Promising Gene Delivery Properties of Polycations Based on 2-(N,N-dimethylamino)ethyl Methacrylate and Polyethylene Glycol Monomethyl Ether Methacrylate Copolymers
Abstract
1. Introduction
2. Materials and Methods
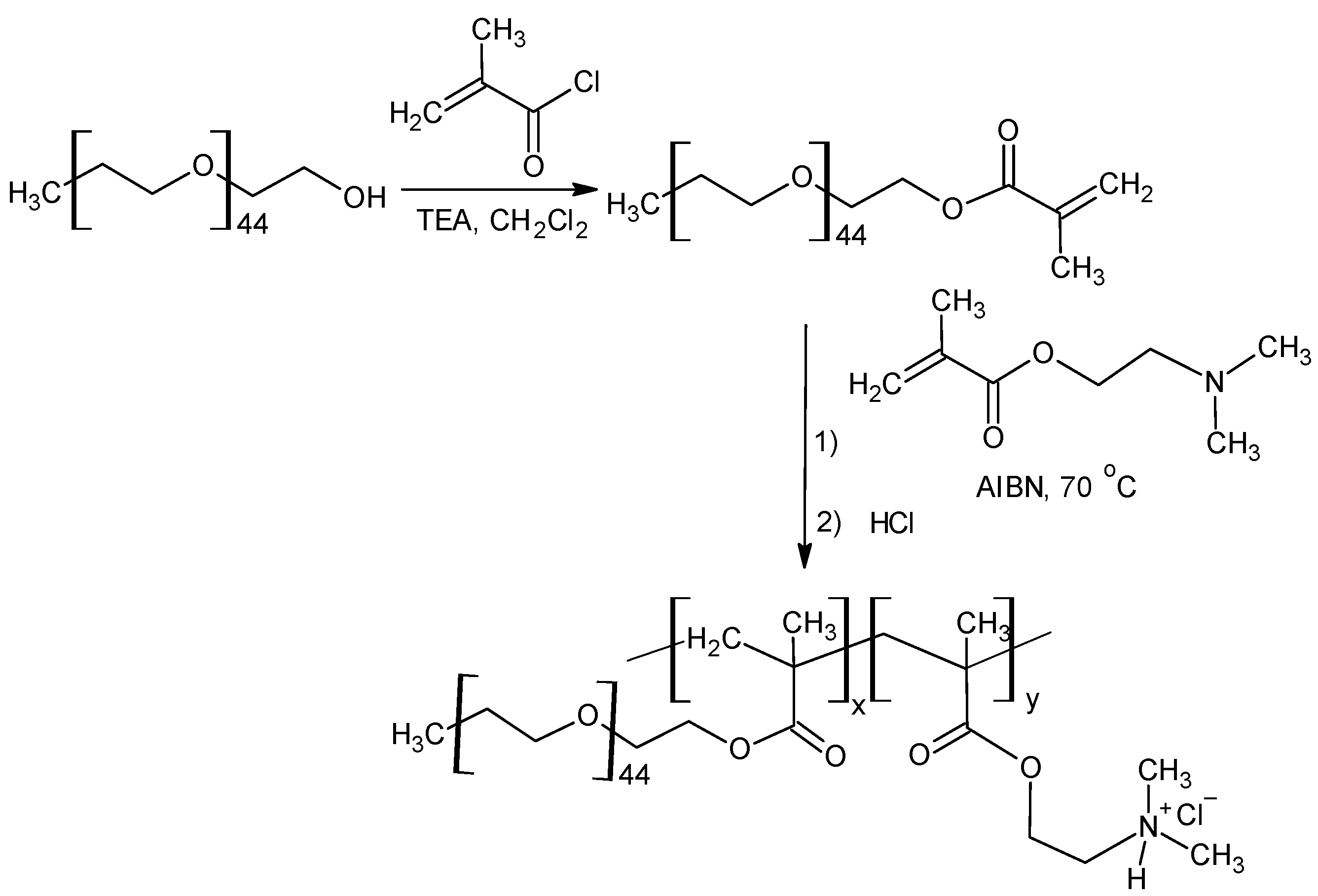
2.1. Synthesis of Polyethylene Glycol Monomethyl ether Methacrylate (PEGMA)
2.2. Synthesis of Poly(2-(N,N-Dimethylamino)ethyl Methacrylate)-co-PEGMA) (PDMAEMA-co-PEO)
2.3. Characterization
2.3.1. NMR Spectroscopy
2.3.2. Sedimentation
2.3.3. Potentiometric Titration
2.3.4. Dynamic Light Scattering
2.3.5. Electrophoretic Mobility
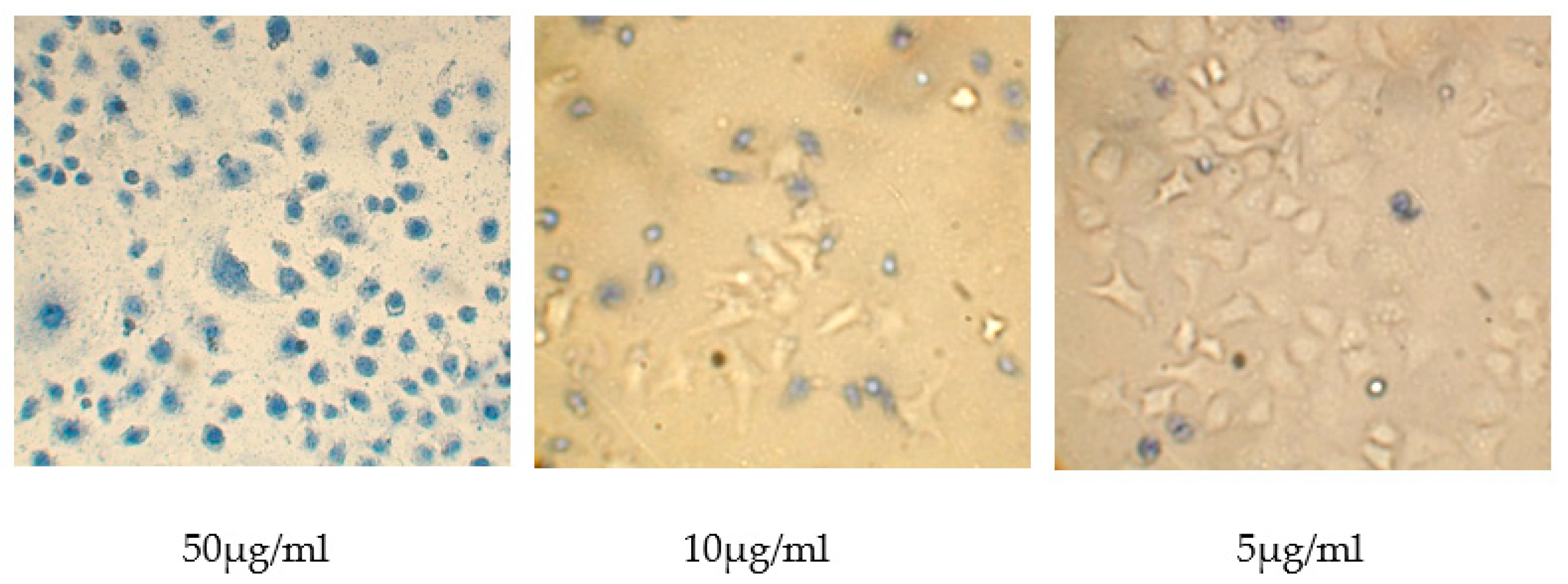
2.3.6. Determination of Cytotoxicity of Polycation-DNA Complexes
2.3.7. Transfection of Polycation PDMAEMA-co-PEO/DNA Complexes into Eukaryotic Cells
3. Results and Discussion
3.1. Study of the Molecular Characteristics of PDMAEMA-co-PEO Polycations by Sedimentation Analysis
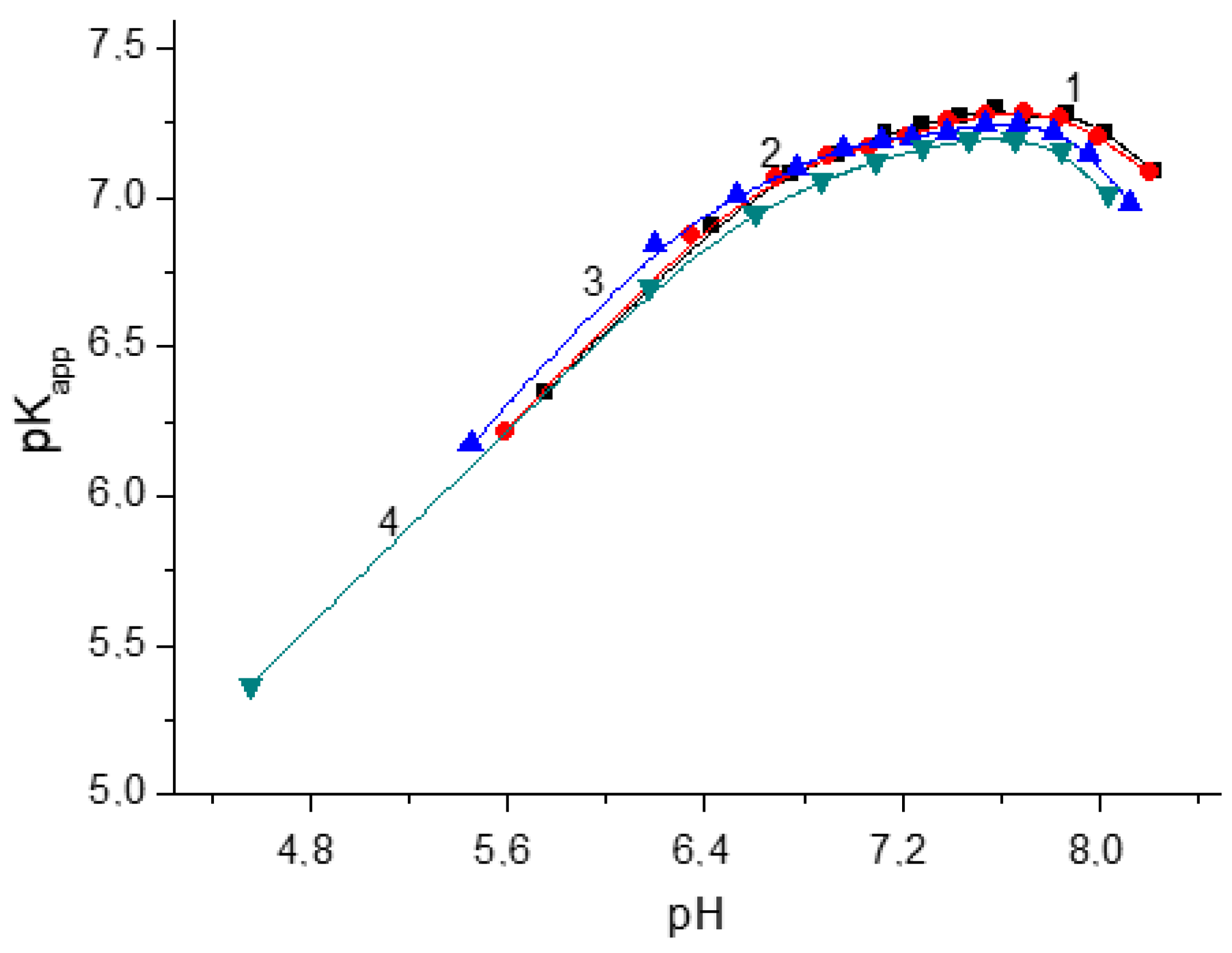
3.2. Study of the Dependence of the Degree of Ionization (α) and Apparent Dissociation Constants pKapp of Polycations on the pH of Aqueous Solutions by Potentiometric Titration
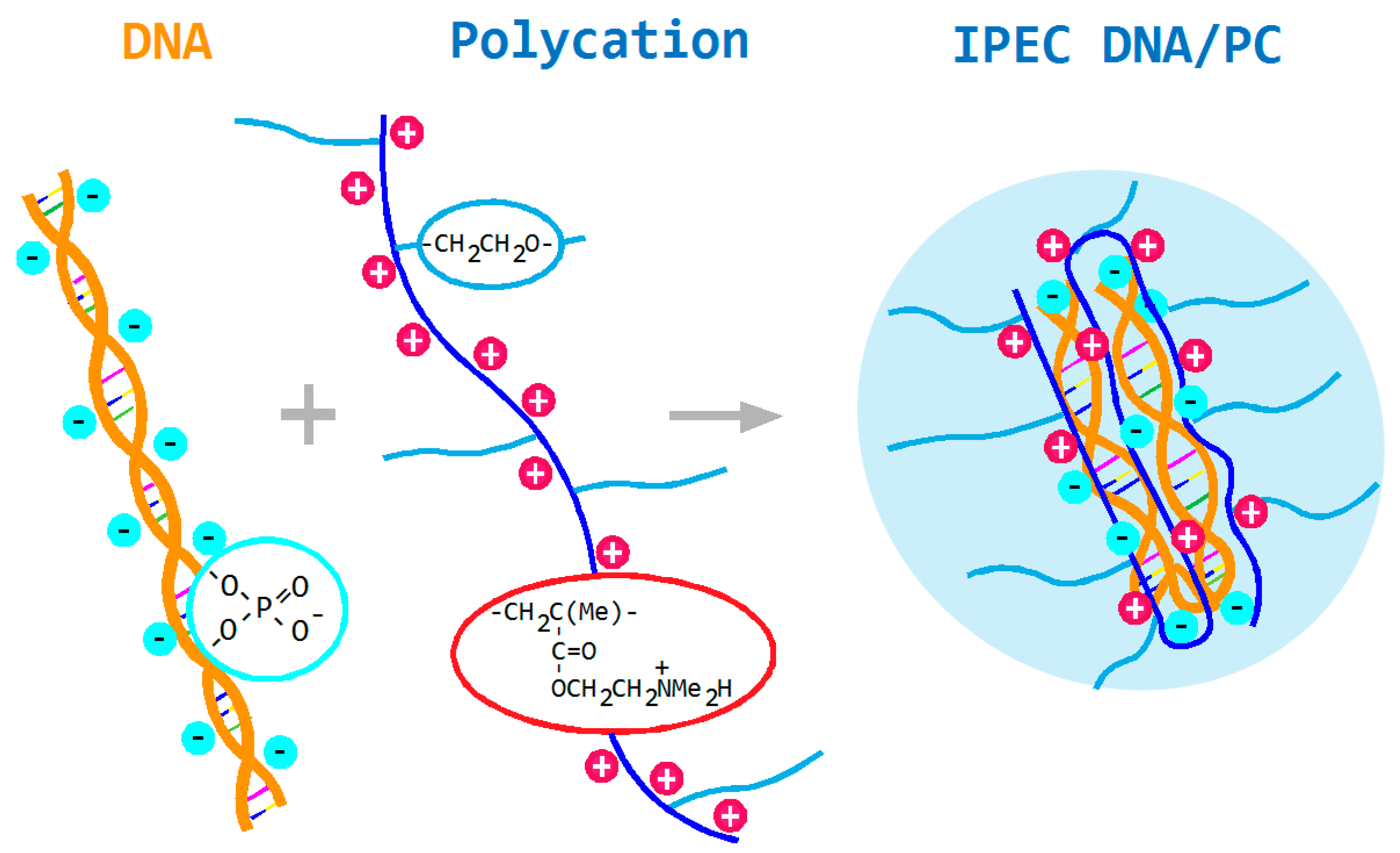
3.3. Study of the Electrophoretic Mobility of Interpolyelectrolyte Complexes of DNA with PDMAEMA-co-PEO Polycations by Laser Microelectrophoresis
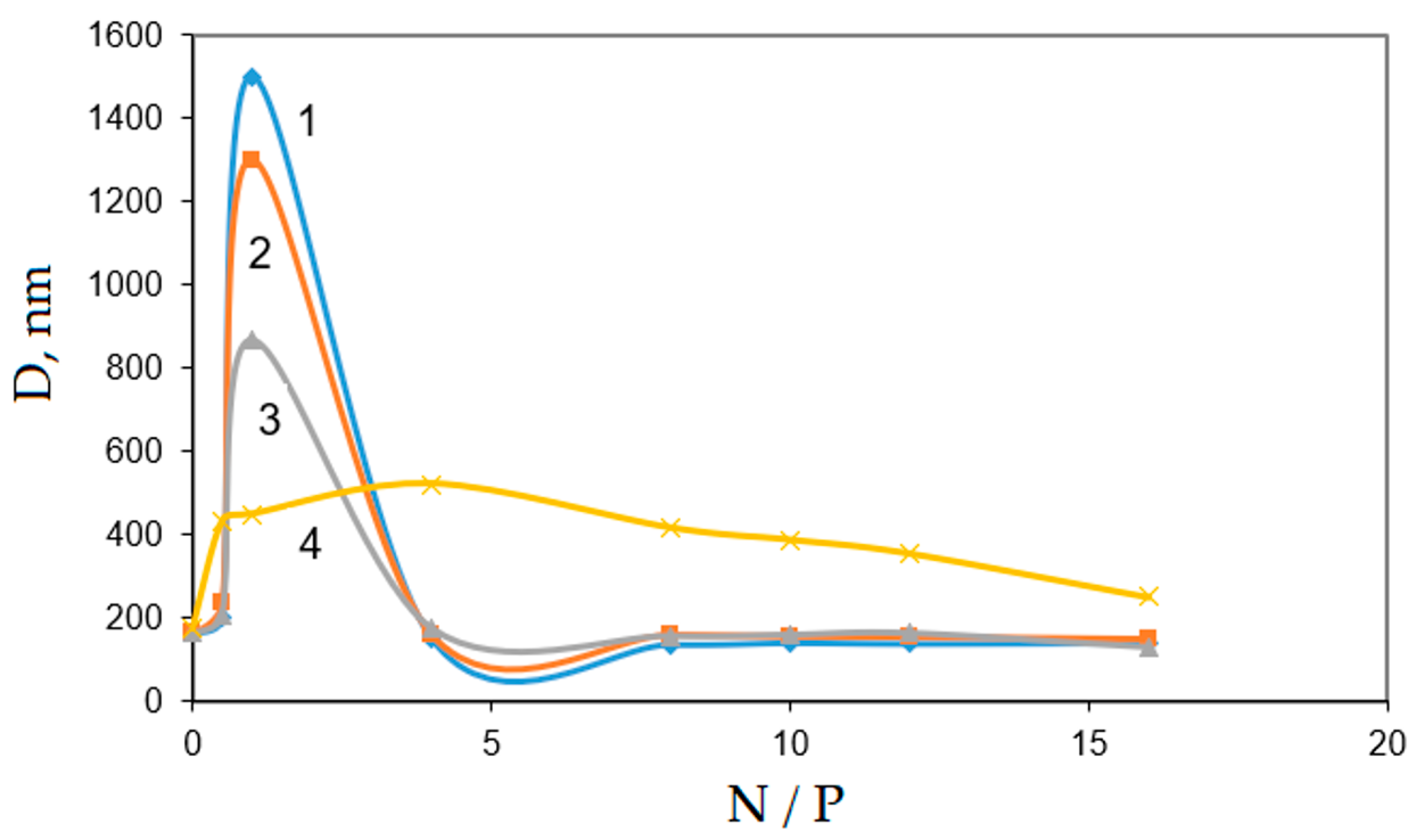
3.4. Investigation of the Diameters of Interpolyelectrolyte DNA Complexes with PDMAEMA-co-PEO Polycations by Dynamic Light Scattering
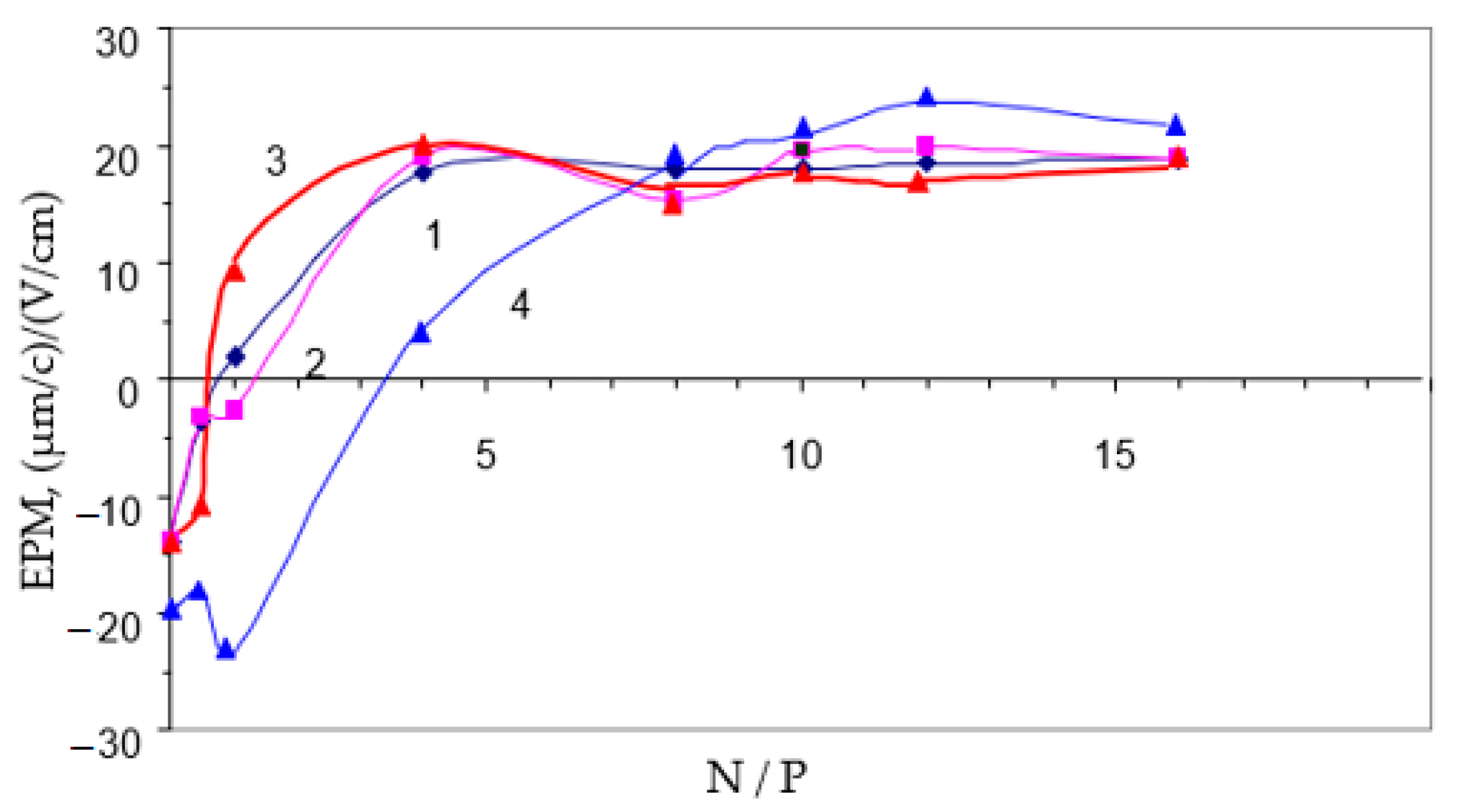
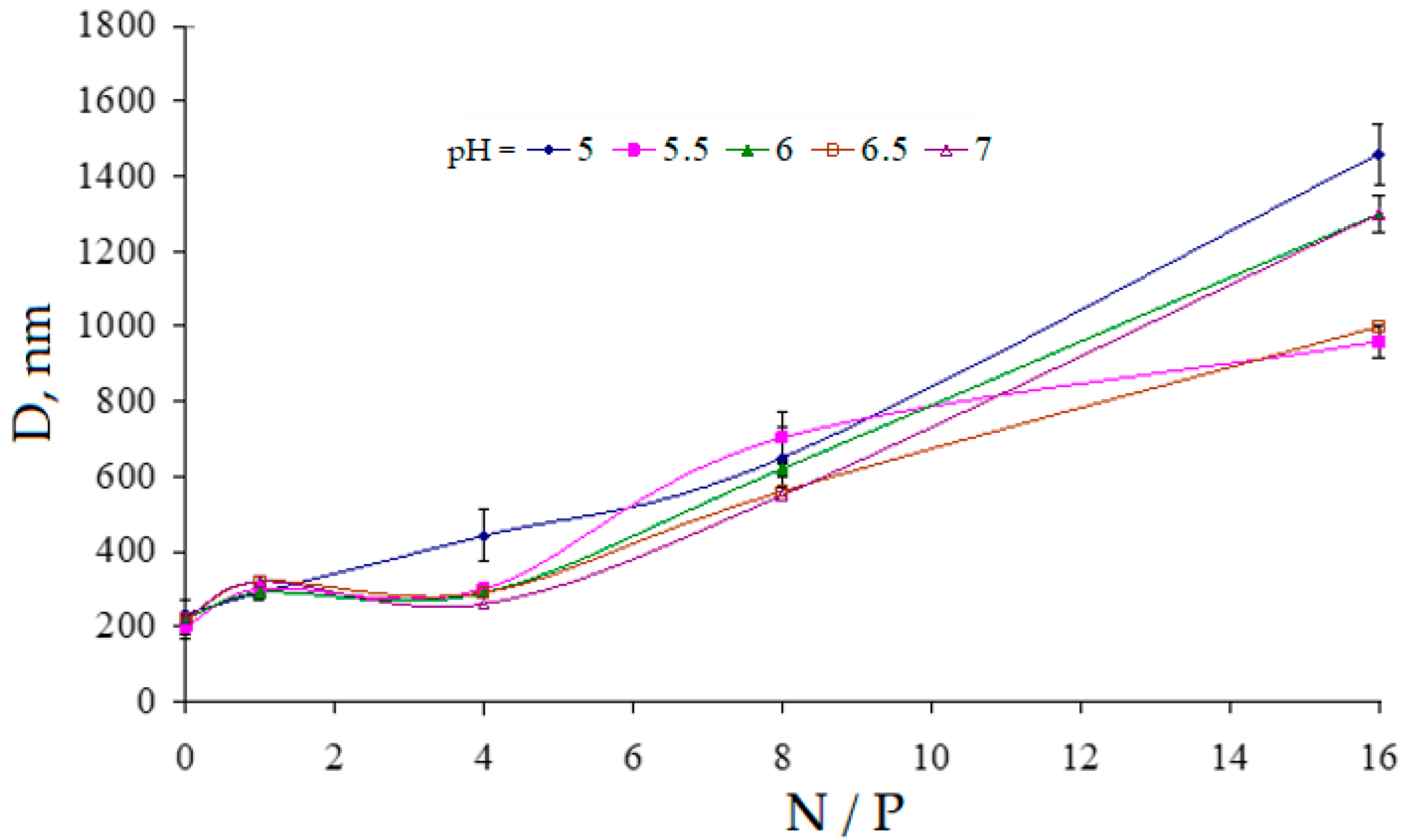
3.5. Study of the Electrophoretic Mobility of Interpolyelectrolyte Complexes of DNA with the Polycation PDMAEMA-co-PEO-2 by Laser Microelectrophoresis at Different pH Values in Aqueous Solutions
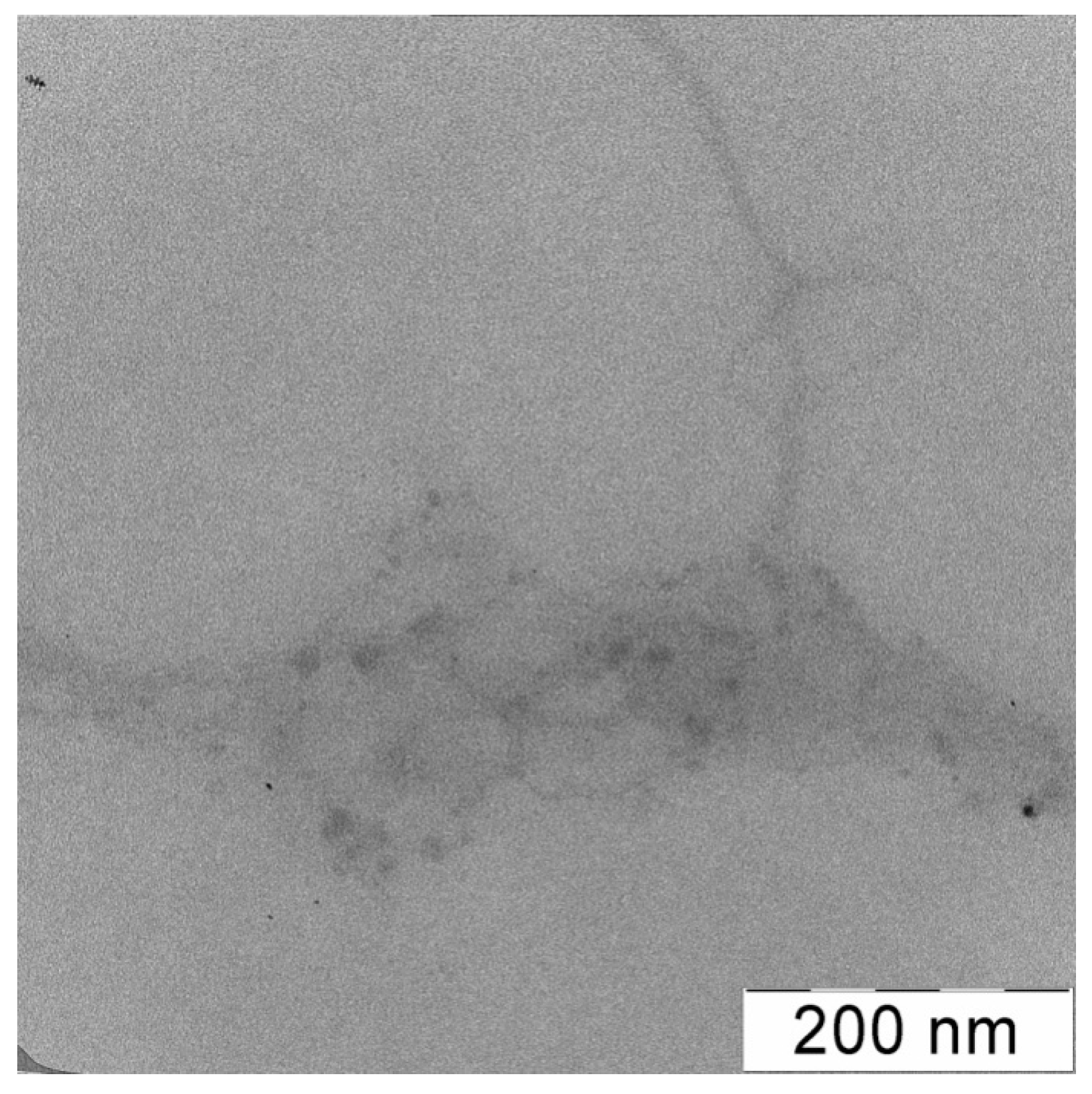
3.6. Study of the Morphology of Interpolyelectrolyte Complexes of DNA with Polycation PDMAEMA-co-PEO-2 by Transmission Electron Microscopy
