echanes, C., Terraza, C. A., Tundidor-Camba, A., Coll, D., & Ortiz, P. A. (2023). Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides. Polymers, 15(14), 3069. https://doi.org/10.3390/polym15143069






