Ductile Effect of PGA/PCL Blending Plastics Using a Novel Ionic Chain Extender with Non-Covalent Bonds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chain Extenders
2.3. Compounding of PGA/PCL Blending Plastics with/without Chain Extenders
2.4. Characterization
3. Results
3.1. Chemical Structure of the Chain Extenders which Functionalized into Different Groups
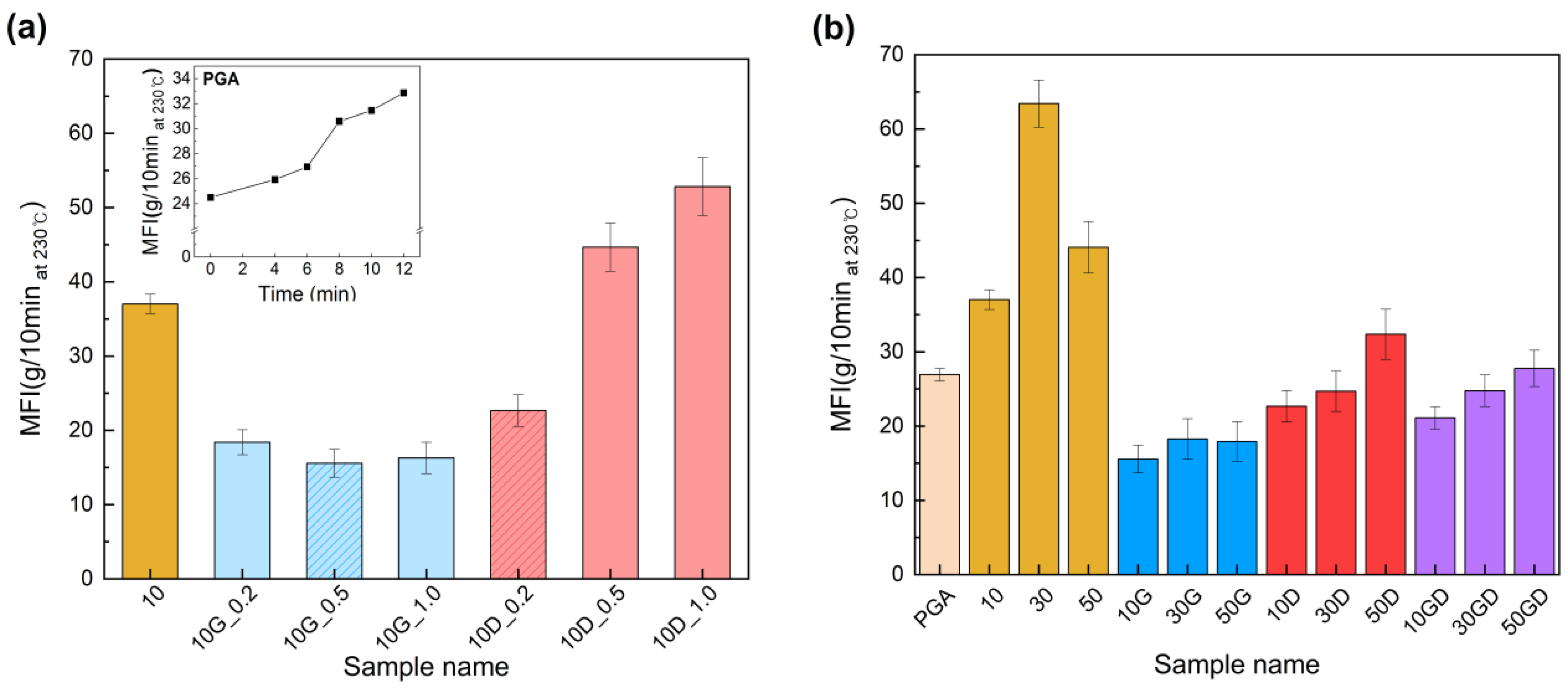
3.2. Evaluation of Changes in Melt Flow Index Characteristics according to Heat Exposure Times and Chain Extender Contents
3.3. Crystallinity of PGA/PCL Blending Plastics according to Chain Extenders
3.4. Ductile properties of PGA/PCL Blending Plastics with Novel Chain Extenders
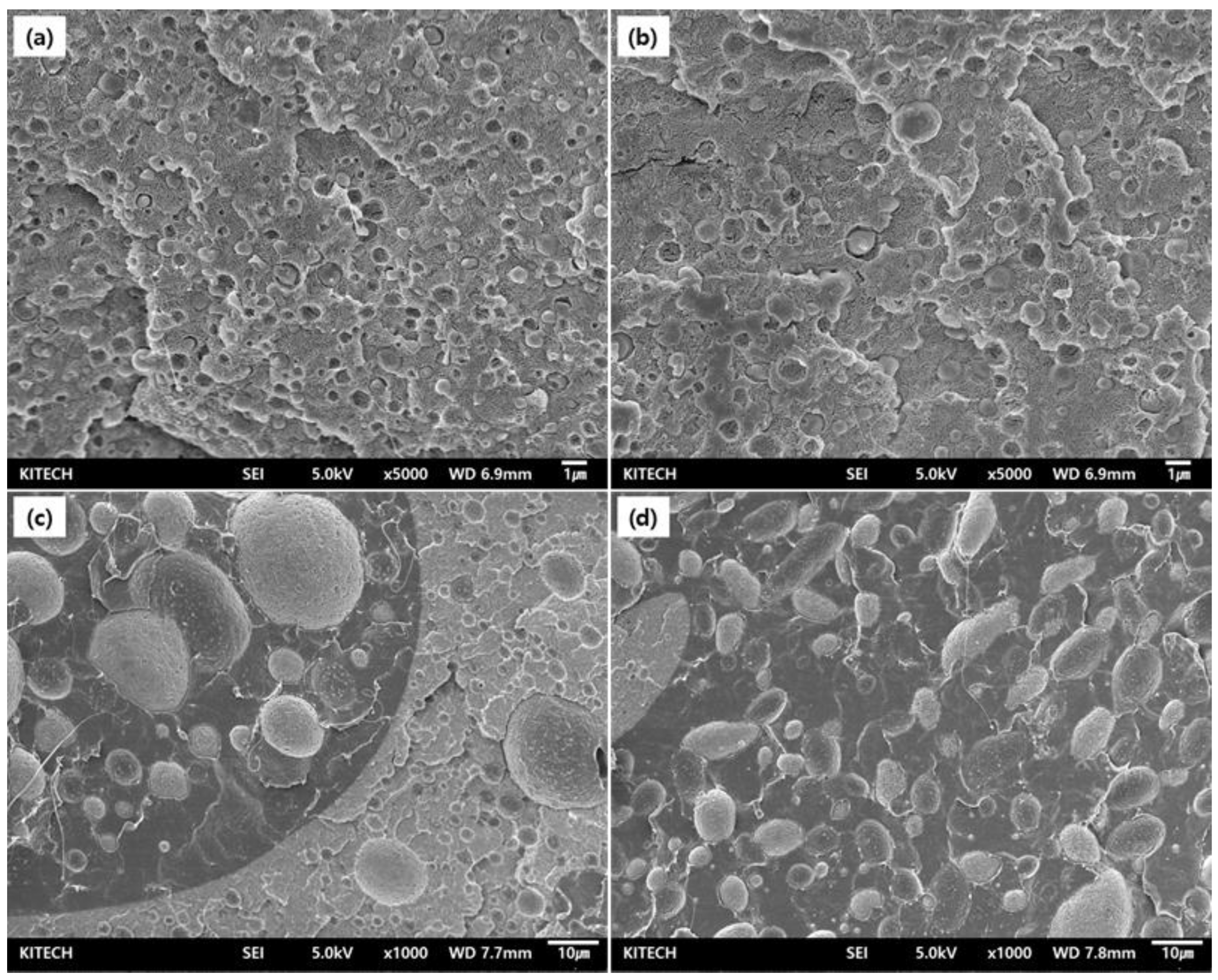
3.5. Morphology Behavior of PGA/PCL Blending Polymers Matrix According to Type of Chain Extenders
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green. Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly (lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Xi, Z.; Zhao, L.; Cen, L.; Yang, Y. A Comparable Study of Polyglycolic acid’s Degradation on macrophages’ Activation. Mater. Sci. Eng. C 2020, 109, 110574. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (lactic Acid) and Poly (glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Yamane, K.; Sato, H.; Ichikawa, Y.; Sunagawa, K.; Shigaki, Y. Development of an industrial production technology for high-molecular-weight polyglycolic acid. Polym. J. 2014, 46, 769–775. [Google Scholar] [CrossRef]
- Schmidt, C.; Behl, M.; Lendlein, A.; Beuermann, S. Synthesis of High Molecular Weight Polyglycolide in Supercritical Carbon Dioxide. RSC Adv. 2014, 4, 35099–35105. [Google Scholar] [CrossRef]
- Göktürk, E.; Pemba, A.G.; Miller, S.A. Polyglycolic Acid from the Direct Polymerization of Renewable C1 Feedstocks. Polym. Chem. 2015, 6, 3918–3925. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Ellingford, C.; Farris, S.; O’Sullivan, C.; Tan, B.; Sun, Z.; McNally, T.; Wan, C. Electron beam-mediated cross-linking of blown film-extruded biodegradable PGA/PBAT blends toward high toughness and low oxygen permeation. ACS Sustain. Chem. Eng. 2022, 10, 1267–1276. [Google Scholar] [CrossRef]