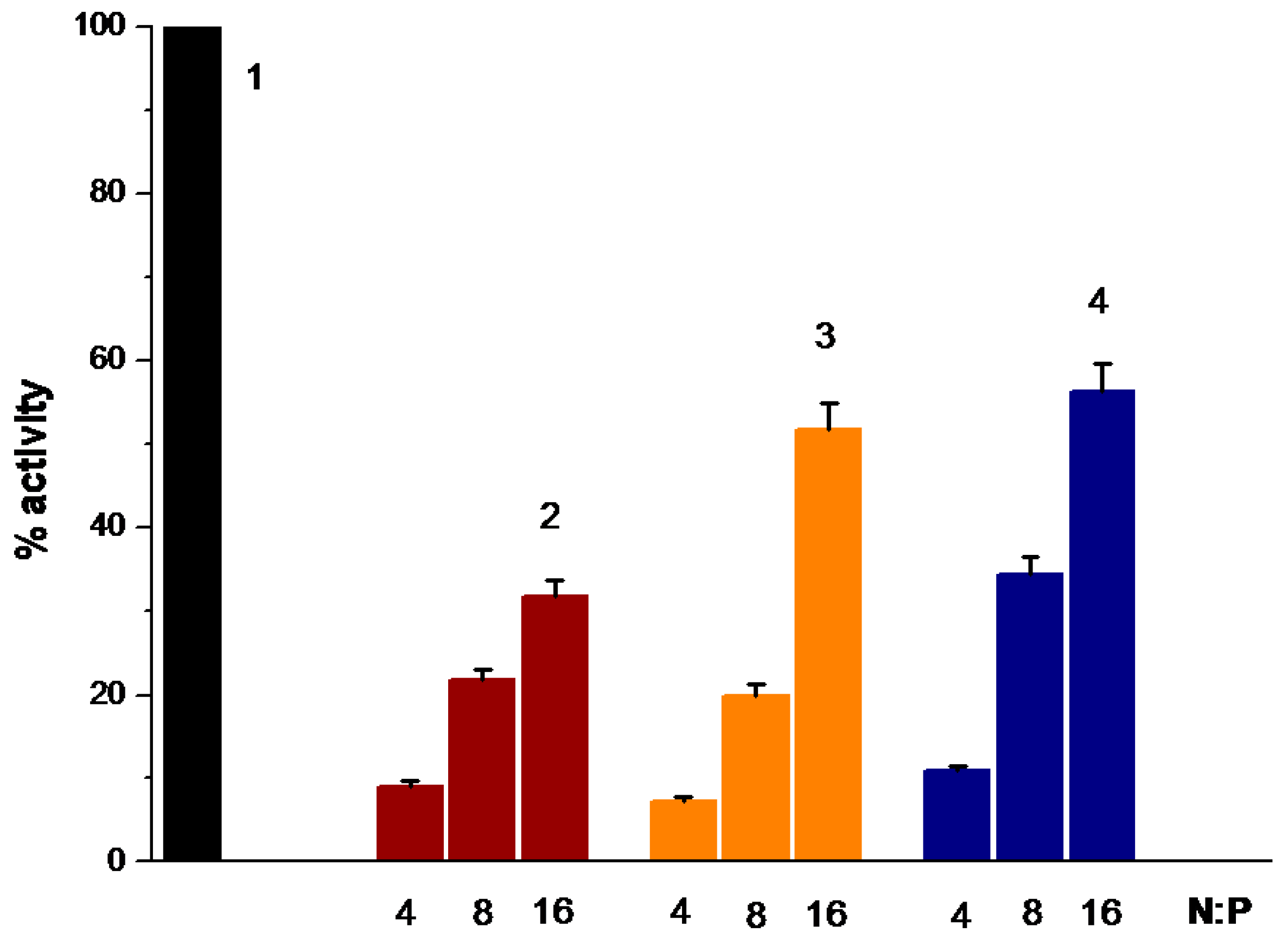
3.7. Study of the Process of Transfection of Interpolyelectrolyte Complexes with Polycations PDMAEMA-co-PEO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseinkhani, H. DNA nanoparticles for gene delivery to cells and tissue. Int. J. Nanotechnol. 2006, 3, 416. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Klinman, D.M. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev. Vaccines 2013, 13, 299–312. [Google Scholar] [CrossRef]
- Uchida, S. Delivery Systems of Plasmid DNA and Messenger RNA for Advanced Therapies. Pharmaceutics 2022, 14, 810. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 1–24. [Google Scholar] [CrossRef]
- Obara, W.; Kanehira, M.; Katagiri, T.; Kato, R.; Kato, Y.; Takata, R. Present status and future perspective of peptide-based vaccine therapy for urological cancer. Cancer Sci. 2018, 109, 550–559. [Google Scholar] [CrossRef]
- Lim, M.; Badruddoza, A.Z.M.; Firdous, J.; Azad, M.; Mannan, A.; Al-Hilal, T.A.; Cho, C.-S.; Islam, M.A. Engineered Nanodelivery Systems to Improve DNA Vaccine Technologies. Pharmaceutics 2020, 12, 30. [Google Scholar] [CrossRef]
- Meng, F.; Yun, Z.; Yan, G.; Wang, G.; Lin, C. Engineering of anticancer drugs entrapped polymeric nanoparticles for the treatment of colorectal cancer therapy. Process Biochem. 2021, 111, 36–42. [Google Scholar] [CrossRef]
- Davis, H.L. Plasmid DNA expression systems for the purpose of immunization. Curr. Opin. Biotechnol. 1997, 8, 635–640. [Google Scholar] [CrossRef]
- Lewis, J.P.; Babiuk, L.A. DNA vaccines: A review. Adv. Virus. Res. 1999, 54, 129–188. [Google Scholar] [PubMed]
- Goudy, K.S.; Wang, B.; Tisch, R. Gene gun-mediated DNA vaccination enhances antigen-specific immunotherapy at a late preclinical stage of type 1 diabetes in nonobese diabetic mice. Clin. Immunol. 2008, 129, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Jinturkar, K.A.; Rathi, M.N.; Misra, A. Gene Delivery Using Physical Methods. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 83–126. [Google Scholar] [CrossRef]
- Lukashev, A.N.; Zamyatnin, A.A. Viral vectors for gene therapy: Current state and clinical perspectives. Biochem. Biokhimiia 2016, 81, 700–708. [Google Scholar] [CrossRef]
- Mollé, L.M.; Smyth, C.H.; Yuen, D.; Johnston, A.P.R. Nanoparticles for vaccine and gene therapy: Overcoming the barriers to nucleic acid delivery. WIREs Nanomed. Nanobiotechnology 2022, 14, 3–38. [Google Scholar] [CrossRef]
- Cherng, J.Y. Investigation of DNA Spectral Conformational Changes and Polymer Buffering Capacity in Relation to Transfection Efficiency of DNA/Polymer Complexes. J. Pharm. Pharm. Sci. 2009, 12, 346–356. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Xu, B.; Wu, M.; Yan, X.; Zhong, L.; Cai, H.; Wang, T.; Wang, Q.; Long, F.; et al. Polymer-based nanoparticles for chemo/gene-therapy: Evaluation its therapeutic efficacy and toxicity against colorectal carcinoma. Biomed. Pharmacother. 2019, 118, 109257. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Green, J.; Chan, J.; Langer, R.; Anderson, D.G. Polymeric Materials for Gene Delivery and DNA Vaccination. Adv. Mater. 2009, 21, 847–867. [Google Scholar] [CrossRef]
- Gebhart, C.L.; Kabanov, A.V. Evaluation of polyplexes as gene transfer agents. J. Control. Release 2001, 73, 401–416. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Zhang, R.; Hu, F.; Zhang, T.; Liu, Y.; Han, M.H.; You, F.; Yang, Y.; Zheng, W. A novel liposome-polymer hybrid nanoparticles delivering a multi-epitope self-replication DNA vaccine and its preliminary immune evaluation in experimental animals. Nanomed. Nanotechnol. Biol. Med. 2021, 35, 102338. [Google Scholar] [CrossRef]