- Rigotti, D.; Soccio, M.; Dorigato, A.; Gazzano, M.; Siracusa, V.; Fredi, G.; Lotti, N. Novel biobased polylactic acid/poly (pentamethylene 2, 5-furanoate) blends for sustainable food packaging. ACS Sustain. Chem. Eng. 2021, 9, 13742–13750. [Google Scholar] [CrossRef]
- Nakafuku, C.; Yoshimura, H. Melting parameters of poly (glycolic acid). Polymer 2014, 45, 3583–3585. [Google Scholar] [CrossRef]
- Gautier, E.; Fuertes, P.; Cassagnau, P.; Pascault, J.P.; Fleury, E. Synthesis and rheology of biodegradable poly (glycolic acid) prepared by melt ring-opening polymerization of glycolide. J. Polym. Sci. Part A 2009, 47, 1440–1449. [Google Scholar] [CrossRef]
- Wang, K.; Shen, J.; Ma, Z.; Zhang, Y.; Xu, N.; Pang, S. Preparation and Properties of Poly (ethylene glycol-co-cyclohexane-1, 4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends. Polymers 2021, 13, 452. [Google Scholar] [CrossRef]
- Liu, G.C.; He, Y.S.; Zeng, J.B.; Xu, Y.; Wang, Y.Z. In situ formed crosslinked polyurethane toughened polylactide. Polym. Chem. 2014, 5, 2530–2539. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, S. Properties of maleic anhydride-modified lignin nanoparticles/polybutylene adipate-co-terephthalate composites. J. Appl. Polym. Sci. 2020, 137, 49025. [Google Scholar] [CrossRef]
- Pinheiro, I.F.; Ferreira, F.V.; Alves, G.F.; Rodolfo, A.; Morales, A.R.; Mei, L.H.I. Biodegradable PBAT-based nanocomposites reinforced with functionalized cellulose nanocrystals from Pseudobombax munguba: Rheological, thermal, mechanical and biodegradability properties. J. Polym. Environ. 2019, 27, 757–766. [Google Scholar] [CrossRef]
- Lai, L.; Li, J.; Liu, P.; Wu, L.; Severtson, S.J.; Wang, W.J. Mechanically reinforced biodegradable Poly (butylene adipate-co-terephthalate) with interactive nanoinclusions. Polymer 2020, 197, 122518. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Cheng, J.; Anstey, A.; Mohanty, A.K.; Misra, M. Development of toughened blends of poly (lactic acid) and poly (butylene adipate-co-terephthalate) for 3d printing applications: Compatibilization methods and material performance evaluation. ACS Sustain. Chem. Eng. 2020, 8, 6576–6589. [Google Scholar] [CrossRef]