- Chen, C.-K.; Huang, P.-K.; Law, W.-C.; Chu, C.-H.; Chen, N.-T.; Lo, L.-W. Biodegradable Polymers for Gene-Delivery Applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Tajmir-Riahi, H.-A.; Pillai, C.K.S. Biodegradable Polymers for Gene Delivery. Molecules 2019, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy. Molecules 2020, 25, 2866–2889. [Google Scholar] [CrossRef]
- Loh, X.J.; Ong, S.J.; Tung, Y.T.; Choo, H.T. Co-delivery of drug and DNA from cationic dual-responsive micelles derived from poly(DMAEMA-co-PPGMA). Mater. Sci. Eng. C 2013, 33, 4545–4550. [Google Scholar] [CrossRef]
- Hinton, T.M.; Guerrero-Sanchez, C.; Graham, J.E.; Le, T.; Muir, B.W.; Shi, S.; Tizard, M.L.; Gunatillake, P.A.; McLean, K.M.; Thang, S.H. The effect of RAFT-derived cationic block copolymer structure on gene silencing efficiency. Biomaterials 2012, 33, 7631–7642. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Kabanov, V.A. DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconjug. Chem. 1995, 6, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Lopukhov, A.V.; Yang, Z.; Haney, M.J.; Bronich, T.K.; Sokolsky-Papkov, M.; Batrakova, E.V.; Klyachko, N.L.; Kabanov, A.V. Mannosylated Cationic Copolymers for Gene Delivery to Macrophages. Macromol. Biosci. 2021, 21, 2000371. [Google Scholar] [CrossRef]
- Roques, C.; Fattal, E.; Fromes, Y. Comparison of toxicity and transfection efficiency of amphiphilic block copolymers and polycationic polymers in striated muscles. J. Gene Med. 2009, 11, 240–249. [Google Scholar] [CrossRef]
- Calejo, M.T.; Cardoso, A.M.S.; Kjøniksen, A.-L.; Zhu, K.; Morais, C.M.; Sande, S.A.; de Lima, M.C.P.; Jurado, A.; Nyström, B. Temperature-responsive cationic block copolymers as nanocarriers for gene delivery. Int. J. Pharm. 2013, 448, 105–114. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, M.J.; Shi, J.F.; Wang, S.P.; Zhong, X.M.; Wu, Y.H.; Qu, Y.; Le Gao, H.; Zhang, J.M. pH-sensitive nano-polyelectrolyte complexes with arthritic macrophage-targeting delivery of triptolide. Int. J. Pharm. 2023, 632, 122572. [Google Scholar] [CrossRef]
- Askarian, S.; Abnous, K.; Taghavi, S.; Oskuee, R.K.; Ramezani, M. Cellular delivery of shRNA using aptamer-conjugated PLL-alkyl-PEI nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Afrouz, M.; Ahmadi-Nouraldinvand, F.; Amani, A.; Zahedian, H.; Elias, S.G.; Arabnejad, F.; Yaghoubi, H.; Farshad, O.; Farazi, N.; Jalali, A.; et al. Preparation and characterization of magnetic PEG-PEI-PLA-PEI-PEG/Fe3O4-PCL/DNA micelles for gene delivery into MCF-7 cells. J. Drug Deliv. Sci. Technol. 2023, 79, 104016. [Google Scholar] [CrossRef]
- Viana, D.B.; Mathieu-Gaedke, M.; Leão, N.M.; Böker, A.; Soares, D.C.F.; Glebe, U.; Tebaldi, M.L. Hybrid protein-polymer nanoparticles based on P(NVCL-co-DMAEMA) loaded with cisplatin as a potential anti-cancer agent. J. Drug Deliv. Sci. Technol. 2023, 79, 103995. [Google Scholar] [CrossRef]
- Santo, D.; Cordeiro, R.A.; Sousa, A.; Serra, A.; Coelho, J.F.; Faneca, H. Combination of Poly[(2-dimethylamino)ethyl methacrylate] and Poly(β-amino ester) Results in a Strong and Synergistic Transfection Activity. Biomacromolecules 2017, 18, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Polyester-based nanoparticles for nucleic acid delivery. Mater. Sci. Eng. C 2018, 92, 983–994. [Google Scholar] [CrossRef]
- Obata, M.; Kobori, T.; Hirohara, S.; Tanihara, M. Aqueous RAFT synthesis of block and statistical copolymers of 2-(α-d-mannopyranosyloxy)ethyl methacrylate with 2-(N,N-dimethylamino)ethyl methacrylate and their application for nonviral gene delivery. Polym. Chem. 2014, 6, 1793–1804. [Google Scholar] [CrossRef]
- Hsiue, G.-H.; Chiang, H.-Z.; Wang, C.-H.; Juang, T.-M. Nonviral Gene Carriers Based on Diblock Copolymers of Poly(2-ethyl-2-oxazoline) and Linear Polyethylenimine. Bioconjug. Chem. 2006, 17, 781–786. [Google Scholar] [CrossRef]