- Hedrick, M.M.; Wu, F.; Mohanty, A.K.; Misra, M. Morphology and performance relationship studies on biodegradable ternary blends of poly (3-hydroxybutyrate-co-3-hydroxyvalerate), polylactic acid, and polypropylene carbonate. RSC Adv. 2020, 10, 44624–44632. [Google Scholar] [CrossRef]
- Kumar, V.; Sehgal, R.; Gupta, R. Blends and composites of polyhydroxyalkanoates (PHAs) and their applications. Eur. Polym. J. 2021, 161, 110824. [Google Scholar] [CrossRef]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Poly (ε-caprolactone)/poly (lactic acid) blends compatibilized by peroxide initiators: Comparison of two strategies. Polymers 2020, 12, 228. [Google Scholar] [CrossRef]
- Nishimura, F.; Hoshina, H.; Ozaki, Y.; Sato, H. Isothermal crystallization of poly (glycolic acid) studied by terahertz and infrared spectroscopy and SAXS/WAXD simultaneous measurements. Polym. J. 2019, 51, 237–245. [Google Scholar] [CrossRef]
- Sato, H.; Miyada, M.; Yamamoto, S.; Reddy, K.R.; Ozaki, Y. C–H⋯O (ether) hydrogen bonding along the (110) direction in polyglycolic acid studied by infrared spectroscopy, wide-angle X-ray diffraction, quantum chemical calculations and natural bond orbital calculations. RSC Adv. 2016, 6, 16817–16823. [Google Scholar] [CrossRef]
- Rasselet, D.; Caro-Bretelle, A.S.; Taguet, A.; Lopez-Cuesta, J.M. Reactive compatibilization of PLA/PA11 blends and their application in additive manufacturing. Materials 2019, 12, 485. [Google Scholar] [CrossRef]
- Kahraman, Y.; Özdemir, B.; Gümüş, B.E.; Nofar, M. Morphological, rheological, and mechanical properties of PLA/TPU/nanoclay blends compatibilized with epoxy-based Joncryl chain extender. Colloid Polym. Sci. 2023, 301, 51–62. [Google Scholar] [CrossRef]
- Matumba, K.I.; Motloung, M.P.; Ojijo, V.; Ray, S.S.; Sadiku, E.R. Investigation of the Effects of Chain Extender on Material Properties of PLA/PCL and PLA/PEG Blends: Comparative Study between Polycaprolactone and Polyethylene Glycol. Polymers 2023, 15, 2230. [Google Scholar] [CrossRef] [PubMed]
- Palsikowski, P.A.; Kuchnier, C.N.; Pinheiro, I.F.; Morales, A.R. Biodegradation in soil of PLA/PBAT blends compatibilized with chain extender. J. Polym. Environ. 2018, 26, 330–341. [Google Scholar] [CrossRef]