- Bhattacharya, S. Fabrication and characterization of chitosan-based polymeric nanoparticles of Imatinib for colorectal cancer targeting application. Int. J. Biol. Macromol. 2020, 151, 104–115. [Google Scholar] [CrossRef]
- Layman, J.M.; Ramirez, S.M.; Green, M.D.; Long, T.E. Influence of Polycation Molecular Weight on Poly(2-dimethylaminoethyl methacrylate)-Mediated DNA Delivery In Vitro. Biomacromolecules 2009, 10, 1244–1252. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Lang, X.; Chen, Z.; Zeng, H.; Chang, Y.; Nie, Y. A 3D-printed PCL/PEI/DNA bioactive scaffold for chemotherapy drug capture in vivo. Int. J. Biol. Macromol. 2023, 236, 123942. [Google Scholar] [CrossRef]
- Dutta, P.; Dey, J.; Shome, A.; Das, P.K. Nanostructure formation in aqueous solution of amphiphilic copolymers of 2-(N,N-dimethylaminoethyl)methacrylate and alkylacrylate: Characterization, antimicrobial activity, DNA binding, and cytotoxicity studies. Int. J. Pharm. 2011, 414, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502. [Google Scholar] [CrossRef] [PubMed]
- Amani, A.; Alizadeh, M.R.; Yaghoubi, H.; Ebrahimi, H.A. Design and fabrication of novel multi-targeted magnetic nanoparticles for gene delivery to breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 61, 102151. [Google Scholar] [CrossRef]
- Loh, X.J. Poly(DMAEMA-co-PPGMA): Dual-responsive “reversible” micelles. J. Appl. Polym. Sci. 2012, 127, 992–1000. [Google Scholar] [CrossRef]
- Artyukhov, A.A.; Nechaeva, A.M.; Shtilman, M.I.; Chistyakov, E.M.; Svistunova, A.Y.; Bagrov, D.V.; Kuskov, A.N.; Docea, A.O.; Tsatsakis, A.M.; Gurevich, L.; et al. Nanoaggregates of Biphilic Carboxyl-Containing Copolymers as Carriers for Ionically Bound Doxorubicin. Materials 2022, 15, 7136. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Harashima, H. An efficient PEGylated gene delivery system with improved targeting: Synergism between octaarginine and a fusogenic peptide. Int. J. Pharm. 2018, 538, 179–187. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Yang, Y.; Li, Y. Folate-PEG modified poly(2-(2-aminoethoxy)ethoxy)phosphazene/DNA nanoparticles for gene delivery: Synthesis, preparation and in vitro transfection efficiency. Int. J. Pharm. 2010, 392, 241–248. [Google Scholar] [CrossRef]
- Mathew, A.; Cao, H.; Collin, E.; Wang, W.; Pandit, A. Hyperbranched PEGmethacrylate linear pDMAEMA block copolymer as an efficient non-viral gene delivery vector. Int. J. Pharm. 2012, 434, 99–105. [Google Scholar] [CrossRef]
- Qiao, Y.; Huang, Y.; Qiu, C.; Yue, X.; Deng, L.; Wan, Y.; Xing, J.; Zhang, C.; Yuan, S.; Dong, A.; et al. The use of PEGylated poly [2-(N,N-dimethylamino) ethyl methacrylate] as a mucosal DNA delivery vector and the activation of innate immunity and improvement of HIV-1-specific immune responses. Biomaterials 2010, 31, 115–123. [Google Scholar] [CrossRef]
- Han, S.; Hagiwara, M.; Ishizone, T. Synthesis of Thermally Sensitive Water-Soluble Polymethacrylates by Living Anionic Polymerizations of Oligo(ethylene glycol) Methyl Ether Methacrylates. Macromolecules 2003, 36, 8312–8319. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Förster, S.; Zisenis, M.; Wenz, E.; Antonietti, M. Micellization of strongly segregated block copolymers. J. Chem. Phys. 1996, 104, 9956–9970. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Chernyshov, D.M.; Timofeeva, G.I.; Dubrovina, L.V.; Valetsky, P.M.; Khokhlov, A.R. Polystyrene-block-Poly(ethylene oxide) Micelles in Aqueous Solution. Langmuir 1999, 15, 6195–6200. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Bronich, T.K.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Block Ionomer Complexes from Polystyrene-block-polyacrylate Anions and N-Cetylpyridinium Cations. Macromolecules 1998, 31, 4511–4515. [Google Scholar] [CrossRef]