- Tatai, L.; Moore, T.G.; Adhikari, R.; Malherbe, F.; Jayasekara, R.; Griffiths, I.; Gunatillake, P.A. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Liu, R.; Liang, Z.; Yang, J.; Zhang, R.; Zhou, Z.; Nie, Y. Design of Self-Healing Rubber by Introducing Ionic Interaction to Construct a Network Composed of Ionic and Covalent Cross-Linking. Ind. Eng. Chem. Res. 2019, 58, 14848–14858. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M. Self-Healing of Polymers via Supramolecular Chemistry. Adv. Mater. Interfaces 2018, 5, 1800384. [Google Scholar] [CrossRef]
- Mai, D.; Mo, J.; Shan, S.; Lin, Y.; Zhang, A. Self-Healing, Self-Adhesive Strain Sensors Made with Carbon Nanotubes/Polysiloxanes Based on Unsaturated Carboxyl–Amine Ionic Interactions. ACS Appl. Mater. Interfaces 2021, 13, 49266–49278. [Google Scholar] [CrossRef]
- Wah, C.A.; Choong, L.Y.; Neon, G.S. Effects of titanate coupling agent on rheological behaviour, dispersion characteristics and mechanical properties of talc filled polypropylene. Eur. Polym. J. 2000, 36, 789–801. [Google Scholar] [CrossRef]
- Liu, Z.; Gilbert, M. Structure and properties of talc-filled polypropylene: Effect of phosphate coating. J. Appl. Polym. Sci. 1996, 59, 1087–1098. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Shi, B.; Mai, D.; Du, X.; Lin, B. Synthesis and thermal analysis of methacrylate ester-based linear triblock copolymers-grafted multiwalled carbon nanotubes. J. Therm. Anal. Calorim. 2015, 119, 2029–2037. [Google Scholar] [CrossRef]
- Comí, M.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Adaptive bio-based polyurethane elastomers engineered by ionic hydrogen bonding interactions. Eur. Polym. J. 2017, 91, 408–419. [Google Scholar] [CrossRef]
- Magazzini, L.; Grilli, S.; Fenni, S.E.; Donetti, A.; Cavallo, D.; Monticelli, O. The Blending of Poly (glycolic acid) with Polycaprolactone and Poly (l-lactide): Promising Combinations. Polymers 2021, 13, 2780. [Google Scholar] [CrossRef] [PubMed]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Lorenzetti, C.; Cavallo, D.; Ocando, C.; Müller, A.J.; Androsch, R. Polymorphism and Multiple Melting Behavior of Bio-Based Poly(propylene 2,5-furandicarboxylate). Biomacromolecules 2020, 21, 2622–2634. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.X.; Chen, H.; Yu, X.L.; Zhao, X.P. Mechanical properties, rheological behaviors, and phase morphologies of high-toughness PLA/PBAT blends by in-situ reactive compatibilization. Compos. Part B Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Sangroniz, L.; Wang, B.; Su, Y.; Liu, G.; Cavallo, D.; Wang, D.; Müller, A.J. Fractionated crystallization in semicrystalline polymers. Prog. Polym. Sci. 2021, 115, 101376. [Google Scholar] [CrossRef]


| Sample Name | PGA (g) | PCL (g) | D-CE *a (g) | G-CE *b (g) | ADR-4368 *c (g) |
|---|---|---|---|---|---|
| PGA | 150 | - | - | - | - |
| 10 | 135 | 15 | - | - | - |
| 30 | 105 | 45 | - | - | - |
| 50 | 75 | 75 | - | - | - |
| 10D (10D_0.2) | 135 | 15 | 0.30 | - | - |
| 10D_0.5 | 135 | 15 | 0.75 | - | - |
| 10D_1.0 | 135 | 15 | 1.50 | - | - |
| 30D | 105 | 45 | 0.30 | - | - |
| 50D | 75 | 75 | 0.30 | - | - |
| 10G_0.2 | 135 | 15 | - | 0.30 | - |
| 10G (10G_0.5) | 135 | 15 | - | 0.75 | - |
| 10G_1.0 | 135 | 15 | - | 1.50 | - |
| 30G | 105 | 45 | - | 0.30 | - |
| 50G | 75 | 75 | - | 0.30 | - |
| 10J | 135 | 15 | - | - | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-J.; Jang, J.; Koh, W.-G.; Lee, J.-Y.; Hwang, K. Ductile Effect of PGA/PCL Blending Plastics Using a Novel Ionic Chain Extender with Non-Covalent Bonds. Polymers 2023, 15, 3025. https://doi.org/10.3390/polym15143025
Kwon H-J, Jang J, Koh W-G, Lee J-Y, Hwang K. Ductile Effect of PGA/PCL Blending Plastics Using a Novel Ionic Chain Extender with Non-Covalent Bonds. Polymers. 2023; 15(14):3025. https://doi.org/10.3390/polym15143025
Chicago/Turabian StyleKwon, Hyuk-Jun, Joseph Jang, Won-Gun Koh, Jun-Young Lee, and Kiseob Hwang. 2023. "Ductile Effect of PGA/PCL Blending Plastics Using a Novel Ionic Chain Extender with Non-Covalent Bonds" Polymers 15, no. 14: 3025. https://doi.org/10.3390/polym15143025
APA StyleKwon, H.-J., Jang, J., Koh, W.-G., Lee, J.-Y., & Hwang, K. (2023). Ductile Effect of PGA/PCL Blending Plastics Using a Novel Ionic Chain Extender with Non-Covalent Bonds. Polymers, 15(14), 3025. https://doi.org/10.3390/polym15143025