| Polymer | y/x * | S0 × 1013, c | D0 × 107, cm2/c | υ, cm3/g | MSD, kD | Rg, nm | C mmol/g |
|---|---|---|---|---|---|---|---|
| PDMAEMA-co-PEO-1 | 78 | 2.35 | 2.43 | 0.847 | 165 | 10 | 5.47 |
| PDMAEMA-co-PEO-2 | 170 | 2.7 | 2.1 | 0.860 | 228 | 12 | 5.9 |
| PDMAEMA-co-PEO-3 | 330 | 2.97 | 1.5 | 0.840 | 392 | 17 | 6.11 |
| PDMAEMA-co-PEO-4 | 40 | 5.26 | 1.5 | 0.845 | 620 | 23 | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loginova, T.P.; Khotina, I.A.; Kabachii, Y.A.; Kochev, S.Y.; Abramov, V.M.; Khlebnikov, V.S.; Kulikova, N.L.; Mezhuev, Y.O. Promising Gene Delivery Properties of Polycations Based on 2-(N,N-dimethylamino)ethyl Methacrylate and Polyethylene Glycol Monomethyl Ether Methacrylate Copolymers. Polymers 2023, 15, 3036. https://doi.org/10.3390/polym15143036
Loginova TP, Khotina IA, Kabachii YA, Kochev SY, Abramov VM, Khlebnikov VS, Kulikova NL, Mezhuev YO. Promising Gene Delivery Properties of Polycations Based on 2-(N,N-dimethylamino)ethyl Methacrylate and Polyethylene Glycol Monomethyl Ether Methacrylate Copolymers. Polymers. 2023; 15(14):3036. https://doi.org/10.3390/polym15143036
Chicago/Turabian StyleLoginova, Tatiana P., Irina A. Khotina, Yurii A. Kabachii, Sergei Yu. Kochev, Vyacheslav M. Abramov, Valentin S. Khlebnikov, Natalia L. Kulikova, and Yaroslav O. Mezhuev. 2023. "Promising Gene Delivery Properties of Polycations Based on 2-(N,N-dimethylamino)ethyl Methacrylate and Polyethylene Glycol Monomethyl Ether Methacrylate Copolymers" Polymers 15, no. 14: 3036. https://doi.org/10.3390/polym15143036
APA StyleLoginova, T. P., Khotina, I. A., Kabachii, Y. A., Kochev, S. Y., Abramov, V. M., Khlebnikov, V. S., Kulikova, N. L., & Mezhuev, Y. O. (2023). Promising Gene Delivery Properties of Polycations Based on 2-(N,N-dimethylamino)ethyl Methacrylate and Polyethylene Glycol Monomethyl Ether Methacrylate Copolymers. Polymers, 15(14), 3036. https://doi.org/10.3390/polym15143036








